Phosphorus Application Decreased Copper Concentration but Not Iron in Maize Grain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Experiment
2.2. Plant and Soil Sampling
2.3. Plant and Soil Analyses
2.4. Statistical Analysis
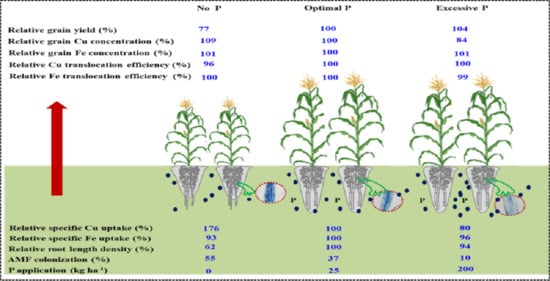
3. Results
3.1. Cu and Fe Concentrations in Grain and Straw as Affected by P Fertilization
3.2. Cu and Fe Content, Ratio of Grain to Shoot Cu and Fe Content as Affected by P Fertilization
3.3. Root Morphology, Specific Cu and Fe Uptake as Affected by P Fertilization
3.4. Shoot Cu and Fe Content, Grain Cu and Fe Concentration Related to Root Length Density and AMF Colonization
3.5. Soil Availability of P, Cu and Fe Concentrations as Affected by P Fertilization
4. Discussion
4.1. Grain Cu and Fe Nutrition as Affected by P Application and Its Effects for Feed
4.2. Does Grain Cu and Fe Nutrition Relate to Uptake or Translocation?
4.3. Cu and Fe Uptake Response to Root Length and AMF Colonization
4.4. Phosphorus Management Based on Yield, Grain Cu and Fe Nutrition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nuss, E.T.; Tanumihardjo, S.A. Quality protein maize for Africa: Closing the protein inadequacy gap in vulnerable populations. Adv. Nutr. 2011, 2, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasanna, B.M.; Vasal, S.K.; Kassahun, B.; Singh, N.N. Quality protein maize. Encycl. Grain Sci. 2001, 81, 212–216. [Google Scholar] [CrossRef] [Green Version]
- Dunn, M.L.; Jain, V.; Klein, B.P. Stability of key micronutrients added to fortified maize flours and corn meal. Ann. N. Y. Acad. Sci. 2014, 1312, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Prasanna, B.M.; Hellin, J.; Bänziger, M. Crops that feed the world 6. Past successes and future challenges to the role played by maize in global food security. Food Secur. 2011, 3, 307–327. [Google Scholar] [CrossRef] [Green Version]
- Kaur, J.; Bhatti, S.; Goyal, M. Influence of copper application on forage yield and quality of Oats fodder in copper deficient soils. Indian J. Anim. Nutr. 2015, 32, 290–294. [Google Scholar]
- Wysocka, D.; Snarska, A.; Sobiech, P. Copper-an essential micronutrient for calves and adult cattle. J. Elementol. 2019, 24, 101–110. [Google Scholar] [CrossRef]
- Xiong, X.; Li, Y.X.; Li, W.; Lin, C.Y.; H, W.; Yang, M. Copper content in animal manures and potential risk of soil copper pollution with animal manure use in agriculture. Resour. Conserv. Recy. 2010, 54, 985–990. [Google Scholar] [CrossRef]
- Dębski, B. Supplementation of pigs diet with zinc and copper as alternative to conventional antimicrobials. Pol. J. Vet. Sci. 2016, 19, 917–924. [Google Scholar] [CrossRef] [Green Version]
- Banaj, D.; Kovacevic, V.; Simic, D.; Seput, M.; Stojic, B. Phosphorus impacts on yield and nutritional status of maize. Cereal Res. Commun. 2006, 34, 393–396. [Google Scholar] [CrossRef]
- Wu, L.; Cui, Z.; Chen, X.; Yue, S.; Sun, Y.; Zhao, R.; Deng, Y.; Zhang, W.; Chen, K. Change in phosphorus requirement with increasing grain yield for Chinese maize production. Field Crops Res. 2015, 180, 216–220. [Google Scholar] [CrossRef]
- Wang, H.; Shi, G.; Zhong, G. Effects of phosphorus supply on copper tolerance and ascorbate-glutathione cycle in maize (Zea Mays L.) seedlings. Sens. Lett. 2012, 10, 670–677. [Google Scholar] [CrossRef]
- Ova, E.A.; Kutman, U.B.; Ozturk, L.; Cakmak, I. High phosphorus supply reduced zinc concentration of wheat in native soil but not in autoclaved soil or nutrient solution. Plant Soil 2015, 393, 147–162. [Google Scholar] [CrossRef]
- Zhang, W.; Chen, X.; Liu, Y.; Liu, D.; Chen, X.; Zou, C. Zinc uptake by roots and accumulation in maize plants as affected by phosphorus application and arbuscular mycorrhizal colonization. Plant Soil 2017, 413, 59–71. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Cui, Z.; Chen, X.; Zou, C. Zinc uptake and accumulation in winter wheat relative to changes in root morphology and mycorrhizal colonization following varying phosphorus application on calcareous soil. Field Crops Res. 2016, 197, 74–82. [Google Scholar] [CrossRef]
- Ao, J.; Fu, J.; Tian, J.; Yan, X.; Liao, H. Genetic variability for root morph-architecture traits and root growth dynamics as related to phosphorus efficiency in soybean. Funct. Plant Biol. 2010, 37, 304–312. [Google Scholar] [CrossRef]
- Gahoonia, T.S.; Nielsen, N.E. Root traits as tools for creating phosphorus efficient crop varieties. Plant Soil 2004, 260, 47–57. [Google Scholar] [CrossRef]
- Oikeh, S.O.; Kling, J.G.; Horst, W.J.; Chude, V.O.; Carsky, R.J. Growth and distribution of maize roots under nitrogen fertilization in plinthite soil. Field Crops Res. 1999, 62, 1–13. [Google Scholar] [CrossRef]
- Mi, G.; Chen, F.; Yuan, L.; Zhang, F. Ideotype root system architecture for maize to achieve high yield and resource use efficiency in intensive cropping systems. Adv. Agron. 2016, 139, 73–97. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, J.; Rengel, Z. Maize responds to low shoot P concentration by altering root morphology rather than increasing root exudation. Plant Soil 2017, 416, 377–389. [Google Scholar] [CrossRef]
- Teng, W.; Deng, Y.; Chen, X.; Xu, X.; Chen, R.; Lv, Y.; Zhao, Y.; Zhao, X.; He, X.; Li, B. Characterization of root response to phosphorus supply from morphology to gene analysis in field-grown wheat. J. Exp. Bot. 2013, 64, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, K.S.; Bharathi, C.; Jegan, A. Response of maize to mycorrhizal colonization at varying levels of zinc and phosphorus. Biol. Fert. Soils 2008, 45, 133–144. [Google Scholar] [CrossRef]
- Watts-Williams, S.J.; Cavagnaro, T.R. Arbuscular mycorrhizas modify tomato responses to soil zinc and phosphorus addition. Biol. Fert. Soils 2012, 48, 285–294. [Google Scholar] [CrossRef]
- Feng, G.; Song, Y.C.; Li, X.L.; Christie, P. Contribution of arbuscular mycorrhizal fungi to utilization of organic sources of phosphorus by red clover in a calcareous soil. Appl. Soil Ecol. 2003, 22, 139–148. [Google Scholar] [CrossRef]
- Li, X.L.; Marschner, H.; George, E. Acquisition of phosphorus and copper by VA-mycorrhizal hyphae and root-shoot transport in while clover. Plant Soil 1991, 136, 49–57. [Google Scholar] [CrossRef]
- Lambert, D.H.; Baker, D.E.; Cole, H. The role of mycorrhizae in the interactions of phosphorus with zinc, copper, and other elements. Soil Sci. Soc. Am. J. 1979, 43, 976–980. [Google Scholar] [CrossRef] [Green Version]
- Liu, A.; Hamel, C.; Hamilton, R.I.; Ma, B.L.; Smith, D.L. Acquisition of Cu, Zn, Mn and Fe by mycorrhizal maize (Zea mays L.) grown in soil at different P and micronutrient levels. Mycorrhiza 2000, 9, 331–336. [Google Scholar] [CrossRef]
- Wang, M.; Christie, P.; Xiao, Z.; Qin, C.; Wang, P.; Liu, J.; Xie, Y.; Xia, R. Arbuscular mycorrhizal enhancement of iron concentration by Poncirus trifoliata L. Raf and Citrus reticulata Blanco grown on sand medium under different pH. Biol. Fert. Soils 2009, 45, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Feng, G.; Chen, X.P.; Zou, C.Q. Arbuscular mycorrhizal fungal colonization is considerable at optimal Olsen-P levels for maximized yields in an intensive wheat-maize cropping system. Field Crops Res. 2017, 209, 1–9. [Google Scholar] [CrossRef]
- Von Wiren, N.; Marschner, H.; Romheld, V. Uptake kinetics of iron-phytosiderophores in two maize genotypes differing in iron efficiency. Physiol. Plantarum 1995, 93, 611–616. [Google Scholar] [CrossRef]
- Curie, C.; Panaviene, Z.; Loulergue, C.; Dellaporta, S.L.; Briat, J.; Walker, E.L. Maize yellow stripe1 encodes a membrane protein directly involved in Fe (III) uptake. Nature 2001, 409, 346–349. [Google Scholar] [CrossRef]
- Jolley, V.D.; Cook, K.A.; Hansen, N.C.; Stevens, W.B. Plant physiological responses for genotypic evaluation of iron efficiency in strategy I and strategy II plants—A review. J. Plant Nutr. 1996, 8–9, 1241–1255. [Google Scholar] [CrossRef]
- Oburger, E.; Gruber, B.; Schindlegger, Y.; Schenkeveld, W.D.; Hann, S.; Kraemer, S.M.; Wenzel, W.W.; Puschenreiter, M. Root exudation of phytosiderophores from soil-grown wheat. New Phytol. 2014, 203, 1161–1174. [Google Scholar] [CrossRef] [Green Version]
- Ptashnyk, M.; Roose, T.; Jones, D.L.; Kirk, G.J. Enhanced zinc uptake by rice through phytosiderophore secretion: A modelling study. Plant Cell Environ. 2011, 34, 2038–2046. [Google Scholar] [CrossRef]
- Olsen, S.R. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; Miscellaneous Paper Institute for Agricultural Research Samaru; US Department of Agriculture: Washington DC, USA, 1954.
- Lindsay, W.L.; Norvell, W.A. Development of a DTPA soil test for zinc, iron, manganese, and copper. Soil Sci. Soc. Am. J. 1978, 42, 421–428. [Google Scholar] [CrossRef]
- Li, D.F.; Wang, K.N.; Qiao, S.Y.; Jia, G.; Jiang, Z.Y.; Chen, Z.L.; Lin, Y.C.; Wu, D.; Zhu, X.M.; Xiong, B.H.; et al. NY/T65-2004 Feeding Standard of Swine; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004. (In Chinese)
- Wen, J.; Cai, H.Y.; Guo, Y.M.; Qi, G.H.; Chen, J.L.; Zhang, G.Z.; Liu, G.H.; Xiong, B.H.; Su, J.S.; Ji, C.; et al. NY/T 33-2004 Feeding Standard of Chicken; Ministry of Agriculture of the People’s Republic of China: Beijing, China, 2004. (In Chinese)
- Gibson, R.S.; Bailey, K.B.; Gibbs, M.; Ferguson, E.L. A review of phytate, iron, zinc, and calcium concentrations in plant-based complementary foods used in low-income countries and implications for bioavailability. Food Nutr. Bull. 2010, 31, S134–S146. [Google Scholar] [CrossRef]
- Raboy, V. Seeds for a better future: ‘low phytate’ grains help to overcome malnutrition and reduce pollution. Trends Plant Sci. 2001, 6, 458–462. [Google Scholar] [CrossRef]
- Haldar, M.; Mandal, L.N. Effect of phosphorus and zinc on the growth and phosphorus, zinc, copper, iron and manganese nutrition of rice. Plant Soil 1981, 59, 415–425. [Google Scholar] [CrossRef]
- Baldi, E.; Miotto, A.; Ceretta, C.A.; Quartieri, M.; Sorrenti, G.; Brunetto, G.; Toselli, M. Soil-applied phosphorous is an effective tool to mitigate the toxicity of copper excess on grapevine grown in rhizobox. Sci. Hortic-Amst. 2018, 227, 102–111. [Google Scholar] [CrossRef]
- Awan, Z.I.; Abbasi, M.K. Interactive effect of phosphorus and copper on maize growth. Pak. J. Agric. Res. 2000, 16, 105–108. [Google Scholar]
- Zhang, Y.; Deng, Y.; Chen, R.; Cui, Z.; Chen, X.; Yost, R.; Zhang, F.; Zou, C. The reduction in zinc concentration of wheat grain upon increased phosphorus-fertilization and its mitigation by foliar zinc application. Plant Soil 2012, 361, 143–152. [Google Scholar] [CrossRef]
- Schroeder, M.S.; Janos, D.P. Plant growth, phosphorus nutrition, and root morphological responses to arbuscular mycorrhizas, phosphorus fertilization, and intraspecific density. Mycorrhiza 2005, 15, 203–216. [Google Scholar] [CrossRef]
- Haynes, R.J.; Ludecke, T.E. Yield, root morphology and chemical composition of two pasture legumes as affected by lime and phosphorus applications to an acid soil. Plant Soil 1981, 62, 241–254. [Google Scholar] [CrossRef]
- Duncan, E.G.; O’Sullivan, C.A.; Roper, M.M.; Palta, J.; Whisson, K.; Peoples, M.B. Yield and nitrogen use efficiency of wheat increased with root length and biomass due to nitrogen, phosphorus, and potassium interactions. J. Plant Nutr. Soil Sc. 2018, 181, 364–373. [Google Scholar] [CrossRef]
- Huang, C.; Webb, M.J.; Graham, R.D. Pot size affects expression of Mn efficiency in barley. Plant Soil 1996, 178, 205–208. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, Y.; Jiang, S.; Deng, Y.; Christie, P.; Murray, P.J.; Li, X.; Zhang, J. Arbuscular mycorrhizal fungi in soil and roots respond differently to phosphorus inputs in an intensively managed calcareous agricultural soil. Sci. Rep. UK 2016, 6, 24902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, P.J.; George, T.S.; Dupuy, L.X.; Karley, A.J.; Valentine, T.A.; Wiesel, L.; Wishart, J. Root traits for infertile soils. Front. Plant Sci. 2013, 4, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Waddell, H.A.; Simpson, R.J.; Ryan, M.H.; Lambers, H.; Garden, D.L.; Richardson, A.E. Root morphology and its contribution to a large root system for phosphorus uptake by Rytidosperma species (wallaby grass). Plant Soil 2017, 412, 7–19. [Google Scholar] [CrossRef]
- Clark, R.B. Differences among mycorrhizal fungi for mineral uptake per root length of switchgrass grown in acidic soil. J. Plant Nutr. 2002, 25, 1753–1772. [Google Scholar] [CrossRef]
- Lehmann, A.; Rillig, M.C. Arbuscular mycorrhizal contribution to copper, manganese and iron nutrient concentrations in crops-A meta-analysis. Soil Biol. Biochem. 2015, 81, 147–158. [Google Scholar] [CrossRef]
- Masalha, J.; Kosegarten, H.; Elmaci, Ö.; Mengel, K. The central role of microbial activity for iron acquisition in maize and sunflower. Biol. Fert. Soils 2000, 30, 433–439. [Google Scholar] [CrossRef]
- MacDonald, G.K.; Bennett, E.M.; Potter, P.A.; Ramankutty, N. Agronomic phosphorus imbalances across the world’s croplands. Proc. Natl. Acad. Sci. USA 2011, 108, 3086–3091. [Google Scholar] [CrossRef] [Green Version]
- Roberts, T.L.; Johnston, A.E. Phosphorus use efficiency and management in agriculture. Resour. Conserv. Recy. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- Lazarte, C.E.; Carlsson, N.; Almgren, A.; Sandberg, A.; Granfeldt, Y. Phytate, zinc, iron and calcium content of common Bolivian food, and implications for mineral bioavailability. J. Food Compos. Anal. 2015, 39, 111–119. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, D.; Liu, Y.; Chen, X.; Zou, C. Overuse of phosphorus fertilizer reduces the grain and flour protein contents and zinc bioavailability of winter wheat (Triticum aestivum L.). J. Agr. Food Chem. 2017, 65, 1473–1482. [Google Scholar] [CrossRef]
- Lambers, H.; Martinoia, E.; Renton, M. Plant adaptations to severely phosphorus-impoverished soils. Curr. Opin. Plant Biol. 2015, 25, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Seguel, A.; Barea, J.M.; Cornejo, P.; Borie, F. Role of arbuscular mycorrhizal symbiosis in phosphorus-uptake efficiency and aluminium tolerance in barley growing in acid soils. Crop Pasture Sci. 2015, 66, 696–705. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Zou, C.; Chen, X.; Liu, Y.; Liu, D.; Yang, H.; Deng, Y.; Chen, X. Phosphorus Application Decreased Copper Concentration but Not Iron in Maize Grain. Agronomy 2020, 10, 1716. https://doi.org/10.3390/agronomy10111716
Zhang W, Zou C, Chen X, Liu Y, Liu D, Yang H, Deng Y, Chen X. Phosphorus Application Decreased Copper Concentration but Not Iron in Maize Grain. Agronomy. 2020; 10(11):1716. https://doi.org/10.3390/agronomy10111716
Chicago/Turabian StyleZhang, Wei, Chunqin Zou, Xiuxiu Chen, Yumin Liu, Dunyi Liu, Huaiyu Yang, Yan Deng, and Xinping Chen. 2020. "Phosphorus Application Decreased Copper Concentration but Not Iron in Maize Grain" Agronomy 10, no. 11: 1716. https://doi.org/10.3390/agronomy10111716
APA StyleZhang, W., Zou, C., Chen, X., Liu, Y., Liu, D., Yang, H., Deng, Y., & Chen, X. (2020). Phosphorus Application Decreased Copper Concentration but Not Iron in Maize Grain. Agronomy, 10(11), 1716. https://doi.org/10.3390/agronomy10111716