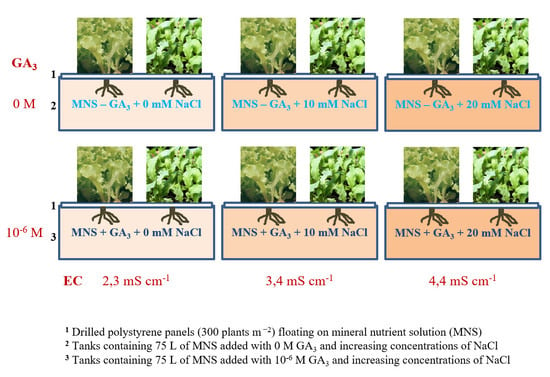
1. Introduction
Currently, one of the major challenges of agricultural research is to meet the increasing food demand while protecting natural resources and improving environmental quality. Nevertheless, agricultural sustainability is threatened by the burgeoning human population and decreasing availability of land for cultivation. The most important factor causing the reduction in cultivated lands is the accumulation of soluble salts [
1]. Soil and water salinity represents one of the major environmental stresses and determines severe reductions in crop productivity and quality [
1,
2]. Salinity negatively influences the germination, growth, physiology, and productivity of crop plants. These negative effects may be triggered by ionic and osmotic stresses, ion toxicity, oxidative damage, and membrane instability and permeability [
3,
4,
5,
6]; salt stress can also affect plant physiology, determining an increased respiration rate, changes in C and N metabolism, modification of mineral uptake and distribution, altered chlorophyll biosynthesis, and inefficiency of photosynthesis [
7,
8]. All these effects result in lowered yield and quality and reduced economic productivity of crops. Vegetable crops have a cash value usually higher than field crops, thus the salt tolerance of vegetable species is important for adequate fulfillment of vegetable demands and to limit the economic effects of salt stress on vegetable growers. Moreover, the high demand for water of vegetable crops, condensed in short production periods, increases their salinity problems [
9].
Soilless cultivation systems (or hydroponics) have been adopted for many vegetable crops to overcome soil salinity and other abiotic and biotic stresses [
10,
11,
12] and to increase yield and quality [
11,
13,
14,
15]. The quality of the water is of paramount importance in the hydroponic cultivation systems in which it is used to prepare nutrient solutions by adding mineral fertilizers that increase its electrical conductivity (EC). Hence, the availability of water with a high salt concentration leads to a nutrient solution with an EC level that trespasses the tolerance level of many vegetable crops [
9]. The majority of high salinity irrigation water occurs in areas located along the sea, especially in Mediterranean areas with intensive agriculture, and in the hot seasons because the intensive use of underground water increases seawater infiltration in the groundwater [
9].
The rising limitations in water quality and availability are increasing the attention in improving the efficiency of water use and enhancing crop tolerance to these stresses. Recently, the mechanisms of salt tolerance in plants has become the aim of many investigations [
16,
17], revealing that the response and adaptation of plants to salinity take place through the activation of stress response mechanisms, which control the ionic/hydraulic re-equilibrium and mediate the detoxification of reactive oxygen species, and the modulation of cell growth or cell division [
18]. These mechanisms are mostly under hormonal control, and the adverse effect of salinity on seed germination and plant growth has been related to modifications of the endogenous levels of phytohormones. In fact, Wang et al. [
19] found that salinity may determine an increase of abscisic acid (ABA) and jasmonic acid (JA) levels and a decrease of indole-3-acetic acid (IAA) and salicylic acid (SA) levels. This evidence suggested that the exogenous application of plant growth regulators (PGRs), such as auxins, gibberellins, or cytokinins, could have beneficial effects in mitigating the repressive effects of salt stress and also enhance germination, growth, development, yield, and quality [
20,
21,
22].
Among these PGRs, gibberellins (GAs) are essential endogenous hormones produced by plants and fungi that control plant development by triggering several physiological mechanisms [
23,
24]. The response of plant tissue to the GA signal can be the modification in gene expression, plant physiology, and morphology [
25]. These effects can also be determined by the application of exogenous gibberellins. Many investigations have focused the attention on the supplementation of gibberellic acid (GA
3), with the aim to improve plant growth and yield and to enhance tolerance to abiotic stresses (e.g., drought, heat, salinity). Exogenous supplementation through foliar spray of GA
3 solutions at low concentrations has proved to determine positive effects on the growth, quality, and salt tolerance of vegetables cultivated in soil or in hydroponic cultivation systems [
26,
27,
28,
29,
30,
31]. Yet, studies on the effects of gibberellic acid application on leafy vegetables grown in hydroponics are rather scant. Miceli et al. [
24,
32] found that the supplementation of 10
−6 M GA
3 through the nutrient solution of hydroponically grown leaf lettuce and rocket can exert a significant effect on the yield, quality, and post-harvest life of these vegetables. Thus, the exogenous application of GA
3 trough the nutrient solution could also affect the salt tolerance of leafy vegetables. Therefore, the aim of this study was to evaluate the feasibility of adding gibberellic acid (10
−6 M GA
3) to the mineral nutrient solution to increase the salt tolerance of leaf lettuce and rocket grown in a floating system.
4. Discussion
The evidence that the salt tolerance mechanism in plants is primarily under hormonal control and that plants’ response to increased salinity is related to changes in endogenous phytohormone levels has led to studies that aimed to re-equilibrate the phytohormone levels of salt-stressed plants through the exogenous supplementation of plant growth regulators [
18,
20,
29,
42]. Considering the plants under field conditions, it has been shown that vigorous plants can better cope with salt stress, possibly by increasing the salinity tolerance threshold or delaying its onset [
43]. The exogenous supplementation of plant growth regulators, such as gibberellic acid (GA
3), can be effective in increasing plant growth and vigor and can affect plant metabolism by enhancing N uptake and assimilation and improving photosynthetic CO
2 fixation [
31,
44,
45], thus helping plants to better cope with salt stress. In a previous work [
24], we found that leaf lettuce and rocket grown in a floating system showed enhanced growth and quality when 10
−6 M GA
3 was added to the MNS. In this experiment, we tested the possibility of increasing the salinity tolerance threshold of these species by supplying 10
−6 M GA
3 through the mineral nutrient solution of a floating system and evaluated the combined effects of salt stress and GA
3 on growth and quality.
Leaf lettuce and rocket were shown to be affected by the salt stress applied to the plants even with a different salinity tolerance threshold. The main visible effect of salinity on vegetables is a reduction of the growth rate, which determines smaller leaves and fruits, shorter plants, and the alteration of other morphological attributes, and ending in a reduction of the plant biomass [
46,
47]. These effects were also found in the tested species. Plant height showed a significant reduction of about 5% in both lettuce and rocket, but the latter recorded this reduction only at the highest salt stress. The salinity of the MNS also affected the fresh biomass of the plants of both species, but even for this parameter, the reduction was slight and related to the salt stress level in rocket plants whereas it reached a significantly lower value at an intermediate salt stress level in lettuce, confirming that the effects of salinity on vegetables depend on its level [
48]. The reduction of fresh biomass could be ascribed to the increased osmotic stress determined by the high EC of the MNS that reduced water uptake and to a modification of plant metabolism and nutrient uptake. Salinity negatively influences the availability of the nutrient elements in the nutrient solution, thus possibly leading to a severe reduction of root growth [
47,
48]. This reduction was more evident for the root fresh weight of lettuce plants and could thus depend on crops and salinity levels. Salt stress can also affect water and nutrient uptake and translocation from the roots to the shoot, determining changes in biomass fractions as shown by the S/R FW ratio of rocket plants. The reduction of the lettuce and rocket fresh biomass induced by the exposure to salinity was mainly due to osmotic effects and changes in the water status of the plants because salt stress did not significantly affect the dry biomass accumulated in the plants of both species. The relative water content of leaves generally decreases in increasing salt stress and this could be due to lower water availability under stress conditions or to disorders of the roots that are not able to sufficiently compensate for the water lost by transpiration [
49,
50].
Inhibition in plant growth was significantly mitigated by the addition of GA
3 to the MNS; the supplementation of gibberellic acid through the nutrient solution was confirmed to have a positive effect on fresh and dry biomass accumulation of the unstressed lettuce and rocket plants [
24] and maintained this positive effect in the 10 and 20 mM salt treatments, with the biomass close to the control plants (fresh weight) or even significantly higher as regards the dry biomass. Plant hormones play an important role in plant growth and development and can alleviate the negative effects of salt stress [
51]; gibberellic acid was found to be helpful in improving the growth of various crops under saline conditions [
18,
31,
51,
52]. The exogenous supply of hormones during salt stress may reduce water loss rates and also determine the increase in the leaf water potential and carbon gain rates [
53]. The exogenous supplementation of GA
3 may exert a positive effect on the growth of the aerial part of the plants by influencing internal resource repartition. The increase of the biomass of aboveground parts may be determined by the rise of many important catabolic pathways (ribose and polyribosome multiplication; DNA, RNA, and protein synthesis) [
54,
55,
56,
57]. The biomass increase in salt-stressed plants treated with GA
3 could also be due to enhanced uptake and use of water and mineral nutrients [
27,
58,
59] and transport of photosynthates [
60,
61,
62], which follow from increased membrane permeability [
63,
64].
The yield and quality of leafy vegetables are strictly related to the characteristics of the aboveground biomass accumulated by the plants, thus the negative effects of salt stress on plant morphology and development severely impact yield and its economic value. Leaf lettuce and rocket cultivated in soilless systems were shown to be sensitive to salinity [
10,
14,
65,
66,
67,
68], and this sensitivity was confirmed in this experiment as they overcame the tolerance threshold starting with the lower NaCl concentration (10 mM; 3.4 mS cm
−1 EC), which determined a yield drop by 11.6% and 24.6% on average for lettuce and rocket, respectively. Moreover, lettuce and rocket plants recorded a significant drop in the minimal processing yield at 20 mM NaCl that further increased the reduction in yielded plants and consequently the marketable portion obtainable from salt-stressed plants.
Even for these parameters, GA
3 treatment successfully mitigated salt stress and enhanced the salt tolerance of the tested crops. The supply of 10
−6 M GA
3 through the nutrient solution increased the yield parameters in salt-stressed plants to similar or even higher levels than control plants (0 mM NaCl; 0 M GA
3). It is well known that the exposure of plants to salinity induces a proportional increase in abscisic acid (ABA) synthesis, which is often related to the leaf water potential [
51]. Thus, the increase of the endogenous ABA may be due to a water deficit rather than specific salt effects [
69]. Abscisic acid is considered the root-to-shoot stress signal and its increased concentration in the xylem was found to be correlated with reduced leaf conductance and general limitation of leaf growth. Since gibberellins stimulate the catabolism of ABA [
70], the exogenous application of gibberellic acid might limit the ABA-mediated negative response of plants to salinity. Moreover, GA
3 has been shown to have many positive direct effects on plant metabolism and plant growth [
24,
28,
31,
62]. Plants supplemented with exogenous GA
3 have shown enhanced activity of carbonic anhydrase (CA), which plays a role in CO
2 fixation in the chloroplast during photosynthesis [
44]. This increased enzymatic activity may improve the CO
2 supply at the site of its fixation and increases the net photosynthetic rate, thus increasing biomass accumulation and yield [
44,
71]. Another contribution that could have increased the photosynthetic rate of lettuce and rocket plants could be ascribed to the positive effect of GA
3 on stomatal conductance. This effect was found in the unstressed plants of both leafy vegetables, and even in salt stress conditions in lettuce plants as also found in tomato grown in soilless cultivation at low salinity [
18]. Plants grown under salt stress try to compensate for the reduced ability to take up water with stomatal closure, which reduces the volume of air exchanged with the environment. This mechanism can be an efficient system to have more economical water utilization and may limit the harmful salt ions’ uptake [
72]. Nonetheless, the reduction in stomatal conductance and the low intercellular CO
2 led to lower photosynthetic rates and limited growth [
73], hence the positive effects of GA
3 on stomatal opening and photosynthetic rates resulted in enhanced water use efficiency and consequently in improved nitrogen use efficiency even if to a different extent for the leafy vegetable tested.
As well as stomatal closure, salt stress affects the rate at which plants produce new leaves and influences other leaf characteristics that may determine a negative impact on photosynthesis and plant growth, such as leaf number and leaf expansion and mesophyll anatomy [
74,
75]. These modifications may vary among species as they can cope with salt stress through different tolerance strategies. Leaf morphology changes were also found in this experiment for lettuce and rocket floating on MNS with high EC. In fact, a reduction in the total leaf area per plant was recorded for both species and was due to variation of the leaf morphology (leaf width in lettuce and petiole length in rocket), leaf area, and leaf number per plant. Moreover, salt-stressed lettuce leaves were thicker than control leaves as shown by the lower SLA. Leaf thickening may negatively influence the diffusion of CO
2 in the mesophyll and this parameter may have a role in limiting the photosynthesis of stressed leaves [
75]. The differences determined by salt stress in SLA between lettuce and rocket could be due to differences in the mechanisms that species activate to overcome salt stress. GA
3 supplementation helped salt-stressed plants in limiting the negative effect of salt stress on leaf development and morphology and confirmed an improvement of these characteristics in control plants [
24]. The application of GA
3 is often simultaneous with an increase in stomatal conductance and a decrease in intercellular CO
2 partial pressure, which increases the net photosynthetic rate [
45]. The positive effect of GA
3 against salt stress could be ascribed to the morphological modifications that it determined in lettuce and rocket plants. These effects of GA
3 supplementation could be very beneficial on leafy vegetables grown in hydroponic floating systems under salt stress, where a high plant density slows air movement inside the canopy, thus negatively affecting CO
2 availability. Moreover, leafy vegetable quality is mostly based on leaf appearance, and the modifications induced by salt stress can negatively influence their market value. In addition to leaf size and morphology, salt stress may also affect leaf color as it can influence chlorophyll synthesis and degradation. Chlorophyll content may be lowered by increasing salinity, but this variation could depend on plant sensitivity or tolerance to salt stress. Leaf lettuce and rocket differed slightly in color changes due to NaCl, thus confirming the differences in species response. Generally, salt stress lowered color lightness and chroma and increased the hue angle, but these variations were very small in both species so that the variation in the photosynthetic pigment content was probably also small. Besides, the increase in leaf thickness recorded in salt-stressed lettuce plants could have determined a chlorophyll accumulation per unit leaf area [
76] that could explain the increase of the hue angle under salt stress conditions.
Even if lettuce and rocket had a similar response to salt stress and GA
3 treatment as regards plant growth and morphology, the experimental factors determined changes in the metabolism that differed in the tested species, as suggested by the differences in SSC, TA, ascorbic acid, and nitrate content. The presence of these chemical components could be modified in response to salt stress. In fact, many plants react to salinity by activating or improving some metabolic pathways or the biosynthesis of secondary metabolites; among these, soluble solids, sugars, organic acids, proteins, and amino acids may play a role in plant turgor maintenance, acting as osmolytes or osmoregulators [
48,
77]. These metabolites are often responsible for the nutritive quality of fruits and vegetables, thus their presence could enhance the market value of the products, offsetting the economic losses caused by yield reductions due to salinity [
48,
78]. The increase of SSC in salt-stressed lettuce leaves confirmed that this species reacted as others by accumulating soluble carbohydrates in response to salinity [
78,
79]. The increase of soluble solids in rocket leaves was very low, thus indicating that rocket plants activated other mechanisms to reduce their osmotic potential and to maintain plant turgor. Moreover, GA
3 treatment determined a significant decrease of SSC in rocket plants irrespective of salt stress that could be due to increased growth promoted by the exogenous supply of GA
3, which increased carbohydrate utilization.
Plants react to the increase of reactive oxygen species under salt stress by activating certain antioxidative enzymes (catalase, peroxidase, glutathione reductase, and superoxide dismutase) and the scavenging of free radicals could also cause a decrease in total ascorbate, total glutathione, and α-tocopherol levels [
80,
81]. Leaf lettuce and rocket showed a reduction of the ascorbic acid content in salt-stressed plants that was probably caused by the counteracting of reactive oxygen species. GA
3 treatment acted with an opposite trend on the ascorbic acid content of lettuce and rocket, showing again that the contribution of gibberellic acid in counteracting salt stress could involve different mechanisms in different species. Moreover, ascorbic acid accumulation could strictly depend on nitrogen availability and translocation, thus the different responses of the ascorbic acid content to the GA
3 treatment could be related to the differences in the nitrogen metabolism of lettuce and rocket plants determined by GA
3 supplementation as revealed by the differing nitrate accumulation. High availability of nitrogen seems to be related to a lower concentration of vitamin C in some fruits and vegetables, with variations that could depend on species, climate, and other factors [
82,
83]. This was confirmed in this work as the level of ascorbic acid was negatively related to the nitrate content of the leaves.
As already stated, the salinity of soil solution or irrigation water can determine imbalances in nutrient uptake, making the absorption of essential cations and anions, such as K
+ and NO
3−, problematic to plants [
84,
85]. Leafy vegetables may accumulate a great amount of nitrates in the leaves, causing a reduction of their nutritional quality as nitrates can be harmful to human health [
86,
87,
88]. Furthermore, if the nitrate content of lettuce and rocket overcomes the thresholds imposed by EU regulations, they can even lose marketability [
89]. Salt stress can determine a reduction of nitrate reductase activity in some species, leading to nitrate accumulation [
90]. Nevertheless, the reduction of nitrate uptake caused by moderate salinity could help in improving the nutritional quality and economic value of some leafy vegetables. This goal was obtained in rocket plants grown with 20 mM NaCl in the MNS whereas salt stress did not affect the nitrate content of lettuce, showing that the root system of these species could differ in uptake capacity. Differences were also found in their response to GA
3 treatments as regards the nitrate content of the leaves as also found in previous works [
24,
32]. As already discussed, GA
3 positively affected NUE and this may be due to the effect of gibberellic acid on improving nitrogen utilization by enhancing N metabolism and its redistribution in the plants [
31]. The exogenous GA
3 supplementation stimulated plant growth, thus increasing the needs of nitrogen for tissue development. It is known that GA
3 has a role in regulating the distribution of the assimilates from the photosynthetic tissue to the shoot apex and the young leaves, aiding in the utilization of nitrogen and thus increasing plant biomass and yield [
31]. Gibberellins can also influence the activity of nitrate reductase, an enzyme that is crucial for the nitrogen metabolism of plants [
91,
92]. The positive effect of GA
3 on nitrate reductase of rocket plants could have determined a reduction of nitrate accumulation even under salt stress. On the contrary, lettuce plants supplied with GA
3 could have increased the nitrate content during growth thanks to a more efficient translocation from roots to leaves where nitrate competed with sodium ions for accumulation in the vacuoles, alleviating ionic and osmotic stress and maintaining plant turgor [
9].
The severity of salinity effects may vary greatly according to species or even to varieties within a species and it may be moderated or accentuated by environmental or agronomic factors [
47]. These differences and the modification to salt tolerance were shown well by the PCA analysis that underlined the different responses of lettuce and rocket to salt stress and showed that the supply of exogenous GA
3 counterbalanced the salinity, acting on different plant adaptation systems. Moreover, the effects of GA
3 treatment had a different extent in the tested species, thus confirming that the response to exogenous GA
3 may vary according to species [
24,
32,
93].