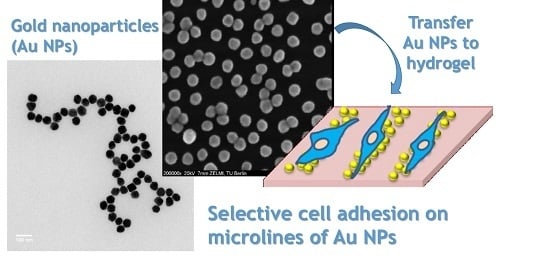
Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumental
2.3. Preparation of Hydrogels
2.3.1. 3BC-UV Hydrogel
2.3.2. PEG575 Hydrogel
2.3.3. 8PEG-UV Hydrogel
2.3.4. Synthesis of 8PEG-VS-SH Hydrogel
2.4. Deposition of Au NPs on Silicon Wafers
2.5. Fabrication of Micro-Patterned Block Polymer Hydrogel Replicas Transfer of the Patterned Au NPs from Silicon Wafer to 8PEG-VS-SH Hydrogels
2.6. Transfer of the Patterned Au NPs from Silicon Wafer to 8PEG-VS-SH Hydrogels
2.7. Cell Culture
Incubation with L-929 Cells
3. Results and Discussion
3.1. Characterization of Au NPs Immobilized on the Surface of Hydrogels by UV-Vis Spectroscopy
3.2. Characterization of Immobilized Au NPs on the Surface of Hydrogels by SEM
3.3. Synthesis of 8PEG-VS-SH Hydrogel with Patterned Au NPs
3.4. Cell Adhesion and Cell Spreading
3.5. Cell Viability
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Tiwari, P.; Vig, K.; Dennis, V.; Singh, S. Functionalized Gold Nanoparticles and Their Biomedical Applications. Nanomaterials 2011, 1, 31–63. [Google Scholar] [CrossRef] [PubMed]
- Giljohann, D.A.; Seferos, D.S.; Daniel, W.L.; Massich, M.D.; Patel, P.C.; Mirkin, C.A. Gold Nanoparticles for Biology and Medicine. Angew. Chem. Int. Ed. 2010, 49, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Agasti, S.S.; Kim, C.; Li, X.; Rotello, V.M. Gold nanoparticles in chemical and biological sensing. Chem. Rev. 2012, 112, 2739–2779. [Google Scholar] [CrossRef] [PubMed]
- Dykman, L.; Khlebtsov, N. Gold nanoparticles in biomedical applications: Recent advances and perspectives. Chem. Soc. Rev. 2012, 41, 2256–2282. [Google Scholar] [CrossRef] [PubMed]
- Abadeer, N.S.; Murphy, C.J. Recent Progress in Cancer Thermal Therapy Using Gold Nanoparticles. J. Phys. Chem. C 2016, 120, 4691–4716. [Google Scholar] [CrossRef]
- Du, Y.; Luo, X.-L.; Xu, J.-J.; Chen, H.-Y. A simple method to fabricate a chitosan-gold nanoparticles film and its application in glucose biosensor. Bioelectrochemistry 2007, 70, 342–347. [Google Scholar] [CrossRef] [PubMed]
- Nanoarrays, G.; Manipulate, T.; Zhu, M.; Baffou, G.; Meyerbro, N. Adhesion. ACS Nano 2012, 8, 7227–7233. [Google Scholar]
- Tao, Y.; Ju, E.; Ren, J.; Qu, X. Bifunctionalized mesoporous silica-supported gold nanoparticles: Intrinsic oxidase and peroxidase catalytic activities for antibacterial applications. Adv. Mater. 2015, 27, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.A.; Zouani, Q.F.; Glinel, K.; Jonas, A.M.; Durrieu, M.-C. Correction to bioactive chemical nanopatterns impact human mesenchymal stem cell fate. Nano Lett. 2013, 13, 4996. [Google Scholar] [CrossRef]
- Geiger, B.; Spatz, J.P.; Bershadsky, A.D. Environmental sensing through focal adhesions. Nat. Rev. Mol. Cell Biol. 2009, 10, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Yap, F.L.; Zhang, Y. Protein and cell micropatterning and its integration with micro/nanoparticles assembly. Biosens. Bioelectron. 2007, 22, 775–788. [Google Scholar] [CrossRef] [PubMed]
- Saha, K.; Keung, A.J.; Irwin, E.F.; Li, Y.; Little, L.; Schaffer, D.V.; Healy, K.E. Substrate modulus directs neural stem cell behavior. Biophys. J. 2008, 95, 4426–4438. [Google Scholar] [CrossRef] [PubMed]
- Discher, D.E.; Janmey, P.A.; Wang, Y. Tissue cells feel and respond to the stiffness of their substrate. Science 2005, 310, 1139–1143. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Functional PEG-Hydrogels Convey Gold Nanoparticles from Silicon and Aid Cell Adhesion onto the Nanocomposites. Chem. Mater. 2017, 29, 2008–2015. [Google Scholar] [CrossRef]
- Dulgar-Tulloch, A.-J.; Bizios, R.; Siegel, R.W. Human mesenchymal stem cell adhesion and proliferation in response to ceramic chemistry and nanoscale topography. J. Biomed. Mater. Res. Part A 2009, 90, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Li, S.; Yan, C.; Liu, P.; Ding, J. Fabrication of RGD Micro/Nanopattern and Corresponding Study of Stem Cell Differentiation. Nano Lett. 2015, 15, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Grater, S.V.; Corbellini, F.; Rinck, S.; Bock, E.; Kemkemer, R.; Kessler, H.; Ding, J.; Spatz, J.P. Impact of order and disorder in RGD nanopatterns on cell adhesion. Nano Lett. 2009, 9, 1111–1116. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mela, P.; Möller, M.; Lensen, M.C. Microcontact deprinting: A technique to pattern gold nanoparticles. ACS Nano 2009, 3, 1451–1456. [Google Scholar] [CrossRef] [PubMed]
- Seliktar, D. Designing Cell-Compatible Hydrogels. Science 2012, 336, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Anseth, K.S. Hydrogels as extracellular matrix mimics for 3D cell culture. Biotechnol. Bioeng. 2009, 103, 655–663. [Google Scholar] [CrossRef] [PubMed]
- Hennink, W.E.; van Nostrum, C.F. Novel crosslinking methods to design hydrogels. Adv. Drug Deliv. Rev. 2012, 64, 223–236. [Google Scholar] [CrossRef]
- Hoffman, A.S. Hydrogels for biomedical applications. Ann. N. Y. Acad. Sci. 2001, 944, 62–73. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.G.; Lee, K.-H.; Khademhosseini, A.; Lee, S.-H. Microfluidic fabrication of microengineered hydrogels and their application in tissue engineering. Lab Chip 2012, 12, 45. [Google Scholar] [CrossRef] [PubMed]
- Nuttelman, C.R.; Rice, M.A.; Rydholm, A.E.; Salinas, C.N.; Shah, D.N.; Anseth, K.S. Macromolecular monomers for the synthesis of hydrogel niches and their application in cell encapsulation and tissue engineering. Prog. Polym. Sci. 2008, 33, 167–179. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.T.; García, J.R.; Paez, J.I.; Singh, A.; Phelps, E.A.; Weis, S.; Shafiq, Z.; Shekaran, A.; Del Campo, A.; García, A.J. Light-triggered in vivo activation of adhesive peptides regulates cell adhesion, inflammation and vascularization of biomaterials. Nat. Mater. 2015, 14, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Minami, K.; Kasuya, Y.; Yamazaki, T.; Ji, Q.; Nakanishi, W.; Hill, J.P.; Sakai, H.; Ariga, K. Highly Ordered 1D Fullerene Crystals for Concurrent Control of Macroscopic Cellular Orientation and Differentiation toward Large-Scale Tissue Engineering. Adv. Mater. 2015, 27, 4020–4026. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, V.; Kasuya, Y.; Ji, Q.; Sathish, M.; Shrestha, L.K.; Ishihara, S.; Minami, K.; Morita, H.; Yamazaki, T.; Hanagata, N.; et al. Vortex-Aligned Fullerene Nanowhiskers as a Scaffold for Orienting Cell Growth. ACS Appl. Mater. Interfaces 2015, 7, 15667–15673. [Google Scholar] [CrossRef] [PubMed]
- Nurgaziyeva, E.K.; Tatykhanova, G.S.; Mun, G.A.; Khutoryanskiy, V.V.; Kudaibergenov, S.E. Catalytic Properties of Gel-Immobilized Gold Nanoparticles in Decomposition of Hydrogen Peroxide. Ph.D. Thesis, Sumy State University, Sumy, Ukraine, 2015. [Google Scholar]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle-Hydrogel Composites: Concept, Design, and Applications of These Promising, Multi-Functional Materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, S.; Madaboosi, N.; Uhlig, K.; Köhler, D.; Skirtach, A.; Duschl, C.; Möhwald, H.; Volodkin, D.V. Control of cell adhesion by mechanical reinforcement of soft polyelectrolyte films with nanoparticles. Langmuir 2012, 28, 7249–7257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Loebus, A.; de Vicente, G.; Ren, F.; Arafeh, M.; Ouyang, Z.; Lensen, M.C. Synthesis of Poly(ethylene glycol)-based Hydrogels via Amine-Michael Type Addition with Tunable Stiffness and Postgelation Chemical Functionality. Chem. Mater. 2014, 26, 3624–3630. [Google Scholar] [CrossRef]
- Zhang, F.; Sautter, K.; Larsen, A.M.; Findley, D.A.; Davis, R.C.; Samha, H.; Linford, M.R. Chemical Vapor Deposition of Three Aminosilanes on Silicon Dioxide: Surface Characterization, Stability, Effects of Silane Concentration, and Cyanine Dye Adsorption. Langmuir 2010, 26, 14648–14654. [Google Scholar] [CrossRef] [PubMed]
- Strehmel, C.; Zhang, Z.; Strehmel, N.; Lensen, M.C. Cell phenotypic changes of mouse connective tissue fibroblasts (L-929) to poly(ethylene glycol)-based gels. Biomater. Sci. 2013, 1, 850. [Google Scholar] [CrossRef]
- Murphy-Ullrich, J.E. The de-adhesive activity of matricellular proteins: Is intermediate cell adhesion an adaptive state? J. Clin. Investig. 2001, 107, 785–790. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Erpenbeck, L.; Amschler, K.; Mundinger, T.A.; Boehm, H.; Helms, H.-J.; Friede, T.; Andrews, R.K.; Schön, M.P.; Spatz, J.P. Adhesion maturation of neutrophils on nanoscopically presented platelet glycoprotein Ibα. ACS Nano 2013, 7, 9984–9996. [Google Scholar] [CrossRef] [PubMed]
- Kolesnikova, T.A.; Kohler, D.; Skirtach, A.G.; Möhwald, H. Laser-induced cell detachment, patterning, and regrowth on gold nanoparticle functionalized surfaces. ACS Nano 2012, 6, 9585–9595. [Google Scholar] [CrossRef] [PubMed]
- Ye, K.; Wang, X.; Cao, L.; Li, S.; Li, Z.; Yu, L.; Ding, J. Matrix Stiffness and Nanoscale Spatial Organization of Cell-Adhesive Ligands Direct Stem Cell Fate. Nano Lett. 2015, 15, 4720–4729. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kloxin, C.J.; Bowman, C.N.; Anseth, K.S. Mechanical properties of cellularly responsive hydrogels and their experimental determination. Adv. Mater. 2010, 22, 3484–3494. [Google Scholar] [CrossRef] [PubMed]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of Stem Cell Fate by Physical Interactions with the Extracellular Matrix. Cell Stem Cell 2009, 5, 17–26. [Google Scholar] [CrossRef] [PubMed]












| Material | PEG-b-PPG-b-PEG Diacrylate (3BC) | PEG Diacrylate (PEG) | 8-arm PEG Acrylate (8PEG) | 8-arm PEG Vinyl Sulfone Acrylate (8PEG-VS) |
|---|---|---|---|---|
| Structure |  |  |  |  |
| Mw [Da] | 4400 | 575 | 15000 | 15000 |
| Chain Length | n + p ~12; m ~57 | n ~13 | n ~40 | n ~40 |
| PEG [%] | 30 (70 % PPG) | 100 | 100 | 100 |
| State at r.t. | Liquid | Liquid | Solid | Liquid |
| Gel formation | UV | UV | UV | Michael Addition Reaction |
| Hydrogels | Transfer efficiency (%) |
|---|---|
| 3BC-UV | 99.8 ± 0.1 |
| PEG575-UV | 0 |
| 8PEG-UV | 0 |
| 8PEG-VS-SH | 98 ± 1.8 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, F.; Yesildag, C.; Zhang, Z.; Lensen, M.C. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers 2017, 9, 154. https://doi.org/10.3390/polym9050154
Ren F, Yesildag C, Zhang Z, Lensen MC. Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers. 2017; 9(5):154. https://doi.org/10.3390/polym9050154
Chicago/Turabian StyleRen, Fang, Cigdem Yesildag, Zhenfang Zhang, and Marga C. Lensen. 2017. "Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion" Polymers 9, no. 5: 154. https://doi.org/10.3390/polym9050154
APA StyleRen, F., Yesildag, C., Zhang, Z., & Lensen, M. C. (2017). Surface Patterning of Gold Nanoparticles on PEG-Based Hydrogels to Control Cell Adhesion. Polymers, 9(5), 154. https://doi.org/10.3390/polym9050154