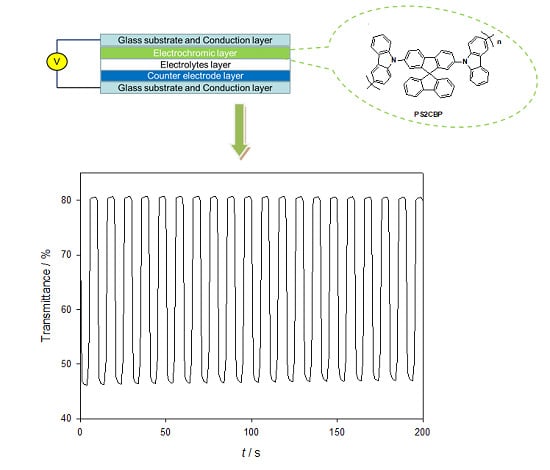
Three Carbazole-Based Polymers as Potential Anodically Coloring Materials for High-Contrast Electrochromic Devices
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Electrochemical Synthesis
2.1.1. Synthesis of 3,6-Di(2-thienyl)carbazole (DTC)
2.1.2. Synthesis of 2,7-Bis(carbazol-9-yl)-9,9-spirobifluorene (S2CBP)
2.1.3. Synthesis of 3,6-Bis(N-carbazolyl)-N-ethylcarbazole (CEC)
2.2. Instrumentation and Measurements
2.3. Construction of ECDs
3. Results and Discussion
3.1. Electrochemical Polymerization of Polymer Films
3.2. Electrochromic Properties of the PDTC, PS2CBP, and PCEC Films
3.3. Spectroelectrochemistry of Electrochromic Devices
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- ÎçIi-Őzkut, M.; Mersini, J.; Őnal, A.M.; Cihaner, A. Substituent and heteroatom effects on the electrochromic properties of similar systems. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 615–621. [Google Scholar] [CrossRef]
- Thakur, V.K.; Ding, G.; Ma, J.; Lee, P.S.; Lu, X. Hybrid materials and polymer electrolytes for electrochromic device applications. Adv. Mater. 2012, 24, 4071–4096. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.S.; Kao, S.Y.; Chang, T.H.; Vittal, R.; Ho, K.C. A high contrast solid-state electrochromic device based on nano-structural Prussian blue and poly(butyl viologen) thin films. Sol. Energy Mater. Sol. Cells 2016, 145, 35–41. [Google Scholar] [CrossRef]
- Chang, K.H.; Wang, H.P.; Wu, T.Y.; Sun, I.W. Optical and electrochromic characterizations of four 2,5-dithienylpyrrole-based conducting polymer films. Electrochim. Acta 2014, 119, 225–235. [Google Scholar] [CrossRef]
- Carbas, B.B.; Kivrak, A.; Teke, E.; Zora, M.; Önal, A.M. Electrochemical polymerization of a new low-voltage oxidized thienylenepyrrole derivative and its electrochromic device application. J. Electroanal. Chem. 2014, 729, 15–20. [Google Scholar] [CrossRef]
- Kuo, C.W.; Hsieh, T.H.; Hsieh, C.K.; Liao, J.W.; Wu, T.Y. Electrosynthesis and characterization of four electrochromic polymers based on carbazole and indole-6-carboxylic acid and their applications in high-contrast electrochromic devices. J. Electrochem. Soc. 2014, 161, D782–D790. [Google Scholar] [CrossRef]
- Liu, Y.; Chao, D.; Yao, H. New triphenylamine-based poly(amine-imide)s with carbazole-substituents for electrochromic applications. Org. Electron. 2014, 15, 1422–1431. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.W. Electrochemical synthesis of electrochromic polycarbazole films from N-phenyl-3,6-bis(N-carbazolyl)carbazoles. Polym. Chem. 2016, 7, 198–211. [Google Scholar] [CrossRef]
- Cui, C.; Xu, C.; Xu, L.; Zhao, J.; Liu, R.; Liu, J.; He, Q.; Wang, H. Electrosynthesis and characterization of a multielectrochromic copolymer of 1,4-bis(2-thienyl)-naphthalene with 2,2′-bithiophene. Opt. Mater. 2011, 33, 1792–1799. [Google Scholar] [CrossRef]
- Balan, A.; Gunbas, G.; Durmus, A.; Toppare, L. Donor–acceptor polymer with benzotriazole moiety: Enhancing the electrochromic properties of the “donor unit”. Chem. Mater. 2008, 20, 7510–7513. [Google Scholar] [CrossRef]
- Wang, G.; Fu, X.; Huang, J.; Wu, L.; Deng, J. Synthesis, electrochemical and fluorescence properties of three new dithienylpyrroles bearing aromatic amine units. J. Electroanal. Chem. 2011, 661, 351–358. [Google Scholar] [CrossRef]
- Otley, M.T.; Alamer, F.A.; Zhu, Y.; Singhaviranon, A.; Zhang, X.; Li, M.; Kumar, A.; Sotzing, G.A. Acrylated poly(3,4-propylenedioxythiophene) for enhancement of lifetime and optical properties for single-layer electrochromic devices. ACS Appl. Mater. Interfaces 2014, 6, 1734–1739. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.Y.; Su, Y.S. Electrochemical synthesis and characterization of 1,4-benzodioxan-based electrochromic polymer and its application in electrochromic devices. J. Electrochem. Soc. 2015, 162, G103–G112. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.L.; Lin, Y.C.; Chang, J.K.; Chen, H.R.; Wu, T.Y. Copolymers based on 1,3-bis(carbazol-9-yl)benzene and three 3,4-ethylenedioxythiophene derivatives as potential anodically coloring copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 368. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wang, H.M. Electrochemically fabricated electrochromic films from 4-(N-carbazolyl)triphenylamine and its dimethoxy derivative. RSC Adv. 2016, 6, 43470–43479. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Lin, S.W. The electrochemical fabrication of electroactive polymer films from diamide- or diimide-cored N-phenylcarbazole dendrons for electrochromic applications. J. Mater. Chem. 2016, 4, 1271–1280. [Google Scholar] [CrossRef]
- Koyuncu, F.B. An ambipolar electrochromic polymer based on carbazole and naphthalene bisimide: Synthesis and electro-optical properties. Electrochim. Acta 2012, 68, 184–191. [Google Scholar] [CrossRef]
- Aydın, A.; Kaya, Í. Syntheses, characterizations and electrochromic applications of polymers derived from carbazole containing thiophene rings in side chain with electrochemical and FeCl3 methods. Org. Electron. 2013, 14, 730–743. [Google Scholar] [CrossRef]
- Wu, T.Y.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, Y.C.; Lai, C.A.; Sun, I.W. Ionic liquids containing an alkyl sulfate group as potential electrolytes. Electrochim. Acta 2010, 55, 4475–4482. [Google Scholar] [CrossRef]
- Sun, I.W.; Wang, H.P.; Teng, H.; Su, S.G.; Lin, Y.C.; Kuo, C.W.; Chen, P.R.; Wu, T.Y. Cyclic ammonium-based ionic liquids as potential electrolytes for dye-sensitized solar cells. Int. J. Electrochem. Sci. 2012, 7, 9748–9764. [Google Scholar]
- Wu, T.Y.; Wang, H.C.; Su, S.G.; Gung, S.T.; Lin, M.W.; Lin, C.B. Characterization of ionic conductivity, viscosity, density, and self-diffusion coefficient for binary mixtures of polyethyleneglycol (or polyethyleneimine) organic solvent with room temperature ionic liquid BMIBF4 (or BMIPF6). J. Taiwan Inst. Chem. Eng. 2010, 41, 315–325. [Google Scholar] [CrossRef]
- Yiğit, D.; Udum, Y.A.; Gűllű, M.; Toppare, L. Electrochemical and spectroelectrochemical studies of poly(2,5-di-2,3-dihydrothieno[3,4-b][1,4]dioxin-5-ylthienyl) derivatives bearing azobenzene, coumarine and fluorescein dyes: Effect of chromophore groups on electrochromic properties. Electrochim. Acta 2014, 147, 669–677. [Google Scholar] [CrossRef]
- Braveenth, R.; Bae, H.W.; Nguyen, Q.P.B.; Ko, H.M.; Lee, C.H.; Kim, H.J.; Kwon, J.H.; Chai, K.Y. Spirobifluorene core-based novel hole transporting materials for red phosphorescence OLEDs. Molecules 2017, 22, 464. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Tang, Y.; Jiang, S.; Chen, H.; Zheng, X.; Wang, X.; Zhao, B.; Tan, S. Efficient triphenylamine-based dyes featuring dual-role carbazole, fluorene and spirobifluorene moieties. Org. Electron. 2011, 12, 125–135. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kumar, A.; Meijer, E.W.; Reynolds, J.R. Enhanced contrast ratio and rapid switching in electrochromics based on poly(3,4-propylenedioxythiophene) derivatives. Adv. Mater. 1999, 11, 1379–1382. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, B.K.; Hao, L.; Lin, K.F.; Sun, I.W. Thermophysical properties of a room temperature ionic liquid (1-methyl-3-pentyl-imidazolium hexafluorophosphate) with poly(ethylene glycol). J. Taiwan Inst. Chem. Eng. 2011, 42, 914–921. [Google Scholar] [CrossRef]
- Wu, T.Y.; Hao, L.; Chen, P.R.; Liao, J.W. Ionic conductivity and transporting properties in LiTFSI-doped bis(trifluoromethanesulfonyl)imide-based ionic liquid electrolyte. Int. J. Electrochem. Sci. 2013, 8, 2606–2624. [Google Scholar]
- Carbas, B.B.; Odabas, S.; Türksoy, F.; Tanyeli, C. Synthesis of a new electrochromic polymer based on tetraphenylethylene cored tetrakis carbazole complex and its electrochromic device application. Electrochim. Acta 2016, 193, 72–79. [Google Scholar] [CrossRef]
- Wu, T.Y.; Liao, J.W.; Chen, C.Y. Electrochemical synthesis, characterization and electrochromic properties of indan and 1,3-benzodioxole-based poly(2,5-dithienylpyrrole) derivatives. Electrochim. Acta 2014, 150, 245–262. [Google Scholar] [CrossRef]
- Hu, B.; Zhang, Y.; Lv, X.; Ouyang, M.; Fu, Z.; Zhang, C. Electrochemical and electrochromic properties of two novel polymers containing carbazole and phenyl-methanone units. J. Electroanal. Chem. 2013, 689, 291–296. [Google Scholar] [CrossRef]
- Zhou, P.; Wan, Z.; Liu, Y.; Jia, C.; Weng, X.; Xie, J.; Deng, L. Synthesis and electrochromic properties of a novel conducting polymer film based on dithiafulvenyl-triphenylamine-di(N-carbazole). Electrochim. Acta 2016, 190, 1015–1024. [Google Scholar] [CrossRef]
- Soganci, T.; Soyleyici, H.C.; Ak, M.; Cetisli, H. An amide substituted dithienylpyrrole based copolymer: Its electrochromic properties physical and analytical electrochemistry, electrocatalysis, and photoelectrochemistry. J. Electrochem. Soc. 2016, 163, H59–H66. [Google Scholar] [CrossRef]
- Wu, T.Y.; Tsao, M.H.; Chen, F.L.; Su, S.G.; Chang, C.W.; Wang, H.P.; Lin, Y.C.; Ou-Yang, W.C.; Sun, I.W. Synthesis and characterization of organic dyes containing various donors and acceptors. Int. J. Mol. Sci. 2010, 11, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Tsao, M.H.; Wu, T.Y.; Wang, H.P.; Sun, I.W.; Su, S.G.; Lin, Y.C.; Chang, C.W. An efficient metal free sensitizer for dye-sensitized solar cells. Mater. Lett. 2011, 65, 583–586. [Google Scholar] [CrossRef]
- Su, Y.S.; Chang, J.C.; Wu, T.Y. Applications of three dithienylpyrroles-based electrochromic polymers in high-contrast electrochromic devices. Polymers 2017, 9, 114. [Google Scholar] [CrossRef]
- Wu, T.Y.; Sheu, R.B.; Chen, Y. Synthesis, optically acid-sensory and electrochemical properties of novel polyoxadiazole derivatives. Macromolecules 2004, 37, 725–733. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chen, Y. Synthesis, optical and electrochemical properties of novel copolymers containing alternate 2,3-quinoxaline and hole-transporting units. J. Polym. Sci. Part. A Polym. Chem. 2002, 40, 4570–4580. [Google Scholar] [CrossRef]
- Guzel, M.; Soganci, T.; Akgun, M.; Ak, M. Carbazole functionalized star shaped triazine monomer and its electrochromic applications. J. Electrochem. Soc. 2015, 162, H527–H534. [Google Scholar] [CrossRef]
- Oğuztűrk, H.E.; Tirkeș, S.; Őnal, A.M. Electrochemical synthesis of new conjugated polymers based on carbazole and furan units. J. Electroanal. Chem. 2015, 750, 1–8. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Wu, L.C. Fluorescent and electrochromic polymers from 2,8-di(carbazol-9-yl)dibenzothiophene and its S, S-dioxide derivative. Dye Pigment. 2016, 134, 51–63. [Google Scholar] [CrossRef]
- Hsiao, S.H.; Hsueh, J.C. Electrochemical synthesis and electrochromic properties of new conjugated polycarbazoles from di(carbazol-9-yl)-substituted triphenylamine and N-phenylcarbazole derivatives. J. Electroanal. Chem. 2015, 758, 100–110. [Google Scholar] [CrossRef]
- Wu, T.Y.; Chung, H.H. Applications of tris(4-(thiophen-2-yl)phenyl)amine- and dithienylpyrrole-based conjugated copolymers in high-contrast electrochromic devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Liu, R.; Liu, J.; He, Q. Electrosyntheses, characterizations and electrochromic properties of a copolymer based on 4,4′-di(N-carbazoyl)biphenyl and 2,2′-bithiophene. Sol. Energy Mater. Sol. Cells 2011, 95, 1867–1874. [Google Scholar] [CrossRef]
- Wang, B.; Zhao, J.; Xiao, J.; Cui, C.; Liu, R. Synthesis and Electropolymerization of 9H-carbazol-9-ylpyrene and its electrochromic properties and electrochromic device application. Int. J. Electrochem. Sci. 2012, 7, 2781–2795. [Google Scholar]
- Xu, C.; Zhao, J.; Wang, M.; Cui, C.; Liu, R. Electrosynthesis and characterization of a donor–acceptor type electrochromic material from poly(4,7-dicarbazol-9-yl-2,1,3-benzothiadia-zole) and its application in electrochromic devices. Thin Solid Films 2013, 527, 232–238. [Google Scholar] [CrossRef]
- Kuo, C.W.; Wu, T.Y.; Huang, M.W. Electrochromic characterizations of copolymers base on 4,4′-bis(N-carbazole)-1,1′-biphenyl and indole-6-carboxylic acid and their application in electrochromic devices. J. Taiwan Inst. Chem. Eng. 2016, 68, 481–488. [Google Scholar] [CrossRef]
- Pander, P.; Data, P.; Turczyn, R.; Lapkowski, M.; Swist, A.; Soloducho, J.; Monkman, A.P. Synthesis and characterization of chalcogenophene-based monomers with pyridine acceptor unit. Electrochim. Acta 2016, 210, 773–782. [Google Scholar] [CrossRef]
- Wu, T.Y.; Li, J.L. Electrochemical synthesis, optical, electrochemical and electrochromic characterizations of indene and 1,2,5-thiadiazole-based poly(2,5-dithienylpyrrole) derivatives. RSC Adv. 2016, 6, 15988–15998. [Google Scholar] [CrossRef]
- Huang, T.T.; Tsai, C.L.; Hsiao, S.H.; Liou, G.S. Linkage and donor-acceptor effects on resistive switching memory devices of 4-(N-carbazolyl)triphenylamine-based polymers. RSC Adv. 2016, 6, 28815–28819. [Google Scholar] [CrossRef]













| Polymer Films and ECDs | 0 V | 1.0 V | 1.2 V | 1.4 V |
|---|---|---|---|---|
| PDTC film |  |  |  |  |
| PS2CBP film |  |  |  |  |
| PCEC film |  |  |  |  |
| PDTC/PProDOT-Et2 ECD |  |  |  |  |
| PS2CBP/PProDOT-Et2 ECD |  |  |  |  |
| PCEC/PProDOT-Et2 ECD |  |  |  |  |
| Polymer Films | E/V | L* | a* | b* |
|---|---|---|---|---|
| PDTC | 0 | 87.23 | −9.03 | 73.27 |
| 0.8 | 51.93 | −1.93 | 9.15 | |
| 1.0 | 40.99 | 8.39 | −13.85 | |
| 1.2 | 41.62 | 6.2 | −12.64 | |
| 1.4 | 45.89 | 2.8 | −9.75 | |
| PS2CBP | 0 | 89.71 | 0.3 | 7.51 |
| 0.8 | 86.31 | −0.97 | 15.64 | |
| 1.0 | 83.25 | −6.74 | 36.93 | |
| 1.2 | 78.29 | −11.76 | 32.53 | |
| 1.4 | 77.82 | −1.2 | 14.99 | |
| 1.6 | 82.04 | 0.96 | 17.56 | |
| PCEC | 0 | 94.61 | 1.18 | 4.37 |
| 0.8 | 92.32 | −3.15 | 13.78 | |
| 1.0 | 88.73 | −8.42 | 21.07 | |
| 1.2 | 82.58 | −11.22 | 10.04 | |
| 1.4 | 81.23 | −8.44 | 8.55 | |
| 1.6 | 85.25 | −4.96 | 13.79 |
| Polymer Films and ECDs | λmax/nm | Cycle No. | ΔT/% | τc/s | τb/s |
|---|---|---|---|---|---|
| PDTC films in [EPI+][TFSI−] | 578 | 1 | 58.79 | 1.91 | 1.93 |
| 50 | 58.11 | 2.04 | 2.01 | ||
| 100 | 58.02 | 2.07 | 2.04 | ||
| 856 | 1 | 66.04 | 1.77 | 1.72 | |
| 50 | 61.77 | 1.64 | 1.69 | ||
| 100 | 56.96 | 1.68 | 1.64 | ||
| PS2CBP films in [EPI+][TFSI−] | 428 | 1 | 39.83 | 1.85 | 1.80 |
| 50 | 33.29 | 2.07 | 1.97 | ||
| 100 | 27.95 | 2.08 | 1.95 | ||
| 1208 | 1 | 63.56 | 2.24 | 1.98 | |
| 50 | 57.94 | 2.23 | 1.91 | ||
| 100 | 50.64 | 2.16 | 1.83 | ||
| PCEC films in [EPI+][TFSI−] | 420 | 1 | 32.41 | 2.21 | 1.77 |
| 50 | 28.27 | 2.24 | 1.74 | ||
| 100 | 26.98 | 2.22 | 1.77 | ||
| 1220 | 1 | 42.36 | 1.99 | 1.72 | |
| 50 | 40.15 | 1.96 | 1.66 | ||
| 100 | 38.59 | 1.90 | 1.63 | ||
| PDTC/PProDOT-Et2 ECD | 592 | 1 | 31.27 | 0.96 | 0.99 |
| 50 | 26.77 | 0.96 | 0.96 | ||
| 100 | 24.85 | 0.99 | 0.97 | ||
| PS2CBP/PProDOT-Et2 ECD | 590 | 1 | 34.45 | 1.06 | 0.99 |
| 50 | 32.67 | 1.04 | 0.99 | ||
| 100 | 31.84 | 1.06 | 1.00 | ||
| PCEC/PProDOT-Et2 ECD | 586 | 1 | 38.25 | 1.01 | 0.96 |
| 50 | 32.19 | 1.01 | 0.93 | ||
| 100 | 29.90 | 0.98 | 0.90 |
| ECDs | E/V | L* | a* | b* |
|---|---|---|---|---|
| PDTC/PProDOT-Et2 | 0 | 47.59 | 0.46 | 12.09 |
| 0.8 | 34.78 | 3.65 | −7.51 | |
| 1.0 | 32.48 | 4.14 | −11.21 | |
| 1.2 | 31.68 | 5.13 | −12.89 | |
| 1.4 | 31.46 | 5.79 | −13.69 | |
| PS2CBP/PProDOT-Et2 | 0 | 70.88 | 8.23 | 19.31 |
| 0.8 | 60.51 | 0.89 | 0.57 | |
| 1.0 | 59.73 | 0.89 | −1.1 | |
| 1.2 | 59.45 | 0.84 | −2.04 | |
| 1.4 | 59.34 | 0.81 | −2.84 | |
| PCEC/PProDOT−Et2 | 0 | 77.37 | −1.62 | 6.39 |
| 0.8 | 64.15 | −0.22 | 0.56 | |
| 1.0 | 56.84 | −2.66 | −6.22 | |
| 1.2 | 52.94 | −3.92 | −8.46 | |
| 1.4 | 51.66 | −4.82 | −7.92 |
| Polymer Films and ECDs | λ/nm | Eg/eV | ΔTmax/% | ΔODmax/% | ηmax/cm2 C−1 |
|---|---|---|---|---|---|
| PDTC | 856 | 2.45 | 66.04 | 76.46 | 167.83 |
| PS2CBP | 1208 | 3.06 | 63.56 | 51.41 | 151.70 |
| PCEC | 1220 | 3.00 | 42.36 | 45.55 | 214.07 |
| PTCPM [29] | 1100 | - | 41 | - | 110.48 |
| PSCz [40] | 762 | 3.26 | 61 | 45.90 | 45 |
| PPhCz-2Cz [41] | 741 | 2.76 | 37 | - | 56 |
| PDTC/PProDOT-Et2 ECD | 592 | - | 31.27 | 35.55 | 345.19 |
| PS2CBP/PProDOT-Et2 ECD | 590 | - | 34.45 | 24.19 | 256.12 |
| PCEC/PProDOT-Et2 ECD | 586 | - | 38.25 | 34.52 | 369.85 |
| P(CBP-co-BT)/PEDOT ECD [43] | 700 | - | 28.6 | - | 234 |
| PMCzP/PEDOT ECD [44] | 623 | - | 23 | - | 290 |
| PCBTD/PEDOT ECD [45] | 620 | - | 49.4 | - | 1728 |
| P(BCz1-co-Inc2)/PProDOT-Et2 ECD [46] | 587 | - | 42 | - | 634 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, Y.-S.; Wu, T.-Y. Three Carbazole-Based Polymers as Potential Anodically Coloring Materials for High-Contrast Electrochromic Devices. Polymers 2017, 9, 284. https://doi.org/10.3390/polym9070284
Su Y-S, Wu T-Y. Three Carbazole-Based Polymers as Potential Anodically Coloring Materials for High-Contrast Electrochromic Devices. Polymers. 2017; 9(7):284. https://doi.org/10.3390/polym9070284
Chicago/Turabian StyleSu, Yuh-Shan, and Tzi-Yi Wu. 2017. "Three Carbazole-Based Polymers as Potential Anodically Coloring Materials for High-Contrast Electrochromic Devices" Polymers 9, no. 7: 284. https://doi.org/10.3390/polym9070284
APA StyleSu, Y. -S., & Wu, T. -Y. (2017). Three Carbazole-Based Polymers as Potential Anodically Coloring Materials for High-Contrast Electrochromic Devices. Polymers, 9(7), 284. https://doi.org/10.3390/polym9070284