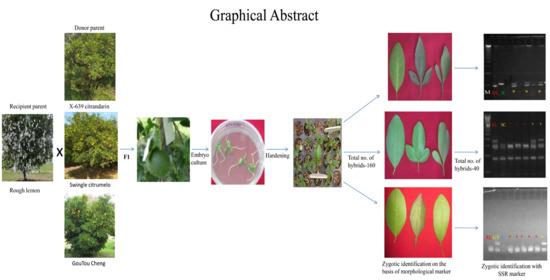
Optimizing Recovery of Hybrid Embryos from Interspecific Citrus Crosses of Polyembryonic Rough Lemon (Citrus jambhiri Lush.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Embryo Rescue and Plant Recovery
2.3. Culture Media
2.4. Hardening and In Vivo Transfer
2.5. SSR Analysis
2.6. Confirmation of Zygotic Seedlings by SSR
2.7. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. Number of Embryos and Plant Recovery
3.2. Embryo Germination
Comparison between In Vitro and In Vivo Germination
3.3. Polyembryony Analysis
3.4. Seedling Growth
3.5. Proportion of Hybrid Seedlings Identified by Morphological Marker
3.6. Verification of Zygotic Seedlings Using SSR Markers
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT Faostat Statistical Database. Food and Agriculture Organization of the United Nations. 2018. Available online: http://faostat.fao.org/faostat (accessed on 13 March 2020).
- Cheng, F.S.Z.; Roose, M.L. Origin and inheritance of dwarfing by the citrus rootstock Poncirus trifoliata ‘Flying Dragon’. J. Am. Soc. Hortic. Sci. 1995, 120, 286–291. [Google Scholar] [CrossRef]
- Medina-Urrutia, V.M. Rootstock research for Mexican lime trees (Citrus aurantifolia Swingle) in Mexico: An overview. In Proceedings of the International Society Citriculture, Wuhan, China, 26–30 October 2008. [Google Scholar]
- Zhu, S.; Wu, B.; Ma, Y.; Chen, J.; Zhonga, G. Obtaining citrus hybrids by in vitro culture of embryos from mature seeds and early identification of hybrid seedlings by allele-specific PCR. Sci. Hortic. 2013, 161, 300–305. [Google Scholar] [CrossRef]
- Davies, F.S.; Albrigo, L.G. Citrus; CAB International: Wallingford, UK, 1994. [Google Scholar]
- Louzada, E.S.; Del, R.H.S.; Xia, D. Preparation and fusion of Citrus sp. microprotoplasts. J. Am. Soc. Hortic. Sci. 2002, 127, 484–488. [Google Scholar] [CrossRef] [Green Version]
- Ollitrault, P.; Dambier, D.; Froelicher, Y.; Carreel, F.; Hont, A.; Luro, F. Somatic hybridization potential for citrus germplasm utilization. Cah. Agric. 2000, 9, 223–236. [Google Scholar]
- Soost, R.K.; Roose, M.L. Citrus. In Fruit Breeding; Janick, J., Moore, J.N., Eds.; Wiley: New York, NY, USA, 1996; pp. 257–323. [Google Scholar]
- Shen, X.; Gmitter, F.G.; Grosser, J.W. Immature embryo rescue and culture. Methods Mol. Boil. 2011, 710, 75–92. [Google Scholar]
- Tan, M.J.; Song, J.; Deng, S. Production of two mandarin × trifoliate orange hybrid population via embryo rescue with verification by SSR analysis. Euphytica 2007, 157, 155–160. [Google Scholar] [CrossRef]
- Yi, H.L.; Deng, X.X.; Fu, C.H. Application of embryo rescue techniques in fruit crops. J. Fruit Sci. 2001, 18, 224–228. [Google Scholar]
- Jaskani, M.J.; Khan, I.A.; Khan, M.M. Fruit set, seed development and embryo germination in interploid crosses of citrus. Sci. Hortic. 2005, 107, 51–57. [Google Scholar] [CrossRef]
- Viloria, Z.; Grosser, J.W.; Bracho, B. Immature embryo rescue, culture and seedling development of acid citrus fruit derived from interploid hybridization. Plant Cell Tissue Organ Cult. 2005, 82, 159–167. [Google Scholar] [CrossRef]
- Caruso, M.; Distefano, G.; Paolo, D.P.; Malfa, S.L.; Russo, G.; Gentile, A.; Recupero, G.R. High resolution melting analysis for early identification of citrus hybrids: A reliable tool to overcome the limitations of morphological markers and assist rootstock breeding. Sci. Hortic. 2014, 180, 199–206. [Google Scholar] [CrossRef]
- Oliveira, A.C.; Garcia, A.N.; Cristofani, M.; Machado, M.A. Identification of citrus hybrids through the combination of leaf apex morphology and SSR markers. Euphytica 2002, 128, 397–403. [Google Scholar] [CrossRef]
- Rao, M.N.; Soneji, J.R.; Chen, C.; Huang, S.; Gmitter, F.G., Jr. Characterization of zygotic and nucellar seedlings from sour orange-like citrus rootstock candidates using RAPD and EST-SSR markers. Tree Genet. Genomes 2008, 4, 113–124. [Google Scholar]
- Dettori, M.T.; Micali, S.; Giovinazzi, J.; Scalabrin, S.; Verde, I.; Cipriani, G. Mining microsatellites in the peach genome: Development of new long-core SSR markers for genetic analyses in five Prunus species. Springer Plus 2015, 10, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lloyd, G.; Mc-Cown, B.H. Commercially feasible micropropagation of mountain laurel, (Kalmia latifolia) by use of shoot tip culture. Int. Plant Prop. Soc. Comb. Proc. 1980, 30, 421–427. [Google Scholar]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus 1990, 12, 13–15. [Google Scholar]
- Chen, C.; Zhou, P.; Choi, Y.A.; Huang, S.; Gmitter, F.G., Jr. Mining and characterizing microsatellites from citrus ESTs. Theor. Appl. Genet. 2006, 112, 1248–1257. [Google Scholar] [CrossRef]
- Kaur, M. Development of Citrus Rootstock Hybrids and Their Molecular Characterization. Ph.D. Thesis, Punjab Agricultural University, Ludhiana, India, 15 July 2017. [Google Scholar]
- Palmieri, D.A.; Novelli, V.M.; Bastianel, M.; Cristofani-Yaly, M.; Astúa-Monge, G.; Carlos, E.F.; Oliveira, A.C.; Machado, M.A. Frequency and distribution of microsatellites from ESTs of citrus. Genet. Mol. Biol. 2007, 30, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Singh, J.; Dhaliwal, H.S.; Thakur, A.; Chhuneja, P.; Sidhu, G.S.; Singh, R. Morphological and genetic diversity in citrus genotypes to substantiate rootstock breeding for root rot resistance. Indian J. Hortic. 2017, 74, 326–333. [Google Scholar] [CrossRef]
- Yildiz, E.; Kaplankiran, M.; Demirkeser, T.H.; Uzun, A.; Toplu, C. Identifcation of zygotic and nucellar individuals produced from several citrus crosses using SSRs markers. Not. Bot. Hortic. Agrobot. 2013, 2, 478–484. [Google Scholar] [CrossRef] [Green Version]
- Cooper, W.C.; Reece, P.C.; Furr, J.R. Citrus breeding in Florida: Past, present and future. Proc. Fla. State Hortic. Soc. 1962, 75, 5–13. [Google Scholar]
- Soost, R.K.; Cameron, J.W. Citrus. In Advances in Fruit Breeding; Janick, J., Moore, J.N., Eds.; Purdue University: West Lafayette, IN, USA, 1975; pp. 507–540. [Google Scholar]
- Perez-Tornero, O.; Porras, I. Assessment of polyembryony in lemon: Rescue and in vitro culture of immature embryos. Plant Cell Tissue Organ Cult. 2008, 93, 173–180. [Google Scholar] [CrossRef]
- Scarano, M.T.; Tusa, N.; Abbate, L.; Lucretti, S.; Nardi, L.; Ferrante, S. Flow cytometry, SSR and modified AFLP markers for the identification of zygotic plantlets in backcrosses between ‘Femminello’ lemon cybrids (2n and 4n) and a diploid clone of ‘Femminello’ lemon (Citrus limon L. Burm. F.) tolerant to Mal secco disease. Plant Sci. 2005, 164, 1009–1017. [Google Scholar] [CrossRef]
- Turgutoglu, E.; Kurt, S.; Demir, G. Effect of GA3 concentrations in basal medium on embryos germination of Cleopatra mandarin × Carrizo citrange and Cleopatra mandarin × Flying Dragon. Ekin J. Crop Breed. Genet. 2015, 1, 17–19. [Google Scholar]
- Chagas, E.A.; Pasqual, M.; Ramos, J.D.; Pio, L.A.S.; Dutra, L.F.; Cazetta, J.O. Activated charcoal and gibberellic acid concentrations on immature embryos culture. Sci. Agrotechnol. 2005, 29, 1125–1131. [Google Scholar]
- Kurt, S.; Ulger, S. Production of common sour orange × Carrizo citrange hybrids using embryo rescue. Int. J. Fruit Sci. 2014, 14, 42–48. [Google Scholar] [CrossRef]
- Sykes, S.R. Characterisation of citrus rootstock germplasm introduced as seeds to Australia from the People’s Republic of China. Sci. Hortic. 2011, 127, 298–304. [Google Scholar] [CrossRef]
- George, E.F.; Hall, M.A.; De Klerk, G.J. Plant propagation by tissue culture- The background. In The Components of Plant Tissue Culture Media I: Macro and Micro-Nutrients; George, E.F., Hall, M.A., de Klerk, G., Eds.; Springer Publishers: Dordrecht, The Netherlands, 2008; pp. 65–113. [Google Scholar]
- Honsho, C.; Tsuruta, K.; Ryuto, K.; Sakata, A.; Kuroki, S.; Nishiwaki, A.; Tetsumura, T. Characterization of seed and embryo abortion during fruit development in citrus cultivars pollinated by ‘Nishiuchi Konatsu’ (Citrus tamurana) and a preliminary trial of embryo rescue in aborting embryos. Acta Hortic. 2015, 1065, 181–186. [Google Scholar] [CrossRef]
- Ribeiro, V.G.; Sanabio, D.; Souza, C.N.D.; Lopes, P.S.N.; Bocardo, M.R.; Pasqual, M. Effects of gibberellic acid (GA3) and activated coal on in vitro culture of Citrus limonia Osbeck × Poncirus trifoliata L. Raf. embryos. Pesq. Agropecu. Bras. Brasilia 2000, 35, 27–30. [Google Scholar] [CrossRef] [Green Version]
- Chagas, E.A.; Pasqual, M.; Ramos, J.D.; Cardoso, P.; Cazetta, J.O.; Figueiredo, M.A.D. Development of globular embryos from the hybridization between ‘Pera Rio’ sweet orange and ‘Ponca’ mandarin. Rev. Bras. Frutic. 2003, 25, 483–488. [Google Scholar] [CrossRef] [Green Version]
- Ollitrault, P.; Guo, W.W.; Grosser, J.W. Somatic hybridization. In Citrus Genetics, Breeding and Biotechnology; Khan, I.A., Ed.; CABI Publishing: Wallingford, UK, 2007; pp. 235–260. [Google Scholar]
- Aleza, P.; Juarez, J.; Ollitrault, P.; Navarro, L. Polyembryony in non-apomictic citrus genotypes. Ann. Bot. 2010, 106, 533–545. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Shivanna, K.R.; Mohan Ram, H.Y. Apomixis and polyembryony in the guggul plant, Commiphora wightii. Ann. Bot. 1996, 78, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Yeung, E.C.; Meinke, D.W. Embryogenesis in angiosperms: Development of the suspensor. Plant Cell Rep. 1993, 5, 1371–1381. [Google Scholar] [CrossRef] [Green Version]
- Kishore, K.; Monika, N.; Rinchen, D.; Lepcha, B.; Pandey, B. Polyembryony and seedling emergence traits in apomictic citrus. Sci. Hortic. 2012, 138, 101–107. [Google Scholar] [CrossRef]
- Raghavan, V. Some reflections on double fertilization, from its discovery to the present. New Phytol. 2003, 159, 565–583. [Google Scholar] [CrossRef]
- Morgante, M.; Olivieri, A.M. PCR-amplified microsatellite as markers in plant genetics. Plant J. 1993, 3, 175–182. [Google Scholar] [CrossRef]
- Ruiz, C.; Breto, M.P.; Asins, M.J. A quick methodology to identify sexual seedlings in citrus breeding programs using SSR markers. Euphytica 2000, 112, 89–94. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.H.; Kim, H.B.; Park, Y.C.; Song, K.J. Some factors affecting the efficiency of hybrid embryo rescue in the ‘Shiranuhi’ mandarin. Hortic. Sci. Technol. 2020, 38, 271–281. [Google Scholar]
- Yun, J.U.; Yang, H.B.; Jung, Y.H.; Yun, S.H.; Kim, K.S.; Kim, C.S.; Song, K.J. Identification of zygotic and nucellar mandarin seedling using randomly amplified polymorphic DNA. Hortic. Environ. Biotechnol. 2007, 48, 171–175. [Google Scholar]
- Jin, S.B.; Yun, S.H.; Park, J.H.; Park, S.M.; Koh, S.W.; Lee, D.H. Early identification of citrus zygotic seedlings using pollen-specific molecular markers. Korean J. Hortic. Sci. Technol. 2015, 33, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Andrade-Rodriguez, M.; Villegas-Monter, A.; Carrillo-Castaneda, G.; Garcia-Velazquez, A. Polyembryony and identification of Volkamerian lemon zygotic and nucellar seedlings using RAPD. Pesq. Agropecu. Bras. 2004, 39, 551–559. [Google Scholar] [CrossRef]
- Carimi, F.; de Pasquale, F.; Puglia, A.M. In vitro rescue of zygotic embryos of sour orange, Citrus aurantium L., and their detection based on RFLP analysis. Plant Breed. 1998, 117, 261–266. [Google Scholar] [CrossRef]





| Media | M1 | M2 | M3 | M4 | G1 | G2 | G3 | G4 | W1 | W2 | W3 | W4 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Basal Medium | MS | WPM | |||||||||||
| Growth regulators | GA3 (mg/L) | - | - | - | - | 1.0 | 1.0 | 1.0 | 1.0 | - | - | - | - |
| ME(g/L) | 0.25 | 0.5 | 0.75 | 1.0 | 0.25 | 0.5 | 0.75 | 1.0 | 0.25 | 0.5 | 0.75 | 1.0 | |
| Growing Conditions | DAP | RL × X | RL × SC | RL × GT | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | Zygotic % | A | B | Zygotic % | A | B | Zygotic % | ||
| In vitro | 65 | 36 | 2.6 ± 0.2 | 22.2 | 29 | 2.0 ± 0.1 | 24.1 | 24 | 1.7 ± 0.8 | 29.2 |
| 80 | 35 | 4.2 ± 0.5 | 34.4 | 26 | 6.5 ± 0.1 | 30.9 | 29 | 3.4 ± 0.5 | 27.6 | |
| 95 | 31 | 6.7 ± 0.5 | 48.5 | 30 | 8.1 ± 1.0 | 56.7 | 20 | 5.6 ± 1.1 | 60.0 | |
| 110 | 30 | 4.3 ± 0.5 | 33.3 | 28 | 5.6 ± 0.6 | 39.3 | 30 | 4.2 ± 0.7 | 23.3 | |
| 125 | 32 | 4.0 ± 0.4 | 25.0 | 36 | 4.4 ± 0.4 | 36.1 | 25 | 3.3 ± 0.5 | 28.0 | |
| 190 | 75 | 3.9 ± 0.4 | 27.7 | 90 | 3.6 ± 0.3 | 31.1 | - | - | - | |
| In soil | 190 | 150 | 2.0 ± 0.2 | 6.7 | 150 | 2.5 ± 0.2 | 3.7 | - | - | |
| Media | Days after Pollination | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RL × X | Mean | RL × SC | Mean | RL × GT | Mean | |||||||||||||
| 65 | 80 | 95 | 110 | 125 | 65 | 80 | 95 | 110 | 125 | 65 | 80 | 95 | 110 | 125 | ||||
| M1 | 35.8 | 50.3 | 75.3 | 65.3 | 62.7 | 57.9 d | 31.5 | 51.2 | 76.6 | 64.9 | 60.8 | 57.0 ef | 33.9 | 48.5 | 74.6 | 63.6 | 60.9 | 56.3 c |
| M2 | 39.1 | 59.6 | 81.4 | 73.7 | 65.8 | 63.9 abcd | 37.7 | 60.5 | 85.9 | 73.8 | 64.9 | 64.6 bcd | 38.7 | 58.7 | 80.0 | 72.2 | 65.5 | 63.0 abc |
| M3 | 43.3 | 66.9 | 92.5 | 77.7 | 70.6 | 70.2 ab | 41.6 | 65.5 | 97.0 | 75.9 | 70.2 | 70.04 a | 41.8 | 65.1 | 90.9 | 75.2 | 69.9 | 68.6 ab |
| M4 | 38.3 | 57.0 | 81.8 | 70.4 | 64.7 | 62.5 bcd | 33.8 | 57.5 | 79.2 | 68.2 | 62.7 | 60.3 def | 37.5 | 56.5 | 79.6 | 68.7 | 64.1 | 61.3 bc |
| G1 | 39.3 | 51.2 | 76.8 | 67.3 | 60.2 | 59.0 d | 31.7 | 52.6 | 78.9 | 64.9 | 61.2 | 57.9 ef | 38.6 | 50.1 | 74.8 | 64.7 | 59.0 | 57.5 c |
| G2 | 42.7 | 63.1 | 83.6 | 72.5 | 67.1 | 65.8 abcd | 38.4 | 61.6 | 87.6 | 73.5 | 67.4 | 65.7 abc | 41.8 | 61.3 | 81.6 | 70.8 | 65.6 | 64.2 abc |
| G3 | 44.1 | 68.3 | 96.2 | 78.3 | 72.1 | 71.8 a | 43.8 | 66.6 | 97.8 | 78.2 | 72.0 | 67.4 ab | 42.1 | 67.5 | 93.7 | 76.7 | 72.0 | 70.4 a |
| G4 | 41.8 | 54.2 | 81.9 | 69.9 | 66.2 | 62.8 bcd | 35.4 | 57.9 | 82.2 | 68.5 | 63.7 | 61.5 cde | 39.6 | 52.9 | 79.7 | 66.9 | 64.9 | 60.8 bc |
| W1 | 35.0 | 50.9 | 76.7 | 67.9 | 61.2 | 58.4 d | 30.5 | 51.0 | 74.6 | 61.9 | 58.8 | 55.3 f | 34.5 | 49.2 | 74.9 | 66.7 | 60.6 | 57.2 c |
| W2 | 38.8 | 55.6 | 81.2 | 72.6 | 64.0 | 62.4 bcd | 35.9 | 61.3 | 85.2 | 71.5 | 63.4 | 63.5 bcd | 37.6 | 54.5 | 79.7 | 70.3 | 62.7 | 61.0 bc |
| W3 | 41.8 | 64.4 | 91.1 | 74.2 | 68.8 | 68.1 abc | 42.3 | 64.1 | 93.7 | 74.2 | 68.1 | 68.5 ab | 40.4 | 63.8 | 89.2 | 72.8 | 68.1 | 66.9 ab |
| W4 | 35.6 | 51.4 | 78.0 | 69.8 | 63.4 | 59.6 cd | 32.9 | 55.4 | 74.8 | 65.2 | 60.4 | 57.7 ef | 35.3 | 50.1 | 76.0 | 68.1 | 61.0 | 58.1 c |
| Mean | 39.6 e | 57.8 d | 83.0 a* | 71.6 b | 65.6 c | 36.3 e | 58.3 d | 84.5 a* | 69.7 b | 64.3 c | 38.5 d | 56.5 c | 81.2 a* | 69.7 b | 64.5 b | |||
| LSD (0.05) | DAP = 5.7 | DAP = 3.4 | DAP = 5.6 | |||||||||||||||
| Media = 8.9 | Media = 5.3 | Media = 8.6 | ||||||||||||||||
| DAP × Media = NS | DAP × Media = NS | DAP × Media = NS | ||||||||||||||||
| Crosses | Number of Seeds Cultured | Germination Rate (%) | Polyembryonic (%) | Monoembryonic (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| In Vitro | In Soil | In Vitro | In Soil | Mean | In Vitro | In Soil | Mean | In Vitro | In Soil | Mean | |
| RL × X | 75 | 150 | 100 ± 0.0 a | 91.3 ± 2.9 a | 95.7 a | 92.0 ± 5.1 a | 71.5 ± 2.4 a | 81.8 a | 8.0 ± 0.5 a | 28.6 ± 2.5 a | 18.3 a |
| RL × SC | 90 | 150 | 100 ± 0.0 a | 89.3 ± 2.9 a | 94.7 a | 97.7 ± 2.4 a | 80.7 ± 4.1 a | 89.2 a | 2.2 ± 0.2 a | 19.4 ± 1.5 a | 10.8 b |
| Mean | 100.0 a | 90.3 b | 95.0 a | 76.1 b | 5.1 b | 24.0 a | |||||
| Source | DF | F-Value | ||
|---|---|---|---|---|
| RL × X | RL × SC | RL × GT | ||
| Media | 11 | 2.2 0.0219 | 7.0 <.0001 | 2.3 0.0146 |
| Days | 4 | 63.1 <0.0001 | 210.7 <0.0001 | 64.4 <0.0001 |
| Media× days | 44 | 0.1 1.0000 | 0.2 1.0000 | 0.1 1.0000 |
| CV | - | 19.3 | 11.5 | 19.2 |
| Crosses | Number of Seedlings Per Seed | Survival % | Plant Height (cm) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| In Vitro | In Soil | Mean | In Vitro | In Soil | Mean | In Vitro | In Soil | Mean | |
| RL × X | 3.1 ± 0.2 a | 1.6 ± 0.1 a | 2.3 b | 80.0 ± 2.1 a | 88.7 ± 2.2 a | 84.3 a | 22.8 ± 3.5 a | 21.3 ± 4.2 a | 22.1 a |
| RL × SC | 3.7 ± 0.2 a | 1.9 ± 0.1 a | 2.8 a | 79.3 ± 2.6 a | 85.3 ± 2.1 a | 82.3 a | 29.6 ± 6.6 a | 19.9 ± 4.1 a | 24.7 a |
| Mean | 3.4 a | 1.7 b | 79.7 a | 87.0 a | 25.4 a | 21.4 a | |||
| Primer Code | Sequence 5′ to 3′ | G + C Content (%) | Annealing Temperature (°C) | Zygotic Seedlings * | ||
|---|---|---|---|---|---|---|
| (RL × X) | (RL × SC) | (RL × GT) | ||||
| F29-F | TTCACCACAAACGAAGACTCAGAC | 41.67 | 60 | 15 (20.0%) | 14 (16.7%) | 9 (10.7%) |
| F29-R | CTGTAATCCACTCGGTAATCCGAC | 45.83 | ||||
| F87-F | ATGAAGGCTTTTTAGAGCCGAGTT | 41.67 | 60 | 8 (10.7%) | 12 (14.3%) | 7 (8.3%) |
| F87-R | ATAATAGGGGCCCACTTGACTTG | 47.83 | ||||
| TAA1-F | AAGAAGAAGAGCCCCCATTAGC | 50.00 | 60 | 11 (14.7%) | 10 (11.9%) | 11 (13.1%) |
| TAA1-R | GACAACATCAACAACAGCAAGAGC | 45.83 | ||||
| CCSMEc4-F | CTTGCTCGAGTCTACGCTCC | 60.00 | 60 | 10 (13.3%) | 12 (14.3%) | 7 (8.3%) |
| CCSMEc4-R | CTTCCTCTTGCGGAGTGTTC | 55.00 | ||||
| CCSME31-F | GGAATTCGAGTTGGAGGTCA | 50.00 | 58 | 13 (17.3%) | 9 (10.7%) | 10 (11.9%) |
| CCSME31-R | ACCACCCATTTGCCTGATAA | 45.00 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, J.; Dhaliwal, H.S.; Thakur, A.; Sidhu, G.S.; Chhuneja, P.; Gmitter, F.G., Jr. Optimizing Recovery of Hybrid Embryos from Interspecific Citrus Crosses of Polyembryonic Rough Lemon (Citrus jambhiri Lush.). Agronomy 2020, 10, 1940. https://doi.org/10.3390/agronomy10121940
Singh J, Dhaliwal HS, Thakur A, Sidhu GS, Chhuneja P, Gmitter FG Jr. Optimizing Recovery of Hybrid Embryos from Interspecific Citrus Crosses of Polyembryonic Rough Lemon (Citrus jambhiri Lush.). Agronomy. 2020; 10(12):1940. https://doi.org/10.3390/agronomy10121940
Chicago/Turabian StyleSingh, Jagveer, Harvinder Singh Dhaliwal, Anirudh Thakur, Gurupkar Singh Sidhu, Parveen Chhuneja, and Frederick G. Gmitter, Jr. 2020. "Optimizing Recovery of Hybrid Embryos from Interspecific Citrus Crosses of Polyembryonic Rough Lemon (Citrus jambhiri Lush.)" Agronomy 10, no. 12: 1940. https://doi.org/10.3390/agronomy10121940
APA StyleSingh, J., Dhaliwal, H. S., Thakur, A., Sidhu, G. S., Chhuneja, P., & Gmitter, F. G., Jr. (2020). Optimizing Recovery of Hybrid Embryos from Interspecific Citrus Crosses of Polyembryonic Rough Lemon (Citrus jambhiri Lush.). Agronomy, 10(12), 1940. https://doi.org/10.3390/agronomy10121940