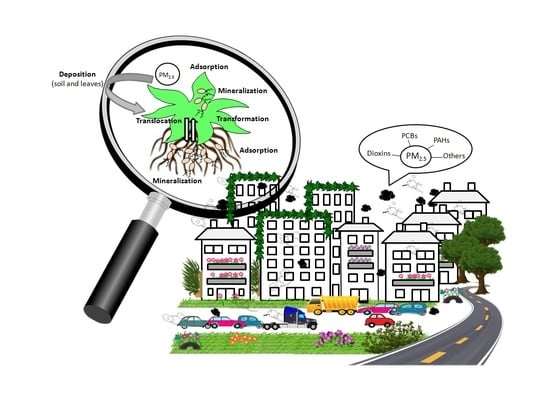
Plant-Bacteria Interactions for the Elimination of Atmospheric Contaminants in Cities
Abstract
:1. Introduction
2. BTEX, PAHs, PCBs and Dioxins: Origin and Toxicity
3. Deposition, Transport and Detoxification of Contaminants in Plants
4. Degradation of BTEX, PAHs, PCBs and Dioxins by Bacteria
5. Plant-Bacteria Associations for the Elimination of Atmospheric Contaminants
5.1. Rhizoremediation
5.2. Phylloremediation
6. Removal of Air Pollutants and the Role of Green Architecture
7. Research Needs
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Muller, N.Z.; Mendelsohn, R. Measuring the Damages of Air Pollution in the United States. J. Environ. Econ. Manag. 2007, 54, 1–14. [Google Scholar] [CrossRef]
- Pimpin, L.; Retat, L.; Fecht, D.; de Preux, L.; Sassi, F.; Gulliver, J.; Belloni, A.; Ferguson, B.; Corbould, E.; Jaccard, A.; et al. Estimating the Costs of Air Pollution to the National Health Service and Social Care: An Assessment and Forecast up to 2035. PLoS Med. 2018, 15, e1002602. [Google Scholar] [CrossRef] [PubMed]
- Di Turo, F.; Proietti, C.; Screpanti, A.; Fornasier, M.F.; Cionni, I.; Favero, G.; De Marco, A. Impacts of Air Pollution on Cultural Heritage Corrosion at European Level: What Has Been Achieved and What Are the Future Scenarios. Environ. Pollut. Barking Essex 1987 2016, 218, 586–594. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Jo, W.-K.; Chun, H.-H. Characteristics of Atmospheric Visibility and Its Relationship with Air Pollution in Korea. J. Environ. Qual. 2014, 43, 1519–1526. [Google Scholar] [CrossRef] [PubMed]
- Miao, W.; Huang, X.; Song, Y. An Economic Assessment of the Health Effects and Crop Yield Losses Caused by Air Pollution in Mainland China. J. Environ. Sci. China 2017, 56, 102–113. [Google Scholar] [CrossRef]
- Stevens, C.J.; Bell, J.N.B.; Brimblecombe, P.; Clark, C.M.; Dise, N.B.; Fowler, D.; Lovett, G.M.; Wolseley, P.A. The Impact of Air Pollution on Terrestrial Managed and Natural Vegetation. Philos. Transact. A Math. Phys. Eng. Sci. 2020, 378, 20190317. [Google Scholar] [CrossRef]
- Dias, D.; Amorim, J.H.; Sá, E.; Borrego, C.; Fontes, T.; Fernandes, P.; Pereira, S.R.; Bandeira, J.; Coelho, M.C.; Tchepel, O. Assessing the Importance of Transportation Activity Data for Urban Emission Inventories. Transp. Res. Part. Transp. Environ. 2018, 62, 27–35. [Google Scholar] [CrossRef]
- Zhang, X.; Murakami, T.; Wang, J.; Aikawa, M. Sources, Species and Secondary Formation of Atmospheric Aerosols and Gaseous Precursors in the Suburb of Kitakyushu, Japan. Sci. Total Environ. 2020, 143001. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.T.; Liu, M.C.; Hung, P.C.; Chang, S.H.; Chang, M.B. PAH Emissions from Coal Combustion and Waste Incineration. J. Hazard. Mater. 2016, 318, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Turpin, B.J. Peer Reviewed: Options for Characterizing Organic Particulate Matter. Environ. Sci. Technol. 1999, 33, 76A–79A. [Google Scholar] [CrossRef]
- Manisalidis, I.; Stavropoulou, E.; Stavropoulos, A.; Bezirtzoglou, E. Environmental and Health Impacts of Air Pollution: A Review. Front. Public Health 2020, 8, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krall, J.R.; Mulholland, J.A.; Russell, A.G.; Balachandran, S.; Winquist, A.; Tolbert, P.E.; Waller, L.A.; Sarnat, S.E. Associations between Source-Specific Fine Particulate Matter and Emergency Department Visits for Respiratory Disease in Four, U.S. Cities. Environ. Health Perspect. 2017, 125, 97–103. [Google Scholar] [CrossRef]
- Robinson, D.L. Composition and Oxidative Potential of PM2.5 Pollution and Health. J. Thorac. Dis. 2017, 9, 444–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murillo, J.H.; Rojas Marin, J.F.; Roman, S.R.; Beita Guerrero, V.H.; Arias, D.S.; Ramos, A.C.; Gonzalez, B.C.; Baumgardner, D.G. Temporal and Spatial Variations in Organic and Elemental Carbon Concentrations in PM10/PM2.5 in the Metropolitan Area of Costa Rica, Central America. Atmos. Pollut. Res. 2013, 4, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Bi, C.; Chen, Y.; Zhao, Z.; Li, Q.; Zhou, Q.; Ye, Z.; Ge, X. Characteristics, Sources and Health Risks of Toxic Species (PCDD/Fs, PAHs and Heavy Metals) in PM2.5 during Fall and Winter in an Industrial Area. Chemosphere 2020, 238, 124620. [Google Scholar] [CrossRef]
- Idris, S.A.A.; Hanafiah, M.M.; Khan, M.F.; Hamid, H.H.A. Indoor Generated PM2.5 Compositions and Volatile Organic Compounds: Potential Sources and Health Risk Implications. Chemosphere 2020, 255, 126932. [Google Scholar] [CrossRef] [PubMed]
- Remizovschi, A.; Carpa, R.; Forray, F.L.; Chiriac, C.; Roba, C.-A.; Beldean-Galea, S.; Andrei, A.-Ș.; Szekeres, E.; Baricz, A.; Lupan, I.; et al. Mud Volcanoes and the Presence of PAHs. Sci. Rep. 2020, 10. [Google Scholar] [CrossRef] [Green Version]
- Luo, J.; Han, Y.; Zhao, Y.; Huang, Y.; Liu, X.; Tao, S.; Liu, J.; Huang, T.; Wang, L.; Chen, K.; et al. Effect of Northern Boreal Forest Fires on PAH Fluctuations across the Arctic. Environ. Pollut. Barking Essex 1987 2020, 261, 114186. [Google Scholar] [CrossRef]
- Samburova, V.; Zielinska, B.; Khlystov, A. Do 16 Polycyclic Aromatic Hydrocarbons Represent PAH Air Toxicity? Toxics 2017, 5, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shen, H.; Huang, Y.; Wang, R.; Zhu, D.; Li, W.; Shen, G.; Wang, B.; Zhang, Y.; Chen, Y.; Lu, Y.; et al. Global Atmospheric Emissions of Polycyclic Aromatic Hydrocarbons from 1960 to 2008 and Future Predictions. Environ. Sci. Technol. 2013, 47, 6415–6424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewa, B.; Danuta, M.-Š. Polycyclic Aromatic Hydrocarbons and PAH-Related DNA Adducts. J. Appl. Genet. 2017, 58, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Fenga, C.; Gangemi, S.; Costa, C. Benzene Exposure Is Associated with Epigenetic Changes (Review). Mol. Med. Rep. 2016, 13, 3401–3405. [Google Scholar] [CrossRef] [Green Version]
- Galbraith, D.; Gross, S.A.; Paustenbach, D. Benzene and Human Health: A Historical Review and Appraisal of Associations with Various Diseases. Crit. Rev. Toxicol. 2010, 40 (Suppl. S2), 1–46. [Google Scholar] [CrossRef]
- Lin, C.-K.; Hsu, Y.-T.; Brown, K.D.; Pokharel, B.; Wei, Y.; Chen, S.-T. Residential Exposure to Petrochemical Industrial Complexes and the Risk of Leukemia: A Systematic Review and Exposure-Response Meta-Analysis. Environ. Pollut. 2020, 258, 113476. [Google Scholar] [CrossRef] [PubMed]
- Vorkamp, K. An Overlooked Environmental Issue? A Review of the Inadvertent Formation of PCB-11 and Other PCB Congeners and Their Occurrence in Consumer Products and in the Environment. Sci. Total Environ. 2016, 541, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Eskenazi, B.; Warner, M.; Brambilla, P.; Signorini, S.; Ames, J.; Mocarelli, P. The Seveso Accident: A Look at 40 years of Health Research and Beyond. Environ. Int. 2018, 121, 71–84. [Google Scholar] [CrossRef] [PubMed]
- Larigot, L.; Juricek, L.; Dairou, J.; Coumoul, X. AhR Signaling Pathways and Regulatory Functions. Biochim. Open 2018, 7, 1–9. [Google Scholar] [CrossRef]
- Jayasundara, N.; Van Tiem Garner, L.; Meyer, J.N.; Erwin, K.N.; Di Giulio, R.T. AHR2-Mediated Transcriptomic Responses Underlying the Synergistic Cardiac Developmental Toxicity of PAHs. Toxicol. Sci. 2015, 143, 469–481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of Inhaled Combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an Environmental Exposure Model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, B.; Chu, C.; Carlin, D.J. Polycyclic Aromatic Hydrocarbons: From Metabolism to Lung Cancer. Toxicol. Sci. Off. J. Soc. Toxicol. 2015, 145, 5–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimada, T.; Fujii-Kuriyama, Y. Metabolic Activation of Polycyclic Aromatic Hydrocarbons to Carcinogens by Cytochromes P450 1A1 And1B. Cancer Sci. 2004, 95, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; Warshawsky, D. Metabolic Activation of Polycyclic and Heterocyclic Aromatic Hydrocarbons and DNA Damage: A Review. Toxicol. Appl. Pharmacol. 2005, 206, 73–93. [Google Scholar] [CrossRef] [PubMed]
- Croutch, C.R.; Lebofsky, M.; Schramm, K.-W.; Terranova, P.F.; Rozman, K.K. 2,3,7,8-Tetrachlorodibenzo-p-Dioxin (TCDD) and 1,2,3,4,7,8-Hexachlorodibenzo-p-Dioxin (HxCDD) Alter Body Weight by Decreasing Insulin-Like Growth Factor I (IGF-I) Signaling. Toxicol. Sci. 2005, 85, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Hashmi, M.Z.; Zhang, J.; Li, B.; Su, X.; Tariq, M.; Ahmad, N.; Malik, R.N.; Ullah, K.; Chen, C.; Shen, C. Effects of Structurally Different Noncoplanar and Coplanar PCBs on HELF Cell Proliferation, Cell Cycle, and Potential Molecular Mechanisms. Environ. Toxicol. 2017, 32, 1183–1190. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, M.; Liu, B.; Wu, J.; Peng, X.; Zhang, Y.; Han, S.; Feng, Y.; Zhu, T. Characterization and Source Apportionment of Size-Segregated Atmospheric Particulate Matter Collected at Ground Level and from the Urban Canopy in Tianjin. Environ. Pollut. 2016, 219, 982–992. [Google Scholar] [CrossRef]
- Mei, D.; Wen, M.; Xu, X.; Zhu, Y.; Xing, F. The Influence of Wind Speed on Airflow and Fine Particle Transport within Different Building Layouts of an Industrial City. J. Air Waste Manag. Assoc. 1995 2018, 68, 1038–1050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Chen, J.; Yang, H.; Li, R.; Yu, Q. Seasonal Variation and Potential Source Regions of PM2.5-Bound PAHs in the Megacity Beijing, China: Impact of Regional Transport. Environ. Pollut. 2017, 231, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, T.; Huang, Y.; Mao, T.; Zhong, N. Size Distribution of Polycyclic Aromatic Hydrocarbons in Urban and Suburban Sites of Beijing, China. Chemosphere 2005, 61, 792–799. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R.; Lammel, G. Adsorptive and Absorptive Contributions to the Gas-Particle Partitioning of Polycyclic Aromatic Hydrocarbons: State of Knowledge and Recommended Parametrization for Modeling. Environ. Sci. Technol. 2004, 38, 3793–3803. [Google Scholar] [CrossRef]
- Mu, Q.; Shiraiwa, M.; Octaviani, M.; Ma, N.; Ding, A.; Su, H.; Lammel, G.; Pöschl, U.; Cheng, Y. Temperature Effect on Phase State and Reactivity Controls Atmospheric Multiphase Chemistry and Transport of PAHs. Sci. Adv. 2018, 4. [Google Scholar] [CrossRef] [Green Version]
- Pullagurala, V.L.R.; Rawat, S.; Adisa, I.O.; Hernandez-Viezcas, J.A.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Plant Uptake and Translocation of Contaminants of Emerging Concern in Soil. Sci. Total Environ. 2018, 636, 1585–1596. [Google Scholar] [CrossRef]
- Collins, C.D.; Finnegan, E. Modeling the Plant Uptake of Organic Chemicals, Including the Soil-Air-Plant Pathway. Environ. Sci. Technol. 2010, 44, 998–1003. [Google Scholar] [CrossRef]
- Takaki, K.; Wade, A.J.; Collins, C.D. Assessment of Plant Uptake Models Used in Exposure Assessment Tools for Soils Contaminated with Organic Pollutants. Environ. Sci. Technol. 2014, 48, 12073–12082. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, S.; Zheng, L.; Li, T.; Sun, Y.; Ma, L.; Lin, Z.; Grathwohl, P.; Lohmann, R. Air-Soil Diffusive Exchange of PAHs in an Urban Park of Shanghai Based on Polyethylene Passive Sampling: Vertical Distribution, Vegetation Influence and Diffusive Flux. Sci. Total Environ. 2019, 689, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fismes, J.; Perrin-Ganier, C.; Empereur-Bissonnet, P.; Morel, J.L. Soil-to-Root Transfer and Translocation of Polycyclic Aromatic Hydrocarbons by Vegetables Grown on Industrial Contaminated Soils. J. Environ. Qual. 2002, 31, 1649–1656. [Google Scholar] [CrossRef]
- Bakker, M.I.; Koerselman, J.W.; Tolls, J.; Kollöffel, C. Localization of Deposited Polycyclic Aromatic Hydrocarbons in Leaves of Plantago. Environ. Toxicol. Chem. 2001, 20, 1112–1116. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Dai, C.; Zhou, Y.; Peng, H.; Yi, K.; Qin, P.; Luo, S.; Zhang, X. Comparisons of Three Plant Species in Accumulating Polycyclic Aromatic Hydrocarbons (PAHs) from the Atmosphere: A Review. Environ. Sci. Pollut. Res. 2018, 25, 16548–16566. [Google Scholar] [CrossRef]
- Howsam, M.; Jones, K.C.; Ineson, P. PAHs Associated with the Leaves of Three Deciduous Tree Species. II: Uptake during a Growing Season. Chemosphere 2001, 44, 155–164. [Google Scholar] [CrossRef]
- Howsam, M.; Jones, K.C.; Ineson, P. PAHs Associated with the Leaves of Three Deciduous Tree Species. I—Concentrations and Profiles. Environ. Pollut. 2000, 108, 413–424. [Google Scholar] [CrossRef]
- Huelster, A.; Mueller, J.F.; Marschner, H. Soil-Plant Transfer of Polychlorinated Dibenzo-p-Dioxins and Dibenzofurans to Vegetables of the Cucumber Family (Cucurbitaceae). Environ. Sci. Technol. 1994, 28, 1110–1115. [Google Scholar] [CrossRef]
- Patowary, R.; Patowary, K.; Devi, A.; Kalita, M.C.; Deka, S. Uptake of Total Petroleum Hydrocarbon (TPH) and Polycyclic Aromatic Hydrocarbons (PAHs) by Oryza sativa L. Grown in Soil Contaminated with Crude Oil. Bull. Environ. Contam. Toxicol. 2017, 98, 120–126. [Google Scholar] [CrossRef]
- Sun, Y.; Zhou, Q. Uptake and Translocation of Benzo[a]Pyrene (B[a]P) in Two Ornamental Plants and Dissipation in Soil. Ecotoxicol. Environ. Saf. 2016, 124, 74–81. [Google Scholar] [CrossRef]
- Kang, F.; Chen, D.; Gao, Y.; Zhang, Y. Distribution of Polycyclic Aromatic Hydrocarbons in Subcellular Root Tissues of Ryegrass (Lolium Multiflorum Lam.). BMC Plant. Biol. 2010, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Gu, R.; Sheng, Y.; Zeng, N.; Zhan, X. Acropetal Translocation of Phenanthrene in Wheat Seedlings: Xylem or Phloem Pathway? Environ. Pollut. 2020, 260, 114055. [Google Scholar] [CrossRef] [PubMed]
- Alves, W.S.; Manoel, E.A.; Santos, N.S.; Nunes, R.O.; Domiciano, G.C.; Soares, M.R. Detection of Polycyclic Aromatic Hydrocarbons (PAHs) in Medicago sativa L. by Fluorescence Microscopy. Micron 2017, 95, 23–30. [Google Scholar] [CrossRef]
- Gao, Y.; Zhu, L. Plant Uptake, Accumulation and Translocation of Phenanthrene and Pyrene in Soils. Chemosphere 2004, 55, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Houshani, M.; Salehi-Lisar, S.Y.; Motafakkerazad, R.; Movafeghi, A. Uptake and distribution of phenanthrene and pyrene in roots and shoots of maize (Zea mays L.). Environ. Sci. Pollut. Res. 2019, 26, 9938–9944. [Google Scholar] [CrossRef] [PubMed]
- Alagić, S.Č.; Jovanović, V.P.S.; Mitić, V.D.; Cvetković, J.S.; Petrović, G.M.; Stojanović, G.S. Bioaccumulation of HMW PAHs in the Roots of Wild Blackberry from the Bor Region (Serbia): Phytoremediation and Biomonitoring Aspects. Sci. Total Environ. 2016, 562, 561–570. [Google Scholar] [CrossRef]
- Wild, E.; Dent, J.; Thomas, G.O.; Jones, K.C. Direct Observation of Organic Contaminant Uptake, Storage, and Metabolism within Plant Roots. Environ. Sci. Technol. 2005, 39, 3695–3702. [Google Scholar] [CrossRef]
- Miller, E.L.; Nason, S.L.; Karthikeyan, K.G.; Pedersen, J.A. Root Uptake of Pharmaceuticals and Personal Care Product Ingredients. Environ. Sci. Technol. 2016, 50, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.; Zhu, M.; Shen, Y.; Yue, L.; Li, J.; Gardea-Torresdey, J.L.; Xu, G. Apoplastic and Symplastic Uptake of Phenanthrene in Wheat Roots. Environ. Pollut. Barking Essex 1987 2018, 233, 331–339. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Liu, X.; Zhang, Y.; Zou, Y.; Yuan, J. Study on Removal of Pyrene by Agropyron cristatum L. in Pyrene–Ni Co-Contaminated Soil. Int. J. Phytoremediation 2020, 22, 313–321. [Google Scholar] [CrossRef]
- Kipopoulou, A.M.; Manoli, E.; Samara, C. Bioconcentration of Polycyclic Aromatic Hydrocarbons in Vegetables Grown in an Industrial Area. Environ. Pollut. 1999, 106, 369–380. [Google Scholar] [CrossRef]
- Pilon-Smits, E. Phytoremediation. Annu. Rev. Plant. Biol. 2005, 56, 15–39. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Garrido, B.; Balseiro-Romero, M.; Kidd, P.S.; Monterroso, C. Effect of Plant Root Exudates on the Desorption of Hexachlorocyclohexane Isomers from Contaminated Soils. Chemosphere 2020, 241, 124920. [Google Scholar] [CrossRef]
- Jia, H.; Lu, H.; Liu, J.; Li, J.; Dai, M.; Yan, C. Effects of Root Exudates on the Leachability, Distribution, and Bioavailability of Phenanthrene and Pyrene from Mangrove Sediments. Environ. Sci. Pollut. Res. Int. 2016, 23, 5566–5576. [Google Scholar] [CrossRef]
- Wang, J.; Chen, X.; Yan, W.; Ning, C.; Gsell, T. Both Artificial Root Exudates and Natural Koelreuteria paniculata Exudates Modify Bacterial Community Structure and Enhance Phenanthrene Biodegradation in Contaminated Soils. Chemosphere 2021, 263, 128041. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sun, J.; Zhu, L. The Role of Artificial Root Exudate Components in Facilitating the Degradation of Pyrene in Soil. Sci. Rep. 2017, 7, 7130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Ren, L.; Ling, W.; Gong, S.; Sun, B.; Zhang, Y. Desorption of Phenanthrene and Pyrene in Soils by Root Exudates. Bioresour. Technol. 2010, 101, 1159–1165. [Google Scholar] [CrossRef]
- Balasubramaniyam, A. The Influence of Plants in the Remediation of Petroleum Hydrocarbon- Contaminated Sites. Pharm. Anal. Chem. Open Access 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Terzaghi, E.; Vitale, C.M.; Salina, G.; Di Guardo, A. Plants Radically Change the Mobility of PCBs in Soil: Role of Different Species and Soil Conditions. J. Hazard. Mater. 2020, 388, 121786. [Google Scholar] [CrossRef]
- Varjani, S.J. Microbial Degradation of Petroleum Hydrocarbons. Bioresour. Technol. 2017, 223, 277–286. [Google Scholar] [CrossRef] [PubMed]
- Shuttleworth, K.L.; Cerniglia, E. Environmental Aspects of PAH Biodegradation. Appl. Biochem. Biotechnol. 1995, 54, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, D.; Ghosh, S.; Dutta, T.K.; Ahn, Y. Current State of Knowledge in Microbial Degradation of Polycyclic Aromatic Hydrocarbons (PAHs): A Review. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieper, D.H. Aerobic Degradation of Polychlorinated Biphenyls. Appl. Microbiol. Biotechnol. 2005, 67, 170–191. [Google Scholar] [CrossRef] [PubMed]
- Gibson, D.T.; Parales, R.E. Aromatic Hydrocarbon Dioxygenases in Environmental Biotechnology. Curr. Opin. Biotechnol. 2000, 11, 236–243. [Google Scholar] [CrossRef]
- Yavas, A.; Icgen, B. Diversity of the Aromatic-Ring-Hydroxylating Dioxygenases in the Monoaromatic Hydrocarbon Degraders Held by a Common Ancestor. Bull. Environ. Contam. Toxicol. 2018, 101, 410–416. [Google Scholar] [CrossRef] [PubMed]
- Kanaly, R.A.; Harayama, S. Advances in the Field of High-Molecular-Weight Polycyclic Aromatic Hydrocarbon Biodegradation by Bacteria. Microb. Biotechnol. 2010, 3, 136–164. [Google Scholar] [CrossRef] [Green Version]
- Wittich, R.M.; Wilkes, H.; Sinnwell, V.; Francke, W.; Fortnagel, P. Metabolism of Dibenzo-p-Dioxin by Sphingomonas sp. Strain RWAppl. Environ. Microbiol. 1992, 58, 1005–1010. [Google Scholar] [CrossRef] [Green Version]
- Wilkes, H.; Wittich, R.M.; Timmis, K.N.; Fortnagel, P.; Francke, W. Degradation of Chlorinated Dibenzofurans and Dibenzo-p-Dioxins by Sphingomonas sp. Strain RWAppl. Environ. Microbiol. 1996, 62, 367–371. [Google Scholar] [CrossRef] [Green Version]
- Chai, B.; Tsoi, T.V.; Iwai, S.; Liu, C.; Fish, J.A.; Gu, C.; Johnson, T.A.; Zylstra, G.; Teppen, B.J.; Li, H.; et al. Sphingomonas wittichii Strain RW1 Genome-Wide Gene Expression Shifts in Response to Dioxins and Clay. PLoS ONE 2016, 11, e0157008. [Google Scholar] [CrossRef] [Green Version]
- Saibu, S.; Adebusoye, S.A.; Oyetibo, G.O.; Rodrigues, D.F. Aerobic Degradation of Dichlorinated Dibenzo-p-Dioxin and Dichlorinated Dibenzofuran by Bacteria Strains Obtained from Tropical Contaminated Soil. Biodegradation 2020, 31, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.; Hu, H.; Wang, W.; Zhou, Z.; Shah, S.B.; Xu, P.; Tang, H. Characterization of a Dibenzofuran-Degrading Strain of Pseudomonas aeruginosa, FA-HZ. Environ. Pollut. 2019, 250, 262–273. [Google Scholar] [CrossRef]
- Nojiri, H.; Omori, T. Molecular Bases of Aerobic Bacterial Degradation of Dioxins: Involvement of Angular Dioxygenation. Biosci. Biotechnol. Biochem. 2002, 66, 2001–2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünz, P.V.; Cook, A.M. Dibenzofuran 4,4a-Dioxygenase from Sphingomonas sp. Strain RW1: Angular Dioxygenation by a Three-Component Enzyme System. J. Bacteriol. 1993, 175, 6467–6475. [Google Scholar] [CrossRef] [Green Version]
- Sander, P.; Wittich, R.M.; Fortnagel, P.; Wilkes, H.; Francke, W. Degradation of 1,2,4-Trichloro- and 1,2,4,5-Tetrachlorobenzene by Pseudomonas strains. Appl. Environ. Microbiol. 1991, 57, 1430–1440. [Google Scholar] [CrossRef] [Green Version]
- Potrawfke, T.; Timmis, K.; Wittich, R. Degradation of 1,2,3,4-Tetrachlorobenzene by Pseudomonas chlororaphis RW. Appl. Environ. Microbiol. 1998, 64, 3798–3806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, K.; Takagi, K.; Iwasaki, A.; Tanaka, N.; Kanesaki, Y.; Martin-Laurent, F.; Igimi, S. Identification of the Hcb Gene Operon Involved in Catalyzing Aerobic Hexachlorobenzene Dechlorination in Nocardioides sp. Strain PD653. Appl. Environ. Microbiol. 2017, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, D.-Z.; Liu, H.; Zhou, N.-Y. Conversion of Sphingobium chlorophenolicum ATCC 39723 to a Hexachlorobenzene Degrader by Metabolic Engineering. Appl. Environ. Microbiol. 2006, 72, 2283–2286. [Google Scholar] [CrossRef] [Green Version]
- Tiedje, J.M.; Stevens, T.O. The Ecology of an Anaerobic Dechlorinating Consortium; Springer US: Boston, MA, USA, 1988. [Google Scholar] [CrossRef]
- Bunge, M.; Lechner, U. Anaerobic Reductive Dehalogenation of Polychlorinated Dioxins. Appl. Microbiol. Biotechnol. 2009, 84, 429–444. [Google Scholar] [CrossRef]
- Nijenhuis, I.; Kuntze, K. Anaerobic Microbial Dehalogenation of Organohalides—State of the Art and Remediation Strategies. Curr. Opin. Biotechnol. 2016, 38, 33–38. [Google Scholar] [CrossRef]
- Kuiper, I.; Lagendijk, E.L.; Bloemberg, G.V.; Lugtenberg, B.J.J. Rhizoremediation: A Beneficial Plant-Microbe Interaction. Mol. Plant. Microbe Interact. 2004, 17, 6–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyens, N.; Thijs, S.; Popek, R.; Witters, N.; Przybysz, A.; Espenshade, J.; Gawronska, H.; Vangronsveld, J.; Gawronski, S. The Role of Plant–Microbe Interactions and Their Exploitation for Phytoremediation of Air Pollutants. Int. J. Mol. Sci. 2015, 16, 25576–25604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Radwan, S.; Sorkhoh, N.; EI-Nemr, I. Oil Biodegradation around Roots. Nature 1995, 376, 302. [Google Scholar] [CrossRef] [PubMed]
- Scharf, B.E.; Hynes, M.F.; Alexandre, G.M. Chemotaxis Signaling Systems in Model Beneficial Plant–Bacteria Associations. Plant. Mol. Biol. 2016, 90, 549–559. [Google Scholar] [CrossRef] [PubMed]
- López-Farfán, D.; Reyes-Darias, J.A.; Matilla, M.A.; Krell, T. Concentration Dependent Effect of Plant Root Exudates on the Chemosensory Systems of Pseudomonas putida KT. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef]
- Pascale, A.; Proietti, S.; Pantelides, I.S.; Stringlis, I.A. Modulation of the Root Microbiome by Plant Molecules: The Basis for Targeted Disease Suppression and Plant Growth Promotion. Front. Plant. Sci. 2020, 10, 1741. [Google Scholar] [CrossRef]
- Matilla, M.A.; Espinosa-Urgel, M.; Rodríguez-Herva, J.J.; Ramos, J.L.; Ramos-González, M. Genomic Analysis Reveals the Major Driving Forces of Bacterial Life in the Rhizosphere. Genome Biol. 2007, 8, R179. [Google Scholar] [CrossRef] [Green Version]
- Burken, J.G. Uptake and Metabolism of Organic Compounds: Green-Liver Model; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2004. [Google Scholar] [CrossRef]
- Rojo, F. Carbon Catabolite Repression in Pseudomonas: Optimizing Metabolic Versatility and Interactions with the Environment. FEMS Microbiol. Rev. 2010, 34, 658–684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rentz, J.A.; Alvarez, P.J.J.; Schnoor, J.L. Repression of Pseudomonas putida Phenanthrene-Degrading Activity by Plant Root Extracts and Exudates. Environ. Microbiol. 2004, 6, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Iyer, B.; Rajput, M.S.; Jog, R.; Joshi, E.; Bharwad, K.; Rajkumar, S. Organic Acid Mediated Repression of Sugar Utilization in Rhizobia. Microbiol. Res. 2016, 192, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, M.; Zhang, M.; Toyota, K. Biodegradation of Volatile Organic Compounds and Their Effects on Biodegradability under Co-Existing Conditions. Microbes Environ. 2017, 32, 188–200. [Google Scholar] [CrossRef] [Green Version]
- Bharwad, K.; Rajkumar, S. Modulation of PQQ-Dependent Glucose Dehydrogenase (MGDH and SGDH) Activity by Succinate in Phosphate Solubilizing Plant Growth Promoting Acinetobacter sp. SK2. 3 Biotech. 2020, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Conde, S.; Molina, L.; González, P.; García-Puente, A.; Segura, A. Degradation of Phenanthrene by Novosphingobium sp. HS2a Improved Plant Growth in PAHs-Contaminated Environments. Appl. Microbiol. Biotechnol. 2016, 100, 10627–10636. [Google Scholar] [CrossRef] [PubMed]
- Bello-Akinosho, M.; Makofane, R.; Adeleke, R.; Thantsha, M.; Pillay, M.; Chirima, G.J. Potential of Polycyclic Aromatic Hydrocarbon-Degrading Bacterial Isolates to Contribute to Soil Fertility. BioMed Res. Int. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bulgarelli, D.; Rott, M.; Schlaeppi, K.; Ver Loren van Themaat, E.; Ahmadinejad, N.; Assenza, F.; Rauf, P.; Huettel, B.; Reinhardt, R.; Schmelzer, E.; et al. Revealing Structure and Assembly Cues for Arabidopsis Root-Inhabiting Bacterial Microbiota. Nature 2012, 488, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Sasse, J.; Martinoia, E.; Northen, T. Feed Your Friends: Do Plant Exudates Shape the Root Microbiome? Trends Plant. Sci. 2018, 23, 25–41. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Cañizares, C.; Jorrín, B.; Poole, P.S.; Tkacz, A. Understanding the Holobiont: The Interdependence of Plants and Their Microbiome. Curr. Opin. Microbiol. 2017, 38, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Xiang, Y.; Zhang, Z.; Ling, W.; Gao, Y. Inoculation of a Phenanthrene-Degrading Endophytic Bacterium Reduces the Phenanthrene Level and Alters the Bacterial Community Structure in Wheat. Appl. Microbiol. Biotechnol. 2017, 101, 5199–5212. [Google Scholar] [CrossRef]
- Siciliano, S.D.; Fortin, N.; Mihoc, A.; Wisse, G.; Labelle, S.; Beaumier, D.; Ouellette, D.; Roy, R.; Whyte, L.G.; Banks, M.K.; et al. Selection of Specific Endophytic Bacterial Genotypes by Plants in Response to Soil Contamination. Appl. Environ. Microbiol. 2001, 67, 2469–2475. [Google Scholar] [CrossRef] [Green Version]
- Jiao, S.; Chen, W.; Wei, G. Resilience and Assemblage of Soil Microbiome in Response to Chemical Contamination Combined with Plant Growth. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [Green Version]
- Thijs, S.; Sillen, W.; Rineau, F.; Weyens, N.; Vangronsveld, J. Towards an Enhanced Understanding of Plant–Microbiome Interactions to Improve Phytoremediation: Engineering the Metaorganism. Front. Microbiol. 2016, 7. [Google Scholar] [CrossRef]
- Kaur, N.; Erickson, T.E.; Ball, A.S.; Ryan, M.H. A Review of Germination and Early Growth as a Proxy for Plant Fitness under Petrogenic Contamination—Knowledge Gaps and Recommendations. Sci. Total Environ. 2017, 603–604, 728–744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anyasi, R.O.; Atagana, H.I. Profiling of Plants at Petroleum Contaminated Site for Phytoremediation. Int. J. Phytoremediation 2018, 20, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Böltner, D.; Godoy, P.; Muñoz-Rojas, J.; Duque, E.; Moreno-Morillas, S.; Sánchez, L.; Ramos, J.L. Rhizoremediation of Lindane by Root-Colonizing Sphingomonas. Microb. Biotechnol. 2007, 1, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Zafra, G.; Absalón, Á.E.; Anducho-Reyes, M.Á.; Fernandez, F.J.; Cortés-Espinosa, D.V. Construction of PAH-Degrading Mixed Microbial Consortia by Induced Selection in Soil. Chemosphere 2017, 172, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Uhlik, O.; Wald, J.; Strejcek, M.; Musilova, L.; Ridl, J.; Hroudova, M.; Vlcek, C.; Cardenas, E.; Mackova, M.; Macek, T. Identification of Bacteria Utilizing Biphenyl, Benzoate, and Naphthalene in Long-Term Contaminated Soil. PLoS ONE 2012, 7, e40653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kappell, A.D.; Wei, Y.; Newton, R.J.; Van Nostrand, J.D.; Zhou, J.; McLellan, S.L.; Hristova, K.R. The Polycyclic Aromatic Hydrocarbon Degradation Potential of Gulf of Mexico Native Coastal Microbial Communities after the Deepwater Horizon Oil Spill. Front. Microbiol. 2014, 5, 205. [Google Scholar] [CrossRef]
- Vergani, L.; Mapelli, F.; Suman, J.; Cajthaml, T.; Uhlik, O.; Borin, S. Novel PCB-Degrading Rhodococcus Strains Able to Promote Plant Growth for Assisted Rhizoremediation of Historically Polluted Soils. PLoS ONE 2019, 14, e0221253. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Megharaj, M.; Wu, C.-Y.; Subashchandrabose, S.R.; Dai, C.-C. Endophyte-Assisted Phytoremediation: Mechanisms and Current Application Strategies for Soil Mixed Pollutants. Crit. Rev. Biotechnol. 2020, 40, 31–45. [Google Scholar] [CrossRef]
- Pino, N.J.; Múnera, L.M.; Peñuela, G.A. Phytoremediation of Soil Contaminated with PCBs Using Different Plants and Their Associated Microbial Communities. Int. J. Phytoremediation 2019, 21, 316–324. [Google Scholar] [CrossRef]
- Vergani, L.; Mapelli, F.; Zanardini, E.; Terzaghi, E.; Di Guardo, A.; Morosini, C.; Raspa, G.; Borin, S. Phyto-Rhizoremediation of Polychlorinated Biphenyl Contaminated Soils: An Outlook on Plant-Microbe Beneficial Interactions. Sci. Total Environ. 2017, 575, 1395–1406. [Google Scholar] [CrossRef]
- Correa-García, S.; Pande, P.; Séguin, A.; St-Arnaud, M.; Yergeau, E. Rhizoremediation of Petroleum Hydrocarbons: A Model System for Plant Microbiome Manipulation. Microb. Biotechnol. 2018, 11, 819–832. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Choppala, G.; Kirkham, M.B.; Bolan, N.S. Rhizoremediation as a Green Technology for the Remediation of Petroleum Hydrocarbon-Contaminated Soils. J. Hazard. Mater. 2021, 401, 123282. [Google Scholar] [CrossRef]
- Tu, C.; Ma, L.; Guo, P.; Song, F.; Teng, Y.; Zhang, H.; Luo, Y. Rhizoremediation of a Dioxin-like PCB Polluted Soil by Alfalfa: Dynamic Characterization at Temporal and Spatial Scale. Chemosphere 2017, 189, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, E.; Vergani, L.; Mapelli, F.; Borin, S.; Raspa, G.; Zanardini, E.; Morosini, C.; Anelli, S.; Nastasio, P.; Sale, V.M.; et al. Rhizoremediation of Weathered PCBs in a Heavily Contaminated Agricultural Soil: Results of a Biostimulation Trial in Semi Field Conditions. Sci. Total Environ. 2019, 686, 484–496. [Google Scholar] [CrossRef] [PubMed]
- Ancona, V.; Barra Caracciolo, A.; Grenni, P.; Di Lenola, M.; Campanale, C.; Calabrese, A.; Uricchio, V.F.; Mascolo, G.; Massacci, A. Plant-Assisted Bioremediation of a Historically PCB and Heavy Metal-Contaminated Area in Southern Italy. New Biotechnol. 2017, 38, 65–73. [Google Scholar] [CrossRef]
- Sivaram, A.K.; Subashchandrabose, S.R.; Logeshwaran, P.; Lockington, R.; Naidu, R.; Megharaj, M. Rhizodegradation of PAHs Differentially Altered by C3 and C4 Plants. Sci. Rep. 2020, 10, 16109. [Google Scholar] [CrossRef]
- Balseiro-Romero, M.; Gkorezis, P.; Kidd, P.S.; Vangronsveld, J.; Monterroso, C. Enhanced Degradation of Diesel in the Rhizosphere of after Inoculation with Diesel-Degrading and Plant Growth-Promoting Bacterial Strains. J. Environ. Qual. 2016, 45, 924–932. [Google Scholar] [CrossRef] [PubMed]
- Nichols, T.D.; Wolf, D.C.; Rogers, H.B.; Beyrouty, C.A.; Reynolds, C.M. Rhizosphere Microbial Populations in Contaminated Soils. Water. Air. Soil Pollut. 1997, 95, 165–178. [Google Scholar] [CrossRef]
- Jacobsen, C.S. Plant Protection and Rhizosphere Colonization of Barley by Seed Inoculated Herbicide Degrading Burkholderia (Pseudomonas) cepacia DBO1(PRO101) in 2,4-D Contaminated Soil. Plant. Soil 1997, 189, 139–144. [Google Scholar] [CrossRef]
- Liste, H.-H.; Alexander, M. Plant-Promoted Pyrene Degradation in Soil. Chemosphere 2000, 40, 7–10. [Google Scholar] [CrossRef]
- Kelley, S.L.; Aitchison, E.W.; Deshpande, M.; Schnoor, J.L.; Alvarez, P.J.J. Biodegradation of 1,4-Dioxane in Planted and Unplanted Soil: Effect of Bioaugmentation with Amycolata Sp. CB1190. Water Res. 2001, 35, 3791–3800. [Google Scholar] [CrossRef]
- Kuiper, I.; Bloemberg, G.V.; Lugtenberg, B.J.J. Selection of a Plant-Bacterium Pair as a Novel Tool for Rhizostimulation of Polycyclic Aromatic Hydrocarbon-Degrading Bacteria. Mol. Plant. Microbe Interact. 2001, 14, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Pai, S.G.; Riley, M.B.; Camper, N.D. Microbial Degradation of Mefenoxam in Rhizosphere of Zinnia angustifolia. Chemosphere 2001, 44, 577–582. [Google Scholar] [CrossRef]
- Anokhina, T.O.; Kochetkov, V.V.; Zelenkova, N.F.; Balakshina, V.V.; Boronin, A.M. Biodegradation of Phenanthrene by Pseudomonas Bacteria Bearing Rhizospheric Plasmids in Model Plant–Microbial Associations. Appl. Biochem. Microbiol. 2004, 40, 568–572. [Google Scholar] [CrossRef]
- Johnson, D. Enhanced Dissipation of Chrysene in Planted Soil: The Impact of a Rhizobial Inoculum. Soil Biol. Biochem. 2004, 36, 33–38. [Google Scholar] [CrossRef]
- Sheng, X.; Gong, J. Increased Degradation of Phenanthrene in Soil by Pseudomonas sp. GF3 in the Presence of Wheat. Soil Biol. Biochem. 2006, 38, 2587–2592. [Google Scholar] [CrossRef]
- Child, R.; Miller, C.D.; Liang, Y.; Narasimham, G.; Chatterton, J.; Harrison, P.; Sims, R.C.; Britt, D.; Anderson, A.J. Polycyclic Aromatic Hydrocarbon-Degrading Mycobacterium Isolates: Their Association with Plant Roots. Appl. Microbiol. Biotechnol. 2007, 75, 655–663. [Google Scholar] [CrossRef]
- Muratova, A.; Pozdnyakova, N.; Golubev, S.; Wittenmayer, L.; Makarov, O.; Merbach, W.; Turkovskaya, O. Oxidoreductase Activity of Sorghum Root Exudates in a Phenanthrene-Contaminated Environment. Chemosphere 2009, 74, 1031–1036. [Google Scholar] [CrossRef]
- Andria, V.; Reichenauer, T.; Sessitsch, A. Expression of Alkane Monooxygenase (AlkB) Genes by Plant-Associated Bacteria in the Rhizosphere and Endosphere of Italian Ryegrass (Lolium multiflorum L.) Grown in Diesel Contaminated Soil. Environ. Pollut. Barking Essex 1987 2009, 157, 3347–3350. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.Y.; Bondarenkova, A.D.; Panchenko, L.V.; Turkovskaya, O.V. Use of Integrated Phytoremediation for Cleaning-up of Oil-Sludge-Contaminated Soil. Appl. Biochem. Microbiol. 2010, 46, 789–794. [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Sessitsch, A. The Inoculation Method Affects Colonization and Performance of Bacterial Inoculant Strains in the Phytoremediation of Soil Contaminated with Diesel Oil. Int. J. Phytoremediation 2012, 14, 35–47. [Google Scholar] [CrossRef]
- Chouychai, W.; Thongkukiatkul, A.; Upatham, S.; Pokethitiyook, P.; Kruatrachue, M.; Lee, H. Effect of Corn Plant on Survival and Phenanthrene Degradation Capacity of Pseudomonas sp. UG14Lr in Two Soils. Int. J. Phytoremediation 2012, 14, 585–595. [Google Scholar] [CrossRef]
- Yu, X.Z.; Wu, S.C.; Wu, F.Y.; Wong, M.H. Enhanced Dissipation of PAHs from Soil Using Mycorrhizal Ryegrass and PAH-Degrading Bacteria. J. Hazard. Mater. 2011, 186, 1206–1217. [Google Scholar] [CrossRef]
- Teng, Y.; Shen, Y.; Luo, Y.; Sun, X.; Sun, M.; Fu, D.; Li, Z.; Christie, P. Influence of Rhizobium meliloti on Phytoremediation of Polycyclic Aromatic Hydrocarbons by Alfalfa in an Aged Contaminated Soil. J. Hazard. Mater. 2011, 186, 1271–1276. [Google Scholar] [CrossRef]
- Hong, S.H.; Ryu, H.; Kim, J.; Cho, K.-S. Rhizoremediation of Diesel-Contaminated Soil Using the Plant Growth-Promoting Rhizobacterium Gordonia sp. S2RP-17. Biodegradation 2011, 22, 593–601. [Google Scholar] [CrossRef]
- Afzal, M.; Yousaf, S.; Reichenauer, T.G.; Kuffner, M.; Sessitsch, A. Soil Type Affects Plant Colonization, Activity and Catabolic Gene Expression of Inoculated Bacterial Strains during Phytoremediation of Diesel. J. Hazard. Mater. 2011, 186, 1568–1575. [Google Scholar] [CrossRef] [PubMed]
- Gałązka, A.; Król, M.; Perzyński, A. The Efficiency of Rhizosphere Bioremediation with Azospirillum sp. and Pseudomonas stutzeri in Soils Freshly Contaminated with PAHs and Diesel Fuel. Pol. J. Environ. Stud. 2012, 21, 345–353. [Google Scholar]
- Bisht, S.; Pandey, P.; Kaur, G.; Aggarwal, H.; Sood, A.; Sharma, S.; Kumar, V.; Bisht, N.S. Utilization of Endophytic Strain Bacillus sp. SBER3 for Biodegradation of Polyaromatic Hydrocarbons (PAH) in Soil Model System. Eur. J. Soil Biol. 2014, 60, 67–76. [Google Scholar] [CrossRef]
- Khan, Z.; Roman, D.; Kintz, T.; Alas, M.; Yap, R.; Doty, S. Degradation, Phytoprotection and Phytoremediation of Phenanthrene by Endophyte Pseudomonas putida, PD. Environ. Sci. Technol. 2014, 48. [Google Scholar] [CrossRef]
- Baneshi, M.M.; Rezaei Kalantary, R.; Jonidi Jafari, A.; Nasseri, S.; Jaafarzadeh, N.; Esrafili, A. Effect of Bioaugmentation to Enhance Phytoremediation for Removal of Phenanthrene and Pyrene from Soil with Sorghum and Onobrychis sativa. J. Environ. Health Sci. Eng. 2014, 12, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pizarro-Tobías, P.; Fernández, M.; Niqui, J.L.; Solano, J.; Duque, E.; Ramos, J.-L.; Roca, A. Restoration of a Mediterranean Forest after a Fire: Bioremediation and Rhizoremediation Field-Scale Trial. Microb. Biotechnol. 2015, 8, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Gong, Z.; Miao, R.; Rookes, J.; Cahill, D.; Zhuang, J. Microbial Mechanisms Controlling the Rhizosphere Effect of Ryegrass on Degradation of Polycyclic Aromatic Hydrocarbons in an Aged-Contaminated Agricultural Soil. Soil Biol. Biochem. 2017, 113, 130–142. [Google Scholar] [CrossRef]
- Dealtry, S.; Ghizelini, A.M.; Mendonça-Hagler, L.C.S.; Chaloub, R.M.; Reinert, F.; devCampos, T.M.P.; Gomes, N.C.M.; Smalla, K. Petroleum Contamination and Bioaugmentation in Bacterial Rhizosphere Communities from Avicennia schaueriana. Braz. J. Microbiol. 2018, 49, 757–769. [Google Scholar] [CrossRef]
- Iqbal, A.; Arshad, M.; Karthikeyan, R.; Gentry, T.J.; Rashid, J.; Ahmed, I.; Schwab, A.P. Diesel Degrading Bacterial Endophytes with Plant Growth Promoting Potential Isolated from a Petroleum Storage Facility. 3 Biotech. 2019, 9, 35. [Google Scholar] [CrossRef] [PubMed]
- Jampasri, K.; Pokethitiyook, P.; Poolpak, T.; Kruatrachue, M.; Ounjai, P.; Kumsopa, A. Bacteria-Assisted Phytoremediation of Fuel Oil and Lead Co-Contaminated Soil in the Salt-Stressed Condition by Chromolaena odorata and Micrococcus luteus. Int. J. Phytoremediation 2020, 22, 322–333. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.C.; Cryder, Z.; Khoury, R.; Carlan, C.; Gan, J. Bioremediation of PAH-Contaminated Shooting Range Soil Using Integrated Approaches. Sci. Total Environ. 2020, 726, 138440. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, M.; Jha, P.; Paul, A.T.; Singh, R.P.; Jha, P.N. Evaluation of Biphenyl- and Polychlorinated-Biphenyl (PCB) Degrading Rhodococcus sp. MAPN-1 on Growth of Morus alba by Pot Study. Int. J. Phytoremediation 2020, 22, 1487–1496. [Google Scholar] [CrossRef]
- Simmer, R.; Mathieu, J.; da Silva, M.L.B.; Lashmit, P.; Gopishetty, S.; Alvarez, P.J.J.; Schnoor, J.L. Bioaugmenting the Poplar Rhizosphere to Enhance Treatment of 1,4-Dioxane. Sci. Total Environ. 2020, 744, 140823. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Miao, R.; Guo, M.; Zhou, Y. Effects of Fire Phoenix (a Genotype Mixture of Fesctuca arundinecea L.) and Mycobacterium sp. on the Degradation of PAHs and Bacterial Community in Soil. Environ. Sci. Pollut. Res. Int. 2021. [Google Scholar] [CrossRef] [PubMed]
- Simonich, S.L.; Hites, R.A. Vegetation-Atmosphere Partitioning of Polycyclic Aromatic Hydrocarbons. Environ. Sci. Technol. 1994, 28, 939–943. [Google Scholar] [CrossRef]
- Vorholt, J.A. Microbial Life in the Phyllosphere. Nat. Rev. Microbiol. 2012, 10, 828–840. [Google Scholar] [CrossRef]
- Lindow, S.E.; Brandl, M.T. Microbiology of the Phyllosphere. Appl. Environ. Microbiol. 2003, 69, 1875–1883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koskella, B. The Phyllosphere. Curr. Biol. CB 2020, 30, R1143–R1146. [Google Scholar] [CrossRef]
- Carvalho, S.D.; Castillo, J.A. Influence of Light on Plant-Phyllosphere Interaction. Front. Plant. Sci. 2018, 9, 1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leveau, J.H. A Brief from the Leaf: Latest Research to Inform Our Understanding of the Phyllosphere Microbiome. Curr. Opin. Microbiol. 2019, 49, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Redford, A.J.; Bowers, R.M.; Knight, R.; Linhart, Y.; Fierer, N. The Ecology of the Phyllosphere: Geographic and Phylogenetic Variability in the Distribution of Bacteria on Tree Leaves. Environ. Microbiol. 2010, 12, 2885–2893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kembel, S.W.; O’Connor, T.K.; Arnold, H.K.; Hubbell, S.P.; Wright, S.J.; Green, J.L. Relationships between Phyllosphere Bacterial Communities and Plant Functional Traits in a Neotropical Forest. Proc. Natl. Acad. Sci. USA 2014, 111, 13715–13720. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grady, K.L.; Sorensen, J.W.; Stopnisek, N.; Guittar, J.; Shade, A. Assembly and Seasonality of Core Phyllosphere Microbiota on Perennial Biofuel Crops. Nat. Commun. 2019, 10, 4135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laforest-Lapointe, I.; Messier, C.; Kembel, S.W. Tree Leaf Bacterial Community Structure and Diversity Differ along a Gradient of Urban Intensity. mSystems 2017, 2, e00087-17. [Google Scholar] [CrossRef] [Green Version]
- Shakir, S.; Zaidi, S.S.-A.; de Vries, F.T.; Mansoor, S. Plant Genetic Networks Shaping Phyllosphere Microbial Community. Trends Genet. 2020, S0168952520302468. [Google Scholar] [CrossRef]
- Stone, B.W.G.; Jackson, C.R. Biogeographic Patterns Between Bacterial Phyllosphere Communities of the Southern Magnolia (Magnolia grandiflora) in a Small Forest. Microb. Ecol. 2016, 71, 954–961. [Google Scholar] [CrossRef]
- Bakan, B.; Marion, D. Assembly of the Cutin Polyester: From Cells to Extracellular Cell Walls. Plants 2017, 6, 57. [Google Scholar] [CrossRef] [Green Version]
- Remus-Emsermann, M.N.P.; Schlechter, R.O. Phyllosphere Microbiology: At the Interface between Microbial Individuals and the Plant Host. New Phytol. 2018, 218, 1327–1333. [Google Scholar] [CrossRef]
- Knief, C.; Ramette, A.; Frances, L.; Alonso-Blanco, C.; Vorholt, J.A. Site and Plant Species Are Important Determinants of the Methylobacterium Community Composition in the Plant Phyllosphere. ISME J. 2010, 4, 719–728. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, S.; Hiradate, S.; Koitabashi, M.; Kamo, T.; Tsushima, S. Phyllosphere Methylobacterium Bacteria Contain UVA-Absorbing Compounds. J. Photochem. Photobiol. B 2017, 167, 168–175. [Google Scholar] [CrossRef] [PubMed]
- Vacher, C.; Hampe, A.; Porté, A.J.; Sauer, U.; Compant, S.; Morris, C.E. The Phyllosphere: Microbial Jungle at the Plant–Climate Interface. Annu. Rev. Ecol. Evol. Syst. 2016, 47, 1–24. [Google Scholar] [CrossRef]
- Wei, X.; Lyu, S.; Yu, Y.; Wang, Z.; Liu, H.; Pan, D.; Chen, J. Phylloremediation of Air Pollutants: Exploiting the Potential of Plant Leaves and Leaf-Associated Microbes. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Łukowski, A.; Popek, R.; Karolewski, P. Particulate Matter on Foliage of Betula pendula, Quercus robur, and Tilia cordata: Deposition and Ecophysiology. Environ. Sci. Pollut. Res. Int. 2020, 27, 10296–10307. [Google Scholar] [CrossRef] [Green Version]
- Popek, R.; Łukowski, A.; Bates, C.; Oleksyn, J. Accumulation of Particulate Matter, Heavy Metals, and Polycyclic Aromatic Hydrocarbons on the Leaves of Tilia cordata Mill. in Five Polish Cities with Different Levels of Air Pollution. Int. J. Phytoremediation 2017, 19, 1134–1141. [Google Scholar] [CrossRef]
- Espenshade, J.; Thijs, S.; Gawronski, S.; Bové, H.; Weyens, N.; Vangronsveld, J. Influence of Urbanization on Epiphytic Bacterial Communities of the Platanus × Hispanica Tree Leaves in a Biennial Study. Front. Microbiol. 2019, 10, 675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperato, V.; Kowalkowski, L.; Portillo-Estrada, M.; Gawronski, S.W.; Vangronsveld, J.; Thijs, S. Characterisation of the Carpinus betulus L. Phyllomicrobiome in Urban and Forest Areas. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franzetti, A.; Gandolfi, I.; Bestetti, G.; Padoa Schioppa, E.; Canedoli, C.; Brambilla, D.; Cappelletti, D.; Sebastiani, B.; Federici, E.; Papacchini, M.; et al. Plant-Microorganisms Interaction Promotes Removal of Air Pollutants in Milan (Italy) Urban Area. J. Hazard. Mater. 2020, 384, 121021. [Google Scholar] [CrossRef]
- Gandolfi, I.; Canedoli, C.; Imperato, V.; Tagliaferri, I.; Gkorezis, P.; Vangronsveld, J.; Padoa Schioppa, E.; Papacchini, M.; Bestetti, G.; Franzetti, A. Diversity and Hydrocarbon-Degrading Potential of Epiphytic Microbial Communities on Platanus x Acerifolia Leaves in an Urban Area. Environ. Pollut. 2017, 220, 650–658. [Google Scholar] [CrossRef]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Bacterial Degradation of Airborne Phenol in the Phyllosphere. Environ. Microbiol. 2007, 9, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Sandhu, A.; Halverson, L.J.; Beattie, G.A. Identification and Genetic Characterization of Phenol-Degrading Bacteria from Leaf Microbial Communities. Microb. Ecol. 2009, 57, 276–285. [Google Scholar] [CrossRef] [PubMed]
- Ali, N.; Sorkhoh, N.; Salamah, S.; Eliyas, M.; Radwan, S. The Potential of Epiphytic Hydrocarbon-Utilizing Bacteria on Legume Leaves for Attenuation of Atmospheric Hydrocarbon Pollutants. J. Environ. Manag. 2012, 93, 113–120. [Google Scholar] [CrossRef]
- Sorkhoh, N.A.; Al-Mailem, D.M.; Ali, N.; Al-Awadhi, H.; Salamah, S.; Eliyas, M.; Radwan, S.S. Bioremediation of Volatile Oil Hydrocarbons by Epiphytic Bacteria Associated with American Grass (Cynodon sp.) and Broad Bean (Vicia faba) Leaves. Int. Biodeterior. Biodegrad. 2011, 65, 797–802. [Google Scholar] [CrossRef]
- Waight, K.; Pinyakong, O.; Luepromchai, E. Degradation of Phenanthrene on Plant Leaves by Phyllosphere Bacteria. J. Gen. Appl. Microbiol. 2007, 53, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Sangthong, S.; Suksabye, P.; Thiravetyan, P. Air-Borne Xylene Degradation by Bougainvillea buttiana and the Role of Epiphytic Bacteria in the Degradation. Ecotoxicol. Environ. Saf. 2016, 126, 273–280. [Google Scholar] [CrossRef]
- Yutthammo, C.; Thongthammachat, N.; Pinphanichakarn, P.; Luepromchai, E. Diversity and Activity of PAH-Degrading Bacteria in the Phyllosphere of Ornamental Plants. Microb. Ecol. 2010, 59, 357–368. [Google Scholar] [CrossRef]
- Daudzai, Z.; Treesubsuntorn, C.; Thiravetyan, P. Inoculated Clitoria ternatea with Bacillus cereus ERBP for Enhancing Gaseous Ethylbenzene Phytoremediation: Plant Metabolites and Expression of Ethylbenzene Degradation Genes. Ecotoxicol. Environ. Saf. 2018, 164, 50–60. [Google Scholar] [CrossRef]
- De Kempeneer, L.; Sercu, B.; Vanbrabant, W.; Van Langenhove, H.; Verstraete, W. Bioaugmentation of the Phyllosphere for the Removal of Toluene from Indoor Air. Appl. Microbiol. Biotechnol. 2004, 64, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Terzaghi, E.; De Nicola, F.; Cerabolini, B.E.L.; Posada-Baquero, R.; Ortega-Calvo, J.-J.; Di Guardo, A. Role of Photo- and Biodegradation of Two PAHs on Leaves: Modelling the Impact on Air Quality Ecosystem Services Provided by Urban Trees. Sci. Total Environ. 2020, 739, 139893. [Google Scholar] [CrossRef]
- Pettit, T.; Irga, P.J.; Torpy, F.R. Towards Practical Indoor Air Phytoremediation: A Review. Chemosphere 2018, 208, 960–974. [Google Scholar] [CrossRef] [PubMed]
- Scheublin, T.R.; Deusch, S.; Moreno-Forero, S.K.; Müller, J.A.; van der Meer, J.R.; Leveau, J.H.J. Transcriptional Profiling of Gram-Positive A Rthrobacter in the Phyllosphere: Induction of Pollutant Degradation Genes by Natural Plant Phenolic Compounds. Environ. Microbiol. 2014, 16, 2212–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brilli, F.; Fares, S.; Ghirardo, A.; de Visser, P.; Calatayud, V.; Muñoz, A.; Annesi-Maesano, I.; Sebastiani, F.; Alivernini, A.; Varriale, V.; et al. Plants for Sustainable Improvement of Indoor Air Quality. Trends Plant. Sci. 2018, 23, 507–512. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, J.; Kraakman, N.J.R.; Pérez, C.; Lebrero, R.; Muñoz, R. A State–of–the-Art Review on Indoor Air Pollution and Strategies for Indoor Air Pollution Control. Chemosphere 2021, 262, 128376. [Google Scholar] [CrossRef]
- Salthammer, T. Emerging Indoor Pollutants. Int. J. Hyg. Environ. Health 2020, 224, 113423. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Min, X.; Weng, M. A Review of Polychlorinated Biphenyls (PCBs) Pollution in Indoor Air Environment. J. Air Waste Manag. Assoc. 2016, 66, 941–950. [Google Scholar] [CrossRef] [PubMed]
- Teiri, H.; Pourzamani, H.; Hajizadeh, Y. Phytoremediation of VOCs from Indoor Air by Ornamental Potted Plants: A Pilot Study Using a Palm Species under the Controlled Environment. Chemosphere 2018, 197, 375–381. [Google Scholar] [CrossRef]
- Hörmann, V.; Brenske, K.-R.; Ulrichs, C. Assessment of Filtration Efficiency and Physiological Responses of Selected Plant Species to Indoor Air Pollutants (Toluene and 2-Ethylhexanol) under Chamber Conditions. Environ. Sci. Pollut. Res. 2018, 25, 447–458. [Google Scholar] [CrossRef]
- Oishi, Y. Comparison of Moss and Pine Needles as Bioindicators of Transboundary Polycyclic Aromatic Hydrocarbon Pollution in Central Japan. Environ. Pollut. 2018, 234, 330–338. [Google Scholar] [CrossRef]
- Sari, M.F.; Esen, F.; Tasdemir, Y. Biomonitoring and Source Identification of Polycyclic Aromatic Hydrocarbons (PAHs) Using Pine Tree Components from Three Different Sites in Bursa, Turkey. Arch. Environ. Contam. Toxicol. 2020, 78, 646–657. [Google Scholar] [CrossRef] [PubMed]
- Nowak, D.J.; Crane, D.E.; Stevens, J.C. Air Pollution Removal by Urban Trees and Shrubs in the United States. Urban For Urban Green. 2006, 4, 115–123. [Google Scholar] [CrossRef]
- Lafortezza, R.; Davies, C.; Sanesi, G.; Konijnendijk, C. Green Infrastructure as a Tool to Support Spatial Planning in European Urban Regions. IForest-Biogeosciences and Forrestry. 2013, 6, 102–108. [Google Scholar] [CrossRef] [Green Version]
- Miller, J.R. Biodiversity Conservation and the Extinction of Experience. Trends Ecol. Evol. 2005, 20, 430–434. [Google Scholar] [CrossRef]
- Lafortezza, R.; Carrus, G.; Sanesi, G.; Davies, C. Benefits and Well-Being Perceived by People Visiting Green Spaces in Periods of Heat Stress. Urban For Urban Green. 2009, 8, 97–108. [Google Scholar] [CrossRef]
- Adinolfi, C.; Suárez-Cáceres, G.P.; Cariñanos, P. Relation between Visitors’ Behaviour and Characteristics of Green Spaces in the City of Granada, South-Eastern Spain. Urban For Urban Green. 2014, 13, 534–542. [Google Scholar] [CrossRef]
- Bolund, P.; Hunhammar, S. Ecosystem Services in Urban Areas. Ecol. Econ. 1999, 29, 293–301. [Google Scholar] [CrossRef]
- Morancho, A.B. A Hedonic Valuation of Urban Green Areas. Landsc. Urban. Plan. 2003, 66, 35–41. [Google Scholar] [CrossRef]
- Jim, C.Y.; Chen, W.Y. External Effects of Neighbourhood Parks and Landscape Elements on High-Rise Residential Value. Land Use Policy 2010, 27, 662–670. [Google Scholar] [CrossRef]
- Xu, T.; Sathaye, J.; Akbari, H.; Garg, V.; Tetali, S. Quantifying the Direct Benefits of Cool Roofs in an Urban Setting: Reduced Cooling Energy Use and Lowered Greenhouse Gas Emissions. Build. Environ. 2012, 48, 1–6. [Google Scholar] [CrossRef]
- Lehmann, I.; Mathey, J.; Rößler, S.; Bräuer, A.; Goldberg, V. Urban Vegetation Structure Types as a Methodological Approach for Identifying Ecosystem Services–Application to the Analysis of Micro-Climatic Effects. Ecol. Indic. 2014, 42, 58–72. [Google Scholar] [CrossRef]
- Eigenbrod, F.; Bell, V.A.; Davies, H.N.; Heinemeyer, A.; Armsworth, P.R.; Gaston, K.J. The Impact of Projected Increases in Urbanization on Ecosystem Services. Proc. R. Soc. B Biol. Sci. 2011, 278, 3201–3208. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Lin, J.; Cui, S.; Qiu, Q.; Zhao, Q. Exploring the Relationship between Urban Transportation Energy Consumption and Transition of Settlement Morphology: A Case Study on Xiamen Island, China. Habitat Int. 2013, 37, 70–79. [Google Scholar] [CrossRef]
- Bowler, D.E.; Buyung-Ali, L.; Knight, T.M.; Pullin, A.S. Urban Greening to Cool Towns and Cities: A Systematic Review of the Empirical Evidence. Landsc. Urban. Plan. 2010, 97, 147–155. [Google Scholar] [CrossRef]
- Byrne, J.; Sipe, N.; Searle, G. Green around the Gills? The Challenge of Density for Urban Greenspace Planning in SEQ. Aust. Plan. 2010, 47, 162–177. [Google Scholar] [CrossRef] [Green Version]
- Lin, B.; Meyers, J.; Barnett, G. Understanding the Potential Loss and Inequities of Green Space Distribution with Urban Densification. Urban. For. Urban. Green. 2015, 14, 952–958. [Google Scholar] [CrossRef]
- Pérez, G.; Coma, J.; Martorell, I.; Cabeza, L.F. Vertical Greenery Systems (VGS) for Energy Saving in Buildings: A Review. Renew. Sustain. Energy Rev. 2014, 39, 139–165. [Google Scholar] [CrossRef] [Green Version]
- Qiu, L.; Liu, F.; Zhang, X.; Gao, T. Difference of Airborne Particulate Matter Concentration in Urban Space with Different Green Coverage Rates in Baoji, China. Int. J. Environ. Res. Public. Health 2019, 16, 1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, T.; Liu, F.; Wang, Y.; Mu, S.; Qiu, L. Reduction of Atmospheric Suspended Particulate Matter Concentration and Influencing Factors of Green Space in Urban Forest Park. Forests 2020, 11, 950. [Google Scholar] [CrossRef]
- Yoo, S.-Y.; Kim, T.; Ham, S.; Choi, S.; Park, C.-R. Importance of Urban Green at Reduction of Particulate Matters in Sihwa Industrial Complex, Korea. Sustainability 2020, 12, 7647. [Google Scholar] [CrossRef]
- Irga, P.J.; Pettit, T.J.; Torpy, F.R. The Phytoremediation of Indoor Air Pollution: A Review on the Technology Development from the Potted Plant through to Functional Green Wall Biofilters. Rev. Environ. Sci. Biotechnol. 2018, 17, 395–415. [Google Scholar] [CrossRef]
- Guo, L.; Ma, S.; Zhao, D.; Zhao, B.; Xu, B.; Wu, J.; Tong, J.; Chen, D.; Ma, Y.; Li, M.; et al. Experimental Investigation of Vegetative Environment Buffers in Reducing Particulate Matters Emitted from Ventilated Poultry House. J. Air Waste Manag. Assoc. 2019, 69, 934–943. [Google Scholar] [CrossRef] [Green Version]
- Darlington, A.B.; Dat, J.F.; Dixon, M.A. The Biofiltration of Indoor Air: Air Flux and Temperature Influences the Removal of Toluene, Ethylbenzene, and Xylene. Environ. Sci. Technol. 2001, 35, 240–246. [Google Scholar] [CrossRef]
- Darlington, A.; Chan, M.; Malloch, D.; Pilger, C.; Dixon, M.A. The Biofiltration of Indoor Air: Implications for Air Quality. Indoor Air 2000, 10, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Paull, N.J.; Irga, P.J.; Torpy, F.R. Active Green Wall Plant Health Tolerance to Diesel Smoke Exposure. Environ. Pollut. 2018, 240, 448–456. [Google Scholar] [CrossRef]
- Lundholm, J.; MacIvor, J.S.; MacDougall, Z.; Ranalli, M. Plant Species and Functional Group Combinations Affect Green Roof Ecosystem Functions. PLoS ONE 2010, 5, e9677. [Google Scholar] [CrossRef] [Green Version]
- Irga, P.J.; Paull, N.J.; Abdo, P.; Torpy, F.R. An Assessment of the Atmospheric Particle Removal Efficiency of an In-Room Botanical Biofilter System. Build. Environ. 2017, 115, 281–290. [Google Scholar] [CrossRef]
- Torpy, F.R.; Irga, P.J.; Burchett, M.D. Reducing Indoor Air Pollutants Through Biotechnology. In Biotechnologies and Biomimetics for Civil Engineering; Pacheco Torgal, F., Labrincha, J.A., Diamanti, M.V., Yu, C.-P., Lee, H.K., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 181–210. [Google Scholar] [CrossRef]
- Soreanu, G.; Dixon, M.; Darlington, A. Botanical Biofiltration of Indoor Gaseous Pollutants–A Mini-Review. Chem. Eng. J. 2013, 229, 585–594. [Google Scholar] [CrossRef]
- Speak, A.F.; Rothwell, J.J.; Lindley, S.J.; Smith, C.L. Urban Particulate Pollution Reduction by Four Species of Green Roof Vegetation in a UK City. Atmos. Environ. 2012, 61, 283–293. [Google Scholar] [CrossRef]
- Paull, N.J.; Krix, D.; Torpy, F.R.; Irga, P.J. Can Green Walls Reduce Outdoor Ambient Particulate Matter, Noise Pollution and Temperature? Int. J. Environ. Res. Public. Health 2020, 17, 5084. [Google Scholar] [CrossRef]
- Russell, J.A.; Hu, Y.; Chau, L.; Pauliushchyk, M.; Anastopoulos, I.; Anandan, S.; Waring, M.S. Indoor-Biofilter Growth and Exposure to Airborne Chemicals Drive Similar Changes in Plant Root Bacterial Communities. Appl. Environ. Microbiol. 2014, 80, 4805–4813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reese, A.T.; Savage, A.; Youngsteadt, E.; McGuire, K.L.; Koling, A.; Watkins, O.; Frank, S.D.; Dunn, R.R. Urban Stress Is Associated with Variation in Microbial Species Composition-but Not Richness-in Manhattan. ISME J. 2016, 10, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gill, A.S.; Purnell, K.; Palmer, M.I.; Stein, J.; McGuire, K.L. Microbial Composition and Functional Diversity Differ Across Urban Green Infrastructure Types. Front. Microbiol. 2020, 11. [Google Scholar] [CrossRef]
- Lymperopoulou, D.S.; Adams, R.I.; Lindow, S.E. Contribution of Vegetation to the Microbial Composition of Nearby Outdoor Air. Appl. Environ. Microbiol. 2016, 82, 3822–3833. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Ma, J.; Wei, J.; Gong, X.; Yu, X.; Guo, H.; Zhao, Y. Biochar Increases Plant Growth and Alters Microbial Communities via Regulating the Moisture and Temperature of Green Roof Substrates. Sci. Total Environ. 2018, 635, 333–342. [Google Scholar] [CrossRef]
- Molineux, C.J.; Connop, S.P.; Gange, A.C. Manipulating Soil Microbial Communities in Extensive Green Roof Substrates. Sci. Total Environ. 2014, 493, 632–638. [Google Scholar] [CrossRef]
- Kaur, M.; Nagpal, A.K. Evaluation of Air Pollution Tolerance Index and Anticipated Performance Index of Plants and Their Application in Development of Green Space along the Urban Areas. Environ. Sci. Pollut. Res. 2017, 24, 18881–18895. [Google Scholar] [CrossRef] [PubMed]
- Bahadoran, M.; Mortazavi, S.N.; Hajizadeh, Y. Evaluation of Anticipated Performance Index, Biochemical, and Physiological Parameters of Cupressus arizonica Greene and Juniperus excelsa Bieb for Greenbelt Development and Biomonitoring of Air Pollution. Int. J. Phytoremediation 2019, 21, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, H.; Bolinius, D.J.; Jahnke, A.; MacLeod, M. Mass Transfer of Hydrophobic Organic Chemicals between Silicone Sheets and through Plant Leaves and Low-Density Polyethylene. Chemosphere 2016, 164, 683–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantke, P.; Arnot, J.A.; Doucette, W.J. Improving Plant Bioaccumulation Science through Consistent Reporting of Experimental Data. J. Environ. Manage. 2016, 181, 374–384. [Google Scholar] [CrossRef] [Green Version]
- Dupuy, J.; Leglize, P.; Vincent, Q.; Zelko, I.; Mustin, C.; Ouvrard, S.; Sterckeman, T. Effect and Localization of Phenanthrene in Maize Roots. Chemosphere 2016, 149, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Cennerazzo, J.; de Junet, A.; Audinot, J.-N.; Leyval, C. Dynamics of PAHs and Derived Organic Compounds in a Soil-Plant Mesocosm Spiked with 13C-Phenanthrene. Chemosphere 2017, 168, 1619–1627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trine, L.S.D.; Davis, E.L.; Roper, C.; Truong, L.; Tanguay, R.L.; Simonich, S.L.M. Formation of PAH Derivatives and Increased Developmental Toxicity during Steam Enhanced Extraction Remediation of Creosote Contaminated Superfund Soil. Environ. Sci. Technol. 2019, 53, 4460–4469. [Google Scholar] [CrossRef]
- Hassani, M.A.; Durán, P.; Hacquard, S. Microbial Interactions within the Plant Holobiont. Microbiome 2018, 6, 58. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ding, Q.; Chao, Y.; Wei, X.; Wang, S.; Qiu, R. Structural Development and Assembly Patterns of the Root-Associated Microbiomes during Phytoremediation. Sci. Total Environ. 2018, 644, 1591–1601. [Google Scholar] [CrossRef]
- Alkio, M.; Tabuchi, T.M.; Wang, X.; Colón-Carmona, A. Stress Responses to Polycyclic Aromatic Hydrocarbons in Arabidopsis Include Growth Inhibition and Hypersensitive Response-like Symptoms. J. Exp. Bot. 2005, 56, 2983–2994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weisman, D.; Alkio, M.; Colón-Carmona, A. Transcriptional Responses to Polycyclic Aromatic Hydrocarbon-Induced Stress in Arabidopsis thaliana Reveal the Involvement of Hormone and Defense Signaling Pathways. BMC Plant. Biol. 2010, 10, 59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Carvalhais, L.C.; Crawford, M.; Singh, E.; Dennis, P.G.; Pieterse, C.M.J.; Schenk, P.M. Inner Plant Values: Diversity, Colonization and Benefits from Endophytic Bacteria. Front. Microbiol. 2017, 8, 2552. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant. Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [Green Version]
- Ghatak, A.; Chaturvedi, P.; Weckwerth, W. Metabolomics in Plant Stress Physiology. In Plant Genetics and Molecular Biology; Varshney, R.K., Pandey, M.K., Chitikineni, A., Eds.; Advances in Biochemical Engineering/Biotechnology; Springer International Publishing: Cham, Switzerland, 2018; Volume 164, pp. 187–236. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K.; et al. Abiotic Stress Responses and Microbe-Mediated Mitigation in Plants: The Omics Strategies. Front. Plant. Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- De Ridder, K.; Adamec, V.; Bañuelos, A.; Bruse, M.; Bürger, M.; Damsgaard, O.; Dufek, J.; Hirsch, J.; Lefebre, F.; Pérez-Lacorzana, J.M.; et al. An Integrated Methodology to Assess the Benefits of Urban Green Space. Sci. Total Environ. 2004, 334–335, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.K.; Pandey, M.; Tripathi, B.D. Assessment of Air Pollution Tolerance Index of Some Plants to Develop Vertical Gardens near Street Canyons of a Polluted Tropical City. Ecotoxicol. Environ. Saf. 2016, 134, 358–364. [Google Scholar] [CrossRef]
- Morella, N.M.; Weng, F.C.-H.; Joubert, P.M.; Metcalf, C.J.E.; Lindow, S.; Koskella, B. Successive Passaging of a Plant-Associated Microbiome Reveals Robust Habitat and Host Genotype-Dependent Selection. Proc. Natl. Acad. Sci. USA 2020, 117, 1148–1159. [Google Scholar] [CrossRef]



| Plants | Pollutants | Microbes | References |
|---|---|---|---|
| Senecus glaucus | Oil | Arthrobacter | [95] |
| Alfalfa | PAHs | Not identified | [132] |
| Barley | 2,4-D | Burkholderia cepacia | [133] |
| Oat, lupin, rape, pepper, radish, pine | Pyrene | Not identified | [134] |
| Poplar | 2,4-D | Amycolata sp. CB1190 | [135] |
| Lolium multiflorum | PAHs | Pseudmonas putida strain PCL1444 | [136] |
| Zinnia anguistifolia | Mefenoxam | Pseudomonas fluorescens Chrysobacterium indologenes | [137] |
| Hordeum vulgare | Phenanthrene | Pseudomonas fluorescens, Pseudomonas aureofaciens | [138] |
| Trifolium repens | Chrysene | Rhizobium leguminosarum | [139] |
| Triticum aestivum | Phenanthrene | Pseudomonas sp. strain GF3 | [140] |
| Hordeum vulgare | PAHs | Mycobacterium sps. | [141] |
| Sorghum bicolor | Phenanthrene | Sinorhizobium meliloti strain P221 | [142] |
| Lolium multiflorum | Diesel oil | Rhodococcus sp. strain ITRH43 | [143] |
| Secale cereale, medicago sativa | Crude oil | Azospirillum brasilense strain SR80 | [144] |
| Lotus corniculatus | Diesel oil | Pantoea sp. strain BTRH79 | [145] |
| Zea mays | Phenanthrene, pyrene | Pseudomonas sp. strain UG14Lr, Pseudomonas putida strain MUB1 | [146] |
| Lolium multiflorum | PAHs | Acinetobacter sp. | [147] |
| Medicago sativa | PAHs | Rhizobium meliloti strain ACCC 17519 | [148] |
| Zea mays | Diesel oil | Gordonia sp. strain S2RP-17 | [149] |
| Lolium perenne | Diesel oil | Pantoea sp. strain BTRH79 | [150] |
| Festuca | PAHs and diesel | Azospirillum sp. and Pseudomonas stutzeri | [151] |
| Populus deltoides | PAHs | Kurthia sp. Micrococcus sp. Bacillus sp. Dienococcus sp. Endophytic Bacillus sp. | [152] |
| Salix purpurea | Phenanthrene | Pseudomonas putida PD1 | [153] |
| Sorghum and onobrychis sativa | Phenanthrene, pyrene | Bacterial consortium | [154] |
| Annual grasses | Monoaromatics, PAHs | Pseudomonas putida strains | [155] |
| Clover | Phenanthrene | Novosphingobium sp. HS2a | [106] |
| Lolium multiflorum | PAHs | Mycobacterium gilvum | [156] |
| Avicennia schaueriana | Oil | Bacterial consortium | [157] |
| Arabidopsis thaliana | PCBs | Rhodococcus | [121] |
| Echinochloa crus-galli, cynodon dactylon | Monoaromatics | Pseudomonas sp. J10 | [158] |
| Chromolaena odorata | Lead, petroleum | Micrococcus luteus. | [159] |
| Grases | PAHs | Mycobacterium vanbaalenii PYR-1 | [160] |
| Morus alba | Biphenyl | Rhodococcus sp. MAPN-1 | [161] |
| Poplar | 1,4-Dioxane | Mycobacterium dioxanotrophicus PH-06, Pseudonocardia dioxanivorans CB1190 | [162] |
| Fesctuca arundinecea l. | PAHs | Mycobacterium sp. | [163] |
| Plants | Pollutants | Microbes | References |
|---|---|---|---|
| Azalea indica | Toluene | Pseudomonas putida TVA8 | [196] |
| Bean | Phenol | Pseudomonas sp. strain CF600 | [188] |
| Ixora sp | Phenanthrene | Pseudomonas oleovorans Mycobacterium sps. Rhizobium sps. Deinococcus sp. | [192] |
| Ornamental plants | Phenanthrene | Indigenous population | [194] |
| Beans and peas | Hydrocarbon vapors | Not identified | [190] |
| Bougainvillea buttiana | Xylene | Enterobacter cloacae LSRC11, Staphylococus sp. A1, Pseudomonas aeruginosa | [193] |
| Urban trees | Phenanthrene, benzo[a]pyrene | Non identified | [197] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molina, L.; Wittich, R.-M.; van Dillewijn, P.; Segura, A. Plant-Bacteria Interactions for the Elimination of Atmospheric Contaminants in Cities. Agronomy 2021, 11, 493. https://doi.org/10.3390/agronomy11030493
Molina L, Wittich R-M, van Dillewijn P, Segura A. Plant-Bacteria Interactions for the Elimination of Atmospheric Contaminants in Cities. Agronomy. 2021; 11(3):493. https://doi.org/10.3390/agronomy11030493
Chicago/Turabian StyleMolina, Lázaro, Regina-Michaela Wittich, Pieter van Dillewijn, and Ana Segura. 2021. "Plant-Bacteria Interactions for the Elimination of Atmospheric Contaminants in Cities" Agronomy 11, no. 3: 493. https://doi.org/10.3390/agronomy11030493
APA StyleMolina, L., Wittich, R. -M., van Dillewijn, P., & Segura, A. (2021). Plant-Bacteria Interactions for the Elimination of Atmospheric Contaminants in Cities. Agronomy, 11(3), 493. https://doi.org/10.3390/agronomy11030493