Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture
Abstract
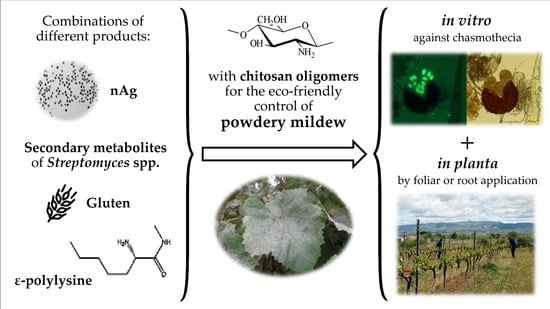
:1. Introduction
2. Material and Methods
2.1. Reagents and Actinobacteria Isolates
2.2. Preparation of Chitosan Oligomers and Secondary Metabolites
2.3. Bioactive Products Tested
2.4. Field Antifungal Activity Test
2.5. In Vitro Test of Antifungal Activity on Ascospores
2.5.1. Isolation of Chasmothecia
2.5.2. Fluorescein Diacetate Staining of Ascospores
2.6. Statistical Treatment
3. Results
3.1. Field Antifungal Activity Tests
3.1.1. Powdery Mildew Control on Leaves
3.1.2. Powdery Mildew Control on Grapevine Clusters
3.2. Effect on Production Quality and Yield
3.3. In Vitro Effect of Antifungal Activity on Ascospores
4. Discussion
4.1. Antifungal Behavior in Field Conditions and Effect on Yield
4.2. In Vitro Efficacy against Chasmothecia
4.3. Justification of the Observed Antifungal Behavior
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galet, P. Précis de Pathologie Viticole, 3rd ed.; Imprimerie JF Impression: Montpellier, France, 1999; p. 296. [Google Scholar]
- Bois, B.; Zito, S.; Calonnec, A. Climate vs grapevine pests and diseases worldwide: The first results of a global survey. OENO One 2017, 51, 133–139. [Google Scholar] [CrossRef]
- Caffarra, A.; Rinaldi, M.; Eccel, E.; Rossi, V.; Pertot, I. Modelling the impact of climate change on the interaction between grapevine and its pests and pathogens: European grapevine moth and powdery mildew. Agric. Ecosyst. Environ. 2012, 148, 89–101. [Google Scholar] [CrossRef]
- Kunova, A.; Pizzatti, C.; Saracchi, M.; Pasquali, M.; Cortesi, P. Grapevine powdery mildew: Fungicides for its management and advances in molecular detection of markers associated with resistance. Microorganisms 2021, 9, 1541. [Google Scholar] [CrossRef] [PubMed]
- Eurostat. The Use of Plant Protection Products in the European Union; Office for Official Publications of the European Communities: Luxembourg, 2007; p. 215. [Google Scholar]
- Rousseau, J.; Chanfreau, S.; Bontemps, É. Les Cépages Résistants and Maladies Cryptogamiques; Groupe ICV: Bordeaux, France, 2013; p. 228. [Google Scholar]
- Sambucci, O.; Alston, J.M.; Fuller, K.B.; Lusk, J. The pecuniary and nonpecuniary costs of powdery mildew and the potential value of resistant grape varieties in California. Am. J. Enol. Vitic. 2019, 70, 177–187. [Google Scholar] [CrossRef]
- Pearson, R.C.; Gadoury, D.M. Cleistothecia, the source of primary inoculum for grape powdery mildew in New York. Phytopathology 1987, 77, 1509–1514. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Pearson, R.C. Germination of ascospores and infection of Vitis by Uncinula necator. Phytopathology 1990, 80, 1198–1203. [Google Scholar] [CrossRef]
- Gadoury, D.M.; Cadle-Davidson, L.; Wilcox, W.F.; Dry, I.B.; Seem, R.C.; Milgroom, M.G. Grapevine powdery mildew (Erysiphe necator): A fascinating system for the study of the biology, ecology and epidemiology of an obligate biotroph. Mol. Plant Pathol. 2012, 13, 1–16. [Google Scholar] [CrossRef]
- Calonnec, A.; Jolivet, J.; Ramaroson, M.L.; Dufour, M.C.; Corio-Costet, M.F. Defence responses of grapevine cultivars to powdery mildew: Ontogenic resistance versus genetic resistance. Plant Pathol. 2021, 70, 1583–1600. [Google Scholar] [CrossRef]
- Ledermann, L.; Daouda, S.; Gouttesoulard, C.; Aarrouf, J.; Urban, L. Flashes of UV-C light stimulate defenses of Vitis vinifera L. ‘Chardonnay’ against Erysiphe necator in greenhouse and vineyard conditions. Plant Dis. 2021, 105, 2106–2113. [Google Scholar] [CrossRef]
- Redl, M.; Sitavanc, L.; Hanousek, F.; Steinkellner, S. A single out-of-season fungicide application reduces the grape powdery mildew inoculum. Crop Prot. 2021, 149, 105760. [Google Scholar] [CrossRef]
- Scott, E.S. 2019 Daniel McAlpine Memorial Lecture. Grapevine powdery mildew: From fundamental plant pathology to new and future technologies. Australas. Plant Pathol. 2021, 50, 1–6. [Google Scholar] [CrossRef]
- Matei, P.; Iacomi, B.; Martín-Gil, J.; Pérez-Lebeña, E.; Ramos-Sánchez, M.; Barrio-Arredondo, M.; Martín-Ramos, P. In vitro antifungal activity of composites of AgNPs and polyphenol inclusion compounds against Fusarium culmorum in different dispersion media. Agronomy 2018, 8, 239. [Google Scholar] [CrossRef] [Green Version]
- Matei, P.; Martín-Gil, J.; Michaela Iacomi, B.; Pérez-Lebeña, E.; Barrio-Arredondo, M.; Martín-Ramos, P. Silver nanoparticles and polyphenol inclusion compounds composites for Phytophthora cinnamomi mycelial growth inhibition. Antibiotics 2018, 7, 76. [Google Scholar] [CrossRef] [Green Version]
- Buzón-Durán, L.; Martín-Gil, J.; Pérez-Lebeña, E.; Ruano-Rosa, D.; Revuelta, J.L.; Casanova-Gascón, J.; Ramos-Sánchez, M.C.; Martín-Ramos, P. Antifungal agents based on chitosan oligomers, ε-polylysine and Streptomyces spp. secondary metabolites against three Botryosphaeriaceae species. Antibiotics 2019, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2017, 36, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Sannan, T.; Kurita, K.; Iwakura, Y. Studies on chitin, 2. Effect of deacetylation on solubility. Die Makromol. Chem. 1976, 177, 3589–3600. [Google Scholar] [CrossRef]
- Sadigh-Eteghad, S.; Dehnad, A.; Shanebandi, D.; Khalili, I.; Razmarayii, N.; Namvaran, A.J.V.R.C. Identification and characterization of a Streptomyces sp. isolate exhibiting activity against multidrug-resistant coagulase-negative Staphylococci. Vet. Res. Commun. 2011, 35, 477–486. [Google Scholar] [CrossRef]
- Pazhanimurugan, R.; Radhakrishnan, M.; Shanmugasundaram, T.; Gopikrishnan, V.; Balagurunathan, R. Terpenoid bioactive compound from Streptomyces rochei (M32): Taxonomy, fermentation and biological activities. World J. Microbiol. Biotechnol. 2016, 32, 161. [Google Scholar] [CrossRef]
- Townsend, G.R.; Heuberger, J.W. Methods for estimating losses caused by diseases in fungicide experiments. Plant Dis. Report. 1943, 27, 340–343. [Google Scholar]
- Cortesi, P.; Bisiach, M.; Ricciolini, M.; Gadoury, D.M. Cleistothecia of Uncinula necator—An additional source of inoculum in Italian vineyards. Plant Dis. 1997, 81, 922–926. [Google Scholar] [CrossRef] [Green Version]
- Widholm, J.M. The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 2009, 47, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Ingham, E.R.; Klein, D.A. Relationship between fluorescein diacetate-stained hyphae and oxygen utilization, glucose utilization, and biomass of submerged fungal batch cultures. Appl. Environ. Microbiol. 1982, 44, 363–370. [Google Scholar] [CrossRef] [Green Version]
- ibidi GmbH. Application Note 33: Live/Dead Staining with FDA and PI; ibidi GmbH: Munich, Germany, 2015. [Google Scholar]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant growth promoting and biocontrol activity of Streptomyces spp. as endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [Green Version]
- Romanazzi, G.; Mancini, V.; Feliziani, E.; Servili, A.; Endeshaw, S.; Neri, D. Impact of alternative fungicides on grape downy mildew control and vine growth and development. Plant Dis. 2016, 100, 739–748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islam, M. Bioactive natural products for managing downy mildew disease in grapevine. In Biocontrol of Major Grapevine Diseases: Leading Research; Compant, S., Mathieu, F., Eds.; CAB International: Wallingford, UK, 2016; pp. 125–149. [Google Scholar]
- Kurth, F.; Mailänder, S.; Bönn, M.; Feldhahn, L.; Herrmann, S.; Große, I.; Buscot, F.; Schrey, S.D.; Tarkka, M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant Microbe Interact. 2014, 27, 891–900. [Google Scholar] [CrossRef] [Green Version]
- LongXian, R.; Du, S.H.; Li, H.P.; Zhen, Z.X.; Jesús, M.-B. Suppression of grape powdery mildew by Streptomyces and Pseudomonas spp. In Proceedings of the XVII Congreso de la Sociedad Española de Fitopatología, Lleida, Spain, 7–10 October 2014; p. 1. [Google Scholar]
- Buzón-Durán, L.; Martín-Gil, J.; Marcos-Robles, J.L.; Fombellida-Villafruela, Á.; Pérez-Lebeña, E.; Martín-Ramos, P. Antifungal activity of chitosan oligomers–amino acid conjugate complexes against Fusarium culmorum in spelt (Triticum spelta L.). Agronomy 2020, 10, 1427. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Langa-Lomba, N.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Pérez-Lebeña, E.; Martín-Ramos, P. On the applicability of chitosan oligomers-amino acid conjugate complexes as eco-friendly fungicides against grapevine trunk pathogens. Agronomy 2021, 11, 324. [Google Scholar] [CrossRef]
- Dos Santos-Silva, C.A.; Zupin, L.; Oliveira-Lima, M.; Vilela, L.M.B.; Bezerra-Neto, J.P.; Ferreira-Neto, J.R.; Ferreira, J.D.C.; de Oliveira-Silva, R.L.; Pires, C.d.J.; Aburjaile, F.F.; et al. Plant antimicrobial peptides: State of the art, in silico prediction and perspectives in the omics era. Bioinform. Biol. Insights 2020, 14, 1–22. [Google Scholar] [CrossRef]
- Sathoff, A.E.; Samac, D.A. Antibacterial activity of plant defensins. Mol. Plant Microbe Interact. 2019, 32, 507–514. [Google Scholar] [CrossRef]
- Su, T.; Han, M.; Cao, D.; Xu, M. Molecular and biological properties of snakins: The foremost cysteine-rich plant host defense peptides. J. Fungi 2020, 6, 220. [Google Scholar] [CrossRef]
- Ahmad, B.; Yao, J.; Zhang, S.; Li, X.; Zhang, X.; Yadav, V.; Wang, X. Genome-wide characterization and expression profiling of GASA genes during different stages of seed development in grapevine (Vitis vinifera L.) predict their involvement in seed development. Int. J. Mol. Sci. 2020, 21, 1088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskandari, S.; Khoshgoftarmanesh, A.H.; Sharifnabi, B. The effect of foliar-applied manganese in mineral and complex forms with amino acids on certain defense mechanisms of cucumber (Cucumis sativus L.) against powdery mildew. J. Plant Growth Regul. 2018, 37, 481–490. [Google Scholar] [CrossRef]
- Buzón-Durán, L.; Pérez-Lebeña, E.; Martín-Gil, J.; Sánchez-Báscones, M.; Martín-Ramos, P. Applications of Streptomyces spp. enhanced compost in sustainable agriculture. In Biology of Composts; Meghvansi, M.A.V., Ed.; Springer Nature: Cham, Switzerland, 2020; Volume 58, pp. 257–291. [Google Scholar]
- Redl, M.; Möth, S.; Koschier, E.; Spangl, B.; Steinkellner, S. Survival and viability of ascospores of Erysiphe necator in Austrian vineyards. Eur. J. Plant Pathol. 2021, 159, 615–626. [Google Scholar] [CrossRef]
- Thiessen, L.D.; Neill, T.M.; Mahaffee, W.F. Interruption and reduction of Erysiphe necator chasmothecia development utilizing fungicidal oil. Plant Health Prog. 2018, 19, 153–155. [Google Scholar] [CrossRef]
- Legler, S.E.; Caffi, T.; Rossi, V. Effect of Different Plant Protection Products on the Sexual Stage of Grapevine Powdery Mildew. In Proceedings of the IOBC/WPRS European Meeting of the Working Group “Integrated Protection and Production in Viticulture, Lacanau, France, 2–5 October 2011; p. 2. [Google Scholar]
- Magarey, P.; Moyer, M. Towards establishing slow input regimes in Australian viticulture 3: Use of ”epi-season” and ”lag phase control” in applying epidemiological knowledge of grapevine powdery mildew, to reduce the number of sprays and inoculum reservoirs for long-term control. In Proceedings of the 6th International Workshop on Grapevine Downy and Powdery Mildew, Bordeaux, France, 4–9 July 2010; pp. 114–116. [Google Scholar]
- Ing, L.Y.; Zin, N.M.; Sarwar, A.; Katas, H. Antifungal activity of chitosan nanoparticles and correlation with their physical properties. Int. J. Biomater. 2012, 2012, 632698. [Google Scholar] [CrossRef]
- Al-Humiany, A.U.-R.A.A. Taificidin1 and Taificidin2, two anti-microbial agents isolated from the fermentation broth of Streptomyces roseodistaticus TA15 and Streptomyces lavendofoliae TA17. Res. J. Microbiol. 2011, 6, 328–342. [Google Scholar] [CrossRef]
- Kim, W.S.; Youn, D.J.; Kim, H.R.; Rhee, S.K.; Choi, E.S. Metabolic conversion of aclacinomycins B and Y to A by pH shift during fermentation with Streptomyces lavendofoliae DKRS. Biotechnol. Tech. 1995, 9, 671–676. [Google Scholar] [CrossRef]
- Le Goff, G.; Ouazzani, J. Natural hydrazine-containing compounds: Biosynthesis, isolation, biological activities and synthesis. Biorg. Med. Chem. 2014, 22, 6529–6544. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Yamazaki, T.; Takahashi, Y.; Hamada, M.; Takeuchi, T.; Aoyagi, T. Piperastatin A, a new selective serine carboxypeptidase inhibitor produced by Actinomycete. I. Taxonomy, production, isolation and biological activities. J. Enzym. Inhib. 2008, 10, 93–103. [Google Scholar] [CrossRef]
- Murakami, S.; Harada, S.; Takahashi, Y.; Naganawa, H.; Takeuchi, T.; Aoyagi, T. Piperastatin B: A new selective serine carboxypeptidase inhibitor from Streptomyces lavendofoliae MJ908-WF13. J. Enzym. Inhib. 2008, 11, 51–66. [Google Scholar] [CrossRef]
- Narayanaswamy, V.K.; Albericio, F.; Coovadia, Y.M.; Kruger, H.G.; Maguire, G.E.M.; Pillay, M.; Govender, T. Total synthesis of a depsidomycin analogue by convergent solid-phase peptide synthesis and macrolactonization strategy for antitubercular activity. J. Pept. Sci. 2011, 17, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Arakawa, K.; Sugino, F.; Kodama, K.; Ishii, T.; Kinashi, H. Cyclization mechanism for the synthesis of macrocyclic antibiotic Lankacidin in Streptomyces rochei. Chem. Biol. 2005, 12, 249–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anukool, U.; Gaze, W.H.; Wellington, E.M.H. In situ monitoring of Streptothricin production by Streptomyces rochei F20 in soil and rhizosphere. Appl. Environ. Microbiol. 2004, 70, 5222–5228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamashita, N.; Shin-Ya, K.; Furihata, K.; Hayakawa, Y.; Seto, H. New Ravidomycin analogues, FE35A and FE35B, apoptosis inducers produced by Streptomyces rochei. J. Antibiot. 1998, 51, 1105–1108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanini, G.S.; Katsifas, E.A.; Savvides, A.L.; Karagouni, A.D. Streptomyces rochei ACTA1551, an indigenous Greek isolate studied as a potential biocontrol agent against Fusarium oxysporum f. sp. lycopersici. BioMed Res. Int. 2013, 2013, 387230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Augustine, S.; Bhavsar, S.; Kapadnis, B. Production of a growth dependent metabolite active against dermatophytes by Streptomyces rochei AK 39. Indian J. Med. Res. 2005, 121, 164–170. [Google Scholar]
- Irdani, T.; Perito, B.; Mastromei, G. Characterization of a Streptomyces rochei endoglucanasea. Ann. N. Y. Acad. Sci. 1996, 782, 173–181. [Google Scholar] [CrossRef]
- Harada, S.; Okada, J.; Takeda, M.; Yamazaki, T. Inclusion compounds of lankacidin-group antibiotics with cyclodextrins. J. Antibiot. 1985, 38, 877–885. [Google Scholar] [CrossRef] [Green Version]




| Treatment | Method of Application | Abbreviation |
|---|---|---|
| COS (20 g∙L−1) | Root | T1R |
| Foliar-spray | T1F | |
| Chasmothecia | T1C | |
| COS (20 g∙L−1) + nAg (0.2 g∙L−1) | Root | T2R |
| Foliar-spray | T2F | |
| Chasmothecia | T2C | |
| COS (20 g∙L−1) + EPL (2 g∙L−1) | Root | T3R |
| Foliar-spray | T3F | |
| Chasmothecia | T3C | |
| COS (20 g∙L−1) + S. rochei metabolites (2 g·L−1), 3:1 (v/v) | Root | T4R |
| Foliar-spray | T4F | |
| Chasmothecia | T4C | |
| COS (20 g∙L−1) + S. lavendofoliae metabolites (2 g·L−1), 3:1 (v/v) | Root | T5R |
| Foliar-spray | T5F | |
| Chasmothecia | T5C | |
| COS (20 g∙L−1) + hydrolyzed gluten (10 g∙L −1) | Root | T6R |
| Foliar-spray | T6F | |
| Chasmothecia | T6C | |
| Freeze-dried S. rochei (0.1 g) + gluten (10 g) * | Root | T7R |
| Freeze-dried S. lavendofoliae (0.1 g) + gluten (10 g) * | Root | T8R |
| Control with conventional treatment (see Table S1) | Foliar-spray | Tconv |
| Untreated control | - | Tcontrol |
| Organs | Sampling Date | |||||
|---|---|---|---|---|---|---|
| 10 June 2019 | 24 June 2019 | 8 July 2019 | 22 July 2019 | 5 August 2019 | 23 August 2019 | |
| Leaves | T5R | T3F | T4R | T5R | T5R | T5R |
| T5F | T7R | T6F | T6R | T6R | T6R | |
| T6R | T5F | Tconv | T6F | Tconv | T6F | |
| T6F | T6R | Tconv | Tconv | |||
| T6F | ||||||
| Grapevine clusters | - | T1R | T6R | T5R | T5R | T5R |
| T1F | T6F | T6F | T5F | T6R | ||
| T4F | T5R | T6R | T7R | T6F | ||
| T6R | ||||||
| Strain | Bioactive Compound | Applications | References |
|---|---|---|---|
| S. lavendofoliae | anthracidine A | antibiotic | [45] |
| aclacinomycin A | [46] | ||
| fosfazinomycins (hydrazides) | antifungal | [47] | |
| piperastatin A | carboxypeptidase inhibitor | [48] | |
| piperastatin B | [49] | ||
| depsidomycin | antimicrobial and immunosuppressive activity | [50] | |
| S. rochei | lancacidine | antibiotic | [51] |
| steptotricin | [52] | ||
| ravidomycin analogues FE35A and FE35B | apoptosis inducers | [53] | |
| borrelidin | antifungal | [54,55,56] | |
| butyrolactol A | |||
| butyrolactol B | |||
| uricase |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruano-Rosa, D.; Sánchez-Hernández, E.; Baquero-Foz, R.; Martín-Ramos, P.; Martín-Gil, J.; Torres-Sánchez, S.; Casanova-Gascón, J. Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture. Agronomy 2022, 12, 495. https://doi.org/10.3390/agronomy12020495
Ruano-Rosa D, Sánchez-Hernández E, Baquero-Foz R, Martín-Ramos P, Martín-Gil J, Torres-Sánchez S, Casanova-Gascón J. Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture. Agronomy. 2022; 12(2):495. https://doi.org/10.3390/agronomy12020495
Chicago/Turabian StyleRuano-Rosa, David, Eva Sánchez-Hernández, Rubén Baquero-Foz, Pablo Martín-Ramos, Jesús Martín-Gil, Sergio Torres-Sánchez, and José Casanova-Gascón. 2022. "Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture" Agronomy 12, no. 2: 495. https://doi.org/10.3390/agronomy12020495
APA StyleRuano-Rosa, D., Sánchez-Hernández, E., Baquero-Foz, R., Martín-Ramos, P., Martín-Gil, J., Torres-Sánchez, S., & Casanova-Gascón, J. (2022). Chitosan-Based Bioactive Formulations for the Control of Powdery Mildew in Viticulture. Agronomy, 12(2), 495. https://doi.org/10.3390/agronomy12020495