Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum
Abstract
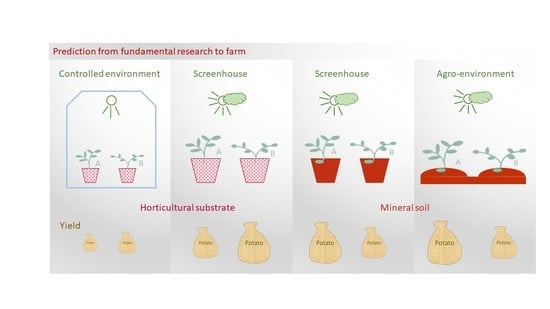
:1. Introduction
2. Materials and Methods
2.1. Experiments on Population 1
2.2. Experiments on Population 2
2.3. Experiments on Population 3
2.4. Statistical Evaluation
3. Results
3.1. Comparison of Potato Cultivars in Field and Pot Experiments
3.2. Effect of Pot Size on Drought Tolerance Assessment
3.3. Effect of Starting Material and Substrate
4. Discussion
4.1. Controlled Water Supply versus Controlled Soil Water Content
4.2. Controlled Condition versus Managed Field Environments
4.3. The Hidden Half: Planting Material, Pot Size, and Soil
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Harrison, M.T.; Tardieu, F.; Dong, Z.S.; Messina, C.D.; Hammer, G.L. Characterizing drought stress and trait influence on maize yield under current and future conditions. Glob. Chang. Biol. 2014, 20, 867–878. [Google Scholar] [CrossRef]
- Cooper, M.; Gho, C.; Leafgren, R.; Tang, T.; Messina, C. Breeding drought-tolerant maize hybrids for the US corn-belt: Discovery to product. J. Exp. Bot. 2014, 65, 6191–6204. [Google Scholar] [CrossRef] [Green Version]
- Monneveux, P.; Ramirez, D.A.; Pino, M.T. Drought tolerance in potato (S. tuberosum L.) Can we learn from drought tolerance research in cereals? Plant Sci. 2013, 205, 76–86. [Google Scholar] [CrossRef]
- Blum, A. Heterosis, stress, and the environment: A possible road map towards the general improvement of crop yield. J. Exp. Bot. 2013, 64, 4829–4837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blum, A.; Shpiler, L.; Golan, G.; Mayer, J. Yield stability and canopy temperature of wheat genotypes under drought-stress. Field Crops Res. 1989, 22, 289–296. [Google Scholar] [CrossRef]
- Slater, A.T.; Cogan, N.O.I.; Forster, J.W.; Hayes, B.J.; Daetwyler, H.D. Improving genetic gain with genomic selection in autotetraploid potato. Plant Genome 2016, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vos, J.; Groenwold, J. Root-growth of potato crops on a marine-clay soil. Plant Soil 1986, 94, 17–33. [Google Scholar] [CrossRef]
- Anithakumari, A.M.; Nataraja, K.N.; Visser, R.G.F.; van der Linden, C.G. Genetic dissection of drought tolerance and recovery potential by quantitative trait locus mapping of a diploid potato population. Mol. Breed. 2012, 30, 1413–1429. [Google Scholar] [CrossRef] [Green Version]
- Obidiegwu, J.; Bryan, G.; Jones, H.; Prashar, A. Coping with drought: Stress and adaptive responses in potato and perspectives for improvement. Front. Plant Sci. 2015, 6, 542. [Google Scholar] [CrossRef] [Green Version]
- Van Loon, C.D. The effect of water stress on potato growth, development, and yield. Am. Potato J. 1981, 58, 51–69. [Google Scholar] [CrossRef]
- Aliche, E.B.; Oortwijn, M.; Theeuwen, T.P.J.M.; Bachem, C.W.B.; Visser, R.G.F.; van der Linden, C.G. Drought response in field grown potatoes and the interactions between canopy growth and yield. Agric. Water Manag. 2018, 206, 20–30. [Google Scholar] [CrossRef]
- Jefferies, R.A.; Mackerron, D.K.L. Aspects of the physiological basis of cultivar differences in yield of potato under droughted and irrigated conditions. Potato Res. 1987, 30, 201–217. [Google Scholar] [CrossRef]
- Schafleitner, R.; Gutierrez, R.; Espino, R.; Gaudin, A.; Pérez, J.; Martínez, M.; Domínguez, A.; Tincopa, L.; Alvarado, C.; Numberto, G.; et al. Field screening for variation of drought tolerance in Solanum tuberosum L. by agronomical, physiological and genetic analysis. Potato Res. 2007, 50, 71–85. [Google Scholar] [CrossRef]
- Sprenger, H.; Rudack, K.; Schudoma, C.; Neumann, A.; Seddig, S.; Peters, R.; Zuther, E.; Kopka, J.; Hincha, D.K.; Walther, D.; et al. Assessment of drought tolerance and its potential yield penalty in potato. Func. Plant Biol. 2015, 42, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Wishart, J.; George, T.S.; Brown, L.K.; White, P.J.; Ramsay, G.; Jones, H.; Gregory, P.J. Field phenotyping of potato to assess root and shoot characteristics associated with drought tolerance. Plant Soil 2014, 378, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Gopal, J. Challenges and way-forward in selection of superior parents, crosses and clones in potato breeding. Potato Res. 2015, 58, 165–188. [Google Scholar] [CrossRef]
- Slater, A.T.; Cogan, N.O.I.; Hayes, B.J.; Schultz, L.; Dale, M.F.B.; Bryan, G.J.; Forster, J.W. Improving breeding efficiency in potato using molecular and quantitative genetics. Theor. Appl. Genet. 2014, 127, 2279–2292. [Google Scholar] [CrossRef] [PubMed]
- Richards, R.A.; Hunt, J.R.; Kirkegaard, J.A.; Passioura, J.B. Yield improvement and adaptation of wheat to water-limited environments in Australia—A case study. Crop Pasture Sci. 2014, 65, 676–689. [Google Scholar] [CrossRef]
- Campos, H.; Cooper, M.; Edmeades, G.; Löffler, C.; Schussler, J.; Ibañez, M. Changes in drought tolerance in maize associated with fifty years of breeding for yield in the U.S. corn belt. Maydica 2006, 51, 369–381. [Google Scholar]
- Gemenet, D.C.; Lindqvist-Kreuze, H.; De Boeck, B.; da Silva Pereira, G.; Mollinari, M.; Zeng, Z.-B.; Craig Yencho, G.; Campos, H. Sequencing depth and genotype quality: Accuracy and breeding operation considerations for genomic selection applications in autopolyploid crops. Theor. Appl. Genet. 2020, 133, 3345–3363. [Google Scholar] [CrossRef] [PubMed]
- Haas, M.; Sprenger, H.; Zuther, E.; Peters, R.; Seddig, S.; Walther, D.; Kopka, J.; Hincha, D.K.; Köhl, K.I. Can metabolite- and transcript-based selection for drought tolerance in Solanum tuberosum replace selection on yield in arid environments? Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef]
- Sprenger, H.; Erban, A.; Seddig, S.; Rudack, K.; Thalhammer, A.; Le, M.Q.; Walther, D.; Zuther, E.; Köhl, K.I.; Kopka, J.; et al. Metabolite and transcript markers for the prediction of potato drought tolerance. Plant Biotech. J. 2018, 16, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tardieu, F. Any trait or trait-related allele can confer drought tolerance: Just design the right drought scenario. J. Exp. Bot. 2012, 63, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Millet, E.J.; Welcker, C.; Kruijer, W.; Negro, S.; Coupel-Ledru, A.; Nicolas, S.D.; Laborde, J.; Bauland, C.; Praud, S.; Ranc, N.; et al. Genome-wide analysis of yield in Europe: Allelic effects vary with drought and heat scenarios. Plant Phys. 2016, 172, 749–764. [Google Scholar] [CrossRef] [PubMed]
- Seki, M.; Narusaka, M.; Ishida, J.; Nanjo, T.; Fujita, M.; Oono, Y.; Kamiya, A.; Nakajima, M.; Enju, A.; Sakurai, T.; et al. Monitoring the expression profiles of 7000 Arabidopsis genes under drought, cold and high-salinity stresses using a full-length cDNA microarray. Plant J. 2002, 31, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Van der Weele, C.M.; Spollen, W.G.; Sharp, R.E.; Baskin, T.I. Growth of Arabidopsis thaliana seedlings under water deficit studied by control of water potential in nutrient-agar media. J. Exp. Bot. 2000, 51, 1555–1562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.X.; Wang, M.C.; Tian, Y.C.; He, W.X.; Han, L.; Xia, G.M. Over-expression of TaMYB33 encoding a novel wheat MYB transcription factor increases salt and drought tolerance in Arabidopsis. Mol. Biol. Rep. 2012, 39, 7183–7192. [Google Scholar] [CrossRef]
- Bu, Q.; Lv, T.; Shen, H.; Phi, L.; Wang, J.; Wang, Z.; Huang, Z.; Xiao, L.; Engineer, C.; Kim, T.H.; et al. Regulation of Drought Tolerance by the F-Box Protein MAX2 in Arabidopsis. Plant Phys. 2014, 164, 424–439. [Google Scholar] [CrossRef] [Green Version]
- Scheffer, F.; Schachtschabel, P. Lehrbuch der Bodenkunde, 11th ed.; Enke: Stuttgart, Germany, 1984. [Google Scholar]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional Analysis of an Arabidopsis Transcription Factor, DREB2A, Involved in Drought-Responsive Gene Expression. J. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef] [Green Version]
- Hummel, I.; Pantin, F.; Sulpice, R.; Piques, M.; Rolland, G.; Dauzat, M.; Christophe, A.; Pervent, M.; Bouteillé, M.; Stitt, M.; et al. Arabidopsis plants acclimate to water deficit at low cost through changes of carbon usage: An integrated perspective using growth, metabolite, enzyme, and gene expression analysis. J. Plant Physiol. 2010, 154, 357–372. [Google Scholar] [CrossRef] [Green Version]
- Muscolo, A.; Junker, A.; Klukas, C.; Weigelt-Fischer, K.; Riewe, D.; Altmann, T. Phenotypic and metabolic responses to drought and salinity of four contrasting lentil accessions. J. Exp. Bot. 2015, 66, 5467–5480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harb, A.; Krishnan, A.; Ambavaram, M.M.R.; Pereira, A. Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. J. Plant Pysiol. 2010, 154, 1254–1271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roca Paixão, J.F.; Gillet, F.-X.; Ribeiro, T.P.; Bournaud, C.; Lourenço-Tessutti, I.T.; Noriega, D.D.; Melo, B.P.D.; de Almeida-Engler, J.; Grossi-de-Sa, M.F. Improved drought stress tolerance in Arabidopsis by CRISPR/dCas9 fusion with a Histone AcetylTransferase. Sci. Rep. 2019, 9, 8080. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimian-Motlagh, S.; Ribone, P.A.; Thirumalaikumar, V.P.; Allu, A.D.; Chan, R.L.; Mueller-Roeber, B.; Balazadeh, S. JUNGBRUNNEN1 Confers Drought Tolerance Downstream of the HD-Zip I Transcription Factor AtHB13. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [Green Version]
- Passioura, J. The drought environment: Physical, biological and agricultural perspectives. J. Exp. Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [Green Version]
- Granier, C.; Aguirrezabal, L.; Chenu, K.; Cookson, S.; Dauzat, M.; Hamard, P.; Thioux, J.; Rolland, G.; Bouchier-Combaud, S.; Lebaudy, A.; et al. PHENOPSIS, an automated platform for reproducible phenotyping of plant responses to soil water deficit in Arabidopsis thaliana permitted the identification of an accession with low sensitivity to soil water deficit. New Phytol. 2006, 16, 623–635. [Google Scholar] [CrossRef]
- Honsdorf, N.; March, T.J.; Berger, B.; Tester, M.; Pillen, K. High-Throughput Phenotyping to Detect Drought Tolerance QTL in Wild Barley Introgression Lines. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [Green Version]
- Dignat, G.; Welcker, C.; Sawkins, M.; Ribaut, J.M.; Tardieu, F. The growths of leaves, shoots, roots and reproductive organs partly share their genetic control in maize plants. Plant Cell Environ. 2013, 36, 1105–1119. [Google Scholar] [CrossRef]
- Bouchabke, O.; Chang, F.; Simon, M.; Voisin, R.; Pelletier, G.; Durand-Tardif, M. Natural variation in Arabidopsis thaliana as a tool for highlighting differential drought responses. PLoS ONE 2008, 3, e1705. [Google Scholar] [CrossRef]
- Degenkolbe, T.; Do, P.T.; Zuther, E.; Rebsilber, D.; Walther, D.; Hincha, D.K.; Köhl, K.I. Expression profiling of rice cultivars differing in their drought tolerance to long-term drought stress. Plant Mol. Biol. 2009, 69, 133–153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passioura, J.B. Phenotyping for drought tolerance in grain crops: When is it useful to breeders? Func. Plant Biol. 2012, 39, 851–859. [Google Scholar] [CrossRef]
- Poorter, H.; Bühler, J.; van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Func. Plant Biol. 2012, 39, 839–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köhl, K.I.; Basler, G.; Luedemann, A.; Selbig, J.; Walther, D. A plant resource and experiment management system based on the Golm Plant Database as a basic tool for omics research. Plant Methods 2008, 4, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuber Yield and Starch Content of 34 Potato Genotypes in 14 Pot and Field Trials with Optimal and Reduced Irrigation Conducted in Northern Germany 2011–2013. 2021. Available online: https://doi.ipk-gatersleben.de/DOI/c4eae50a-02ed-4c6c-b0b5-b5518b732a15/54a99f99-1255-4c3b-9038-929d4439b757/2 (accessed on 7 April 2021). [CrossRef]
- Arend, D.; Junker, A.; Scholz, U.; Schüler, D.; Wylie, J.; Lange, M. PGP repository: A plant phenomics and genomics data publication infrastructure. Database 2016, 2016. [Google Scholar] [CrossRef] [Green Version]
- Selection and Validation Experiment Comparing Phenotypic and Marker-Assisted Selection for Drought Tolerance in Solanum tuberosum ssp. tuberosum. 2018; Available online: https://doi.ipk-gatersleben.de/DOI/afa7cb69-224a-4f3b-b153-a013faff51c2/a0cd444d-0616-4ac3-91f4-e4ba987f5752/2 (accessed on 27 April 2021). [CrossRef]
- Supplemental Material for Publication counfounding Factors in Container-Based Assessment of Drought Tolerance in Solanum tuberosum. 2021. Available online: https://doi.ipk-gatersleben.de/DOI/a9dd6f7c-4457-4326-821c-https://api.asm.skype.com/v1/objects/0-ea-d8-335b5bc69130da0613c030dd77730642/views/imgpsh_mobile_save_anim1eecbba5a05d/001a3730-0874-436f-87d1-2e9a8cc89ee8/2/1847940088 (accessed on 27 April 2021). [CrossRef]
- Vadez, V.; Kholova, J.; Yadav, R.S.; Hash, C.T. Small temporal differences in water uptake among varieties of pearl millet (Pennisetum glaucum (L.) R. Br.) are critical for grain yield under terminal drought. Plant Soil 2013, 371, 447–462. [Google Scholar] [CrossRef] [Green Version]
- Wurr, D.; Fellows, J.; Allen, E. An approach to determining optimum tuber planting densities in early potato varieties. J. Agric. Sci. 1993, 120, 63–70. [Google Scholar] [CrossRef] [Green Version]
- Europlant. Anbauempfehlungen. Available online: https://www.europlant.biz/sortensuche-ergebnis/euroresa/ (accessed on 4 March 2021).
- Iwama, K.; Hukushima, T.; Yoshimura, T.; Nakaseko, K. Influence of planting density on root growth and yield in potato. Jpn. J. Crop Sci. 1993, 62, 628–635. [Google Scholar] [CrossRef] [Green Version]
- Puertolas, J.; Ballester, C.; Elphinstone, E.D.; Dodd, I.C. Two potato (Solanum tuberosum) varieties differ in drought tolerance due to differences in root growth at depth. Func. Plant Biol. 2014, 41, 1107–1118. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, S.H.; Plauborg, F.; Andersen, M.N.; Sepaskhah, A.R.; Jensen, C.R.; Hansen, S. Effects of irrigation strategies and soils on field grown potatoes: Root distribution. Agric. Water Manag. 2011, 98, 1280–1290. [Google Scholar] [CrossRef]
- Svoboda, P.; Raimanová, I.; Duffková, R.; Fučík, P.; Kurešová, G.; Haberle, J. The effects of irrigation on root density profiles of potato, celery, and wheat. Agron. Res. 2020, 18, 567–578. [Google Scholar] [CrossRef]
- Granier, C.; Tardieu, F. Water Deficit and Spatial Pattern of Leaf Development. Variability in Responses Can Be Simulated Using a Simple Model of Leaf Development. J. Plant Physiol. 1999, 119, 609–620. [Google Scholar] [CrossRef] [Green Version]
- FAO Water Development and Management Unit Crop Water Information: Potato. Available online: http://www.fao.org/nr/water/cropinfo_potato.html (accessed on 26 March 2012).
- Ierna, A.; Mauromicale, G. Tuber yield and irrigation water productivity in early potatoes as affected by irrigation regime. Agric. Water Manag. 2012, 115, 276–284. [Google Scholar] [CrossRef]
- Lafitte, H.R.; Li, Z.K.; Vijayakumar, C.H.M.; Gao, Y.M.; Shi, Y.; Xu, J.L.; Fu, B.Y.; Yu, A.J.; Ali, A.J.; Domingo, J.; et al. Improvement of rice drought tolerance through backcross breeding: Evaluation of donors and selection in drought nurseries. Field Crops Res. 2006, 97, 77–86. [Google Scholar] [CrossRef]
- Hatzig, S.V.; Nuppenau, J.N.; Snowdon, R.J.; Schiessl, S.V. Drought stress has transgenerational effects on seeds and seedlings in winter oilseed rape (Brassica napus L.). BMC Plant Biol. 2018, 18. [Google Scholar] [CrossRef] [PubMed]
- Bundig, C.; Vu, T.H.; Meise, P.; Seddig, S.; Schum, A.; Winkelmann, T. Variability in Osmotic Stress Tolerance of Starch Potato Genotypes (Solanum tuberosum L.) as Revealed by an In Vitro Screening: Role of Proline, Osmotic Adjustment and Drought Response in Pot Trials. J. Agron. Crop Sci. 2017, 203, 206–218. [Google Scholar] [CrossRef]
- Galiano, L.; Timofeeva, G.; Saurer, M.; Siegwolf, R.; Martínez-Vilalta, J.; Hommel, R.; Gessler, A. The fate of recently fixed carbon after drought release: Towards unravelling C storage regulation in Tilia platyphyllos and Pinus sylvestris. Plant Cell Environ. 2017, 40, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Serraj, R.; McNally, K.L.; Slamet-Loedin, I.; Kohli, A.; Haefele, S.M.; Atlin, G.; Kumar, A. Drought resistance improvement in rice: An integrated genetic and resource management strategy. Plant Prod. Sci. 2011, 14, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Grzesiak, M.T.; Marcińska, I.; Janowiak, F.; Rzepka, A.; Hura, T. The relationship between seedling growth and grain yield under drought conditions in maize and triticale genotypes. Acta Physiol. Plant. 2012, 34, 1757–1764. [Google Scholar] [CrossRef]
- Poorter, H.; Fiorani, F.; Pieruschka, R.; Wojciechowski, T.; van der Putten, W.H.; Kleyer, M.; Schurr, U.; Postma, J. Pampered inside, pestered outside? Differences and similarities between plants growing in controlled conditions and in the field. New Phytol. 2016, 212, 838–855. [Google Scholar] [CrossRef]
- Köhl, K.I.; Laitinen, R.A. From the greenhouse to the real world—Arabidopsis field trials and applications. In Molecular Mechanisms in Plant Adaptation; Laitinen, R., Ed.; Wiley-Blackwell: Malden, MA, USA, 2015; pp. 217–235. [Google Scholar]
- Mishra, Y.; Johansson Jänkänpää, H.; Kiss, A.Z.; Funk, C.; Schröder, W.P.; Jansson, S. Arabidopsis plants grown in the field and climate chambers significantly differ in leaf morphology and photosystem components. BMC Plant Biol. 2012, 12, 6. [Google Scholar] [CrossRef] [Green Version]
- Annunziata, M.G.; Apelt, F.; Carillo, P.; Krause, U.; Feil, R.; Köhl, K.; Lunn, J.E.; Stitt, M. Response of Arabidopsis primary metabolism and circadian clock to low night temperature in a natural light environment. J. Exp. Bot. 2018, 69, 4881–4895. [Google Scholar] [CrossRef] [PubMed]
- Altmann, T. Pflanzenkulturhalle. Available online: https://www.ipk-gatersleben.de/phaenotypisierung/organismus (accessed on 24 February 2021).
- Folitec Agrarfolienvertriebs GmbH. Available online: http://www.folitec.de/Produkte/Gewaechshausfolie/Lumisol-clear-AF/ (accessed on 9 March 2021).
- Waisel, Y.; Eshel, A.; Kafkafi, U. Plant Roots: The Hidden Half, 2nd ed.; Dekker: New York, NY, USA, 1996; p. 1002. [Google Scholar]
- Passioura, J.B. The perils of pot experiments. Funct. Plant Biol. 2006, 33, 1075–1079. [Google Scholar] [CrossRef] [PubMed]
- Iwama, K. Physiology of the Potato: New Insights into Root System and Repercussions for Crop Management. Potato Res. 2008, 51, 333. [Google Scholar] [CrossRef]
- Boguszewska-Mańkowska, D.; Zarzyńska, K.; Nosalewicz, A. Drought Differentially Affects Root System Size and Architecture of Potato Cultivars with Differing Drought Tolerance. Am. J. Potato Res. 2020, 97, 54–62. [Google Scholar] [CrossRef]
- Stalham, M.A.; Allen, E.J. Effect of variety, irrigation regime and planting date on depth, rate, duration and density of root growth in the potato (Solanum tuberosum) crop. J. Agric. Sci. 2001, 137, 251–270. [Google Scholar] [CrossRef]
- Stalham, M.A.; Allen, E.J. Water uptake in the potato (Solanum tuberosum) crop. J. Agric. Sci. 2004, 142, 373–393. [Google Scholar] [CrossRef]
- Mullin, R.; Blomquist, A.W.; Lauer, F.I. Effect of seed tuber size on first clonal generation yield of potatoes. Am. Potato J. 1966, 43, 418–423. [Google Scholar] [CrossRef]
- Bussan, A.J.; Mitchell, P.D.; Copas, M.E.; Drilias, M.J. Evaluation of the Effect of Density on Potato Yield and Tuber Size Distribution. Crop Sci. 2007, 47, 2462–2472. [Google Scholar] [CrossRef]
- Grieder, C.; Trachsel, S.; Hund, A. Early vertical distribution of roots and its association with drought tolerance in tropical maize. Plant Soil 2014, 377. [Google Scholar] [CrossRef] [Green Version]







| Trial-Code | P | Culture | Location | n | Pl | G | Start Date | End Date | SI |
|---|---|---|---|---|---|---|---|---|---|
| FTS01 | 1 | 46150 | Dethlingen | 2 | 31 | 34 | 11.04.2011 | 02.09.2011 | 0.11 |
| FTS02 | 1 | 44443 | Golm Field | 4 | 8 | 34 | 21.04.2011 | 01.09.2011 | 0.23 |
| FTS05 | 1 | 56726 | Golm Field | 4 | 8 | 34 | 15.04.2012 | 18.08.2012 | 0.13 |
| FTS06 | 1 | 56877 | Dethlingen | 2 | 31 | 34 | 16.04.2012 | 14.09.2012 | 0.09 |
| FTS07 | 1 | 56875 | Groß Lüsewitz | 2 | 6 | 34 | 19.04.2012 | 28.08.2012 | 0.44 |
| FTS12 | 1 | 62326 | Golm Field | 4 | 8 | 34 | 20.04.2013 | 09.08.2013 | 0.33 |
| FTS13 | 1 | 62328 | Dethlingen | 2 | 31 | 34 | 20.04.2013 | 19.09.2013 | 0.56 |
| FTS14 | 1 | 62327 | Groß Lüsewitz | 2 | 6 | 34 | 25.04.2013 | 19.09.2013 | 0.58 |
| PCH03 | 1 | 45990 | Shelter JKI | 8 | 1 | 34 | 27.04.2011 | 15.08.2011 | 0.55 |
| PCH04 | 1 | 56575 | CE MPIMP | 3 | 1 | 34 | 29.02.2012 | 01.06.2012 | 0.78 |
| PCH08 | 1 | 57803 | Shelter JKI | 8 | 1 | 34 | 26.04.2012 | 09.08.2012 | 0.52 |
| PCH09 | 1 | 58243 | CE MPIMP | 3 | 1 | 34 | 27.06.2012 | 02.10.2012 | 0.65 |
| PCH10 | 1 | 60319 | CE MPIMP | 3 | 1 | 34 | 17.10.2012 | 24.01.2013 | 0.69 |
| PCH11 | 1 | 62030 | CE MPIMP | 3 | 1 | 34 | 13.02.2013 | 16.05.2013 | 0.42 |
| BCH15 | 2 | 72247 | Golm FGH | 6 | 1 | 64 | 09.04.2015 | 19.07.2015 | 0.60 |
| FTS16 | 2 | 72482 | Dethlingen | 2 | 16 | 63 | 20.04.2015 | 31.08.2015 | 0.21 |
| FTS17 | 2 | 72275 | Golm Field | 3 | 5 | 64 | 22.04.2015 | 17.08.2015 | 0.73 |
| FTS18 | 2 | 72396 | Groß Lüsewitz | 2 | 6 | 60 | 28.04.2015 | 04.09.2015 | 0.53 |
| PCH19 | 2 | 72292 | JKI Shelter | 4 | 1 | 63 | 12.05.2015 | 10.08.2015 | 0.48 |
| BCH20 | 2 | 76240 | Golm FGH | 5 | 1 | 64 | 14.04.2016 | 17.07.2016 | 0.54 |
| FTS21 | 2 | 76528 | Dethlingen | 2 | 16 | 63 | 19.04.2016 | 01.09.2016 | 0.18 |
| FTS22 | 2 | 76219 | Golm Field | 3 | 8 | 64 | 21.04.2016 | 09.08.2016 | 0.65 |
| FTS23 | 2 | 76529 | Groß Lüsewitz | 2 | 6 | 63 | 02.05.2016 | 10.08.2016 | 0.70 |
| PCH24 | 2 | 76354 | JKI Shelter | 4 | 1 | 63 | 09.05.2016 | 11.08.2016 | 0.68 |
| BTH25 | 3 | 81251 | Golm FGH | 8 | 1 | 20 | 11.04.2017 | 21.07.2017 | 0.58 |
| FTS26 | 3 | 81256 | Golm Field | 2 | 5 | 21 | 25.04.2017 | 07.09.2017 | 0.44 |
| BTH27 | 3 | 85178 | Golm FGH | 8 | 1 | 20 | 17.04.2018 | 09.07.2018 | 0.74 |
| FTS28 | 3 | 85442 | Golm Field | 4 | 5 | 21 | 02.05.2018 | 22.08.2018 | 0.48 |
| BTH29 | 3 | 88022 | Golm FGH | 6 | 1 | 20 | 02.05.2019 | 30.07.2019 | 0.88 |
| BTS30 | 3 | 88022 | Golm FGH | 3 | 1 | 4 | 02.05.2019 | 30.07.2019 | 0.66 |
| BTH31 | 3 | 93383 | Golm FGH | 3 | 1 | 4 | 23.04.2020 | 20.07.2020 | 0.74 |
| BTS32 | 3 | 93383 | Golm FGH | 6 | 1 | 20 | 23.04.2020 | 20.07.2020 | 0.35 |
| Source | DF | F | Prob(SY) |
|---|---|---|---|
| ERROR | 4096 | - | - |
| Type | 1 | 18,902.96 | <0.0001 |
| Treatment | 1 | 1813.26 | <0.0001 |
| Type × Treatment | 1 | 197.46 | <0.0001 |
| Genotype | 33 | 16.25 | <0.0001 |
| Type × Genotype | 33 | 19.11 | <0.0001 |
| Genotype × Treatment | 33 | 1.69 | 0.008275 |
| Type × Genotype × Treatment | 33 | 1.43 | 0.054271 |
| Source | DF | F(SY) | Prob(SY) |
|---|---|---|---|
| ERROR | 4250 | - | - |
| Type | 2 | 3994.22 | <0.0001 |
| Treatment | 1 | 5439.05 | <0.0001 |
| Type×Treatment | 2 | 554.55 | <0.0001 |
| Genotype | 62 | 7.38 | <0.0001 |
| Type × Genotype | 124 | 6.92 | <0.0001 |
| Genotype × Treatment | 62 | 2.24 | <0.0001 |
| Type × Genotype × Treatment | 124 | 0.97 | 0.57963 |
| Source | DF | F(SY) | Prob(SY) | F(FW) | Prob(FW) |
|---|---|---|---|---|---|
| ERROR | 182 | - | - | - | - |
| Genotype | 3 | 20.27 | 0.0000 | 13.45 | 0.0000 |
| Treatment | 1 | 495.80 | 0.0000 | 587.29 | 0.0000 |
| Genotype × Treatment | 3 | 2.42 | 0.0673 | 1.28 | 0.2837 |
| Year | 1 | 81.77 | 0.0000 | 100.43 | 0.0000 |
| Genotype × Year | 3 | 2.03 | 0.1117 | 4.71 | 0.0034 |
| Year × Treatment | 1 | 1.23 | 0.2682 | 0.23 | 0.6331 |
| Genotype × Year × Treatment | 3 | 0.43 | 0.7325 | 0.56 | 0.6435 |
| Type | 1 | 374.95 | 0.0000 | 288.96 | 0.0000 |
| Genotype × Type | 3 | 33.20 | 0.0000 | 25.06 | 0.0000 |
| Type × Treatment | 1 | 2.61 | 0.1079 | 0.29 | 0.5890 |
| Genotype × Type × Treatment | 3 | 0.32 | 0.8079 | 0.53 | 0.6649 |
| Year × Type | 1 | 14.85 | 0.0002 | 1.96 | 0.1627 |
| Genotype × Year × Type | 3 | 5.75 | 0.0009 | 9.61 | 0.0000 |
| Year × Type × Treatment | 1 | 1.82 | 0.1796 | 0.02 | 0.9034 |
| Genotype × Year × Type × Treatment | 3 | 0.14 | 0.9385 | 0.08 | 0.9715 |
| Year | SourGenotypee | DF | F(SY) | Prob(SY) | F(FW) | Prob(FW) |
|---|---|---|---|---|---|---|
| 2019 | ERROR | 48 | - | - | - | - |
| 2019 | Genotype | 3 | 4.38 | 0.008 | 3.84 | 0.015 |
| 2019 | Substrate | 1 | 103.90 | 0.000 | 176.29 | 0.000 |
| 2019 | Genotype × Substrate | 3 | 2.99 | 0.040 | 5.71 | 0.002 |
| 2019 | Treatment | 1 | 208.30 | 0.000 | 143.83 | 0.000 |
| 2019 | Genotype × Treatment | 3 | 2.00 | 0.127 | 1.22 | 0.312 |
| 2019 | Substrate × Treatment | 1 | 7.70 | 0.008 | 1.37 | 0.248 |
| 2019 | Genotype × Substrate × Treatment | 3 | 2.01 | 0.126 | 0.75 | 0.529 |
| 2020 | ERROR | 56 | - | - | - | - |
| 2020 | Genotype | 3 | 11.47 | 0.000 | 5.43 | 0.002 |
| 2020 | Substrate | 1 | 18.34 | 0.000 | 12.50 | 0.001 |
| 2020 | Genotype × Substrate | 3 | 0.70 | 0.556 | 2.75 | 0.051 |
| 2020 | Treatment | 1 | 498.39 | 0.000 | 537.25 | 0.000 |
| 2020 | Genotype × Treatment | 3 | 2.84 | 0.047 | 1.20 | 0.318 |
| 2020 | Substrate × Treatment | 1 | 142.96 | 0.000 | 41.95 | 0.000 |
| 2020 | Genotype × Substrate × Treatment | 3 | 1.50 | 0.224 | 0.99 | 0.404 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Köhl, K.I.; Mulugeta Aneley, G.; Haas, M.; Peters, R. Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum. Agronomy 2021, 11, 865. https://doi.org/10.3390/agronomy11050865
Köhl KI, Mulugeta Aneley G, Haas M, Peters R. Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum. Agronomy. 2021; 11(5):865. https://doi.org/10.3390/agronomy11050865
Chicago/Turabian StyleKöhl, Karin I., Gedif Mulugeta Aneley, Manuela Haas, and Rolf Peters. 2021. "Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum" Agronomy 11, no. 5: 865. https://doi.org/10.3390/agronomy11050865
APA StyleKöhl, K. I., Mulugeta Aneley, G., Haas, M., & Peters, R. (2021). Confounding Factors in Container-Based Drought Tolerance Assessments in Solanum tuberosum. Agronomy, 11(5), 865. https://doi.org/10.3390/agronomy11050865