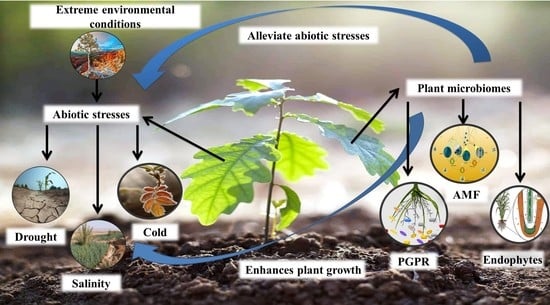
Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses
Abstract
:1. Introduction
2. Climate Change and Loss of Soil Fertility
3. Plant-Associated Microorganisms
4. Salinity, Drought, and Waterlogging
4.1. Salinity
4.2. Drought
4.3. Waterlogging
4.4. Heavy Metals Stress
4.5. Temperature Stress
5. How Do Abiotic Stresses Cause Loss of Plants Productivity?
6. Microbiome and Stresses
7. Plant Microbiome
8. Microbe-Mediated Mitigation of Abiotic Stresses
8.1. Mechanisms of PGPRs
8.2. Direct Mechanisms
9. Indirect Mechanisms
9.1. Mechanisms of AMFs
9.2. Mechanisms of Endophytes
10. Tolerance Mechanism by Plant Microbiome Interaction
11. Role of Microbes in Growth Improvement
12. Role of Microbes in Minerals Improvements in Plants
13. Microbial Plant Biostimulants
14. Synergic Effects of Biochar and Microbes in Ecophysiological Parameters
15. Customized Adjustment of Plant Microbiome: A Revolution in Progress
16. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cooper, R.N.; Houghton, J.T.; McCarthy, J.J.; Metz, B. Climate Change 2001: The Scientific Basis. Foreign Aff. 2002, 81, 208. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014 Synthesis Report Summary Chapter for Policymakers; IPCC: Paris, France, 2014; p. 31. [Google Scholar]
- Compant, S.; van der Heijden, M.G.; Sessitsch, A. Climate change effects on beneficial plant–microorganism interactions. Fed. Eur. Microbiol. Soc. 2010, 73, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Wadgymar, S.M.; Daws, S.C.; Anderson, J.T. Integrating viability and fecundity selection to illuminate the adaptive nature of genetic clines. Evol. Lett. 2017, 1, 26–39. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Leifheit, E.; Verbruggen, E.; Rillig, M. Arbuscular mycorrhizal fungi reduce decomposition of woody plant litter while increasing soil aggregation. Soil Biol. Biochem. 2015, 81, 323–328. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Wagner, M.R.; Lundberg, D.S.; Coleman-Derr, D.; Tringe, S.G.; Dangl, J.L.; Mitchell-Olds, T. Natural soil microbes alter flowering phenology and the intensity of selection on flowering time in a wild arabidopsis relative. Ecol. Lett. 2014, 17, 717–726. [Google Scholar] [CrossRef]
- Gehring, C.A.; Sthultz, C.M.; Flores-Rentería, L.; Whipple, A.V.; Whitham, T.G. Tree genetics defines fungal partner communities that may confer drought tolerance. Proc. Natl. Acad. Sci. USA 2017, 114, 11169–11174. [Google Scholar] [CrossRef]
- Calvo, O.C.; Franzaring, J.; Schmid, I.; Müller, M.; Brohon, N.; Fangmeier, A. Atmospheric CO2 enrichment and drought stress modify root exudation of barley. Glob. Chang. Biol. 2017, 23, 1292–1304. [Google Scholar] [CrossRef]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-mediated stress resistance in plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Sagar, A.; Rathore, P.; Ramteke, P.W.; Ramakrishna, W.; Reddy, M.S.; Pecoraro, L. Plant growth promoting rhizobacteria, arbuscular mycorrhizal fungi and their synergistic interactions to counteract the negative effects of saline soil on agriculture: Key macromolecules and mechanisms. Microorganisms 2021, 9, 1491. [Google Scholar] [CrossRef] [PubMed]
- Evelin, H.; Devi, T.S.; Gupta, S.; Kapoor, R. Mitigation of salinity stress in plants by arbuscular mycorrhizal symbiosis: Current understanding and new challenges. Front. Plant Sci. 2019, 10, 470. [Google Scholar] [CrossRef] [PubMed]
- Porcel, R.; Aroca, R.; Azcon, R.; Ruiz-Lozano, J.M. Regulation of cation transporter genes by the arbuscular mycorrhizal symbiosis in rice plants subjected to salinity suggests improved salt tolerance due to reduced Na+ root-to-shoot distribution. Mycorrhiza 2016, 26, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Al-Arjani, A.-B.F.; Hashem, A.; Abd Allah, E.F. Arbuscular mycorrhizal fungi modulates dynamics tolerance expression to mitigate drought stress in Ephedra foliata boiss. Saudi J. Biol. Sci. 2020, 27, 380–394. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, H.; Zhang, X.; Tang, M. Arbuscular mycorrhizal symbiosis alleviates salt stress in black locust through improved photosynthesis, water status, and K+/Na+ homeostasis. Front. Plant Sci. 2017, 2017, 1739. [Google Scholar] [CrossRef]
- Li, Z.; Wu, N.; Meng, S.; Wu, F.; Liu, T. Arbuscular mycorrhizal fungi (AMF) enhance the tolerance of Euonymus maackii rupr. At a moderate level of salinity. PLoS ONE 2020, 15, e0231497. [Google Scholar] [CrossRef]
- Hartman, K.; Tringe, S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019, 476, 2705–2724. [Google Scholar] [CrossRef]
- Vandenkoornhuyse, P.; Quaiser, A.; Duhamel, M.; Le Van, A.; Dufresne, A. The importance of the microbiome of the plant holobiont. New Phytol. 2015, 206, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Rouydel, Z.; Barin, M.; Rasouli-Sadaghiani, M.H.; Khezri, M.; Vetukuri, R.R.; Kushwaha, S. Harnessing the Potential of Symbiotic Endophytic Fungi and Plant Growth-Promoting Rhizobacteria to Enhance Soil Quality in Saline Soils. Processes 2021, 9, 1810. [Google Scholar] [CrossRef]
- Coleman-Derr, D.; Desgarennes, D.; Fonseca-Garcia, C.; Gross, S.; Clingenpeel, S.; Woyke, T.; North, G.; Visel, A.; Partida-Martinez, L.P.; Tringe, S.G. Plant compartment and biogeography affect microbiome composition in cultivated and native agave species. New Phytol. 2016, 209, 798–811. [Google Scholar] [CrossRef] [PubMed]
- Mathur, P.; Roy, S. Insights into the plant responses to drought and decoding the potential of root associated microbiome for inducing drought tolerance. Physiol. Plant. 2021, 172, 1016–1029. [Google Scholar] [CrossRef] [PubMed]
- Hartman, K.J. Molecular and Experimental Approaches for Exploring the Role of the Soil and Root Microbiome in Agroecosystem Functioning. Ph.D. Dissertation, University of Zurich, Zurich, Switzerland, 2018. [Google Scholar]
- Jiménez-Pérez, M.; Morales-Manzo, I.I.; Ana, F.I.T.A.; Rodríguez-Burruezo, A. Mitigation of drought stress in solanaceae vegetables through symbiosis with plant growth-promoting bacteria and arbuscular mycorrhizal fungi. A review. Sci. J. 2022, 11, 86. [Google Scholar]
- Bulgarelli, D.; Garrido-Oter, R.; Münch, P.C.; Weiman, A.; Dröge, J.; Pan, Y.; McHardy, A.C.; Schulze-Lefert, P. Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 2015, 17, 392–403. [Google Scholar] [CrossRef]
- Gamalero, E.; Glick, B.R. Recent Advances in Bacterial Amelioration of Plant Drought and Salt Stress. Biology 2022, 11, 437. [Google Scholar] [CrossRef]
- Ding, Z.; Kheir, A.M.; Ali, M.G.; Ali, O.A.; Abdelaal, A.I.; Zhou, Z.; Wang, B.; Liu, B.; He, Z. The integrated effect of salinity, organic amendments, phosphorus fertilizers, and deficit irrigation on soil properties, phosphorus fractionation and wheat productivity. Sci. Rep. 2020, 10, 2736. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y. Biotechnological potential of plant-microbe interactions in environmental decontamination. Front. Plant Sci. 2019, 10, 1519. [Google Scholar] [CrossRef]
- Sabra, M.; Aboulnasr, A.; Franken, P.; Perreca, E.; Wright, L.P.; Camehl, I. Beneficial root endophytic fungi increase growth and quality parameters of sweet basil in heavy metal contaminated soil. Front. Plant Sci. 2018, 9, 1726. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- da Silva, A.V.; da Silva, M.K.; Sampaio, E.B.T.; Ferreira, L.F.R.; Passarini, M.R.Z.; de Oliveira, V.M.; Rosa, L.H.; Duarte, A.W.F. Benefits of plant growth-promoting symbiotic microbes in climate change era. In Microbiome under Changing Climate; Woodhead Publishing: Cambridge, UK, 2022; pp. 85–113. [Google Scholar]
- Elsheikh, E.A.; El-Keblawy, A.; Mosa, K.A.; Okoh, A.I.; Saadoun, I. Role of Endophytes and Rhizosphere Microbes in Promoting the Invasion of Exotic Plants in Arid and Semi-Arid Areas: A Review. Sustainability 2021, 13, 13081. [Google Scholar] [CrossRef]
- Gamalero, E.; Bona, E.; Todeschini, V.; Lingua, G. Saline and arid soils: Impact on bacteria, plants, and their interaction. Biology 2020, 9, 116. [Google Scholar] [CrossRef]
- Mainka, T.; Weirathmüller, D.; Herwig, C.; Pflügl, S. Potential applications of halophilic microorganisms for biological treatment of industrial process brines contaminated with aromatics. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab015. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Sharma, P.; Dev, K.; Sourirajan, A. Halophilic bacteria of lunsu produce an array of industrially important enzymes with salt tolerant activity. Biochem. Res. Int. 2016, 2016, 9237418. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Malik, K.A.; Mehnaz, S. Microbiome of halophytes: Diversity and importance for plant health and productivity. Microbiol. Biotechnol. Lett. 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Mahmud, K.; Missaoui, A.; Lee, K.C.; Ghimire, B.; Presley, H.W.; Makaju, S. Rhizosphere microbiome manipulation for sustainable crop production. Curr. Plant Biol. 2021, 27, 100210. [Google Scholar] [CrossRef]
- Mukhtar, S.; Mehnaz, S.; Mirza, M.S.; Malik, K.A. Isolation and characterization of bacteria associated with the rhizosphere of halophytes (Salsola stocksii and Atriplex amnicola) for production of hydrolytic enzymes. Braz. J. Microbiol. 2019, 50, 85–97. [Google Scholar] [CrossRef]
- Andronov, E.; Petrova, S.; Pinaev, A.; Pershina, E.; Rakhimgalieva, S.Z.; Akhmedenov, K.; Gorobets, A.; Sergaliev, N.K. Analysis of the structure of microbial community in soils with different degrees of salinization using t-rflp and real-time pcr techniques. Eurasian Soil Sci. 2012, 45, 147–156. [Google Scholar] [CrossRef]
- Bouasria, A.; Mustafa, T.; De Bello, F.; Zinger, L.; Lemperiere, G.; Geremia, R.A.; Choler, P. Changes in root-associated microbial communities are determined by species-specific plant growth responses to stress and disturbance. Eur. J. Soil Biol. 2012, 52, 59–66. [Google Scholar] [CrossRef]
- Fitzpatrick, C.R.; Copeland, J.; Wang, P.W.; Guttman, D.S.; Kotanen, P.M.; Johnson, M.T. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA 2018, 115, E1157–E1165. [Google Scholar] [CrossRef]
- Bauer, P.S. Dissipative dynamical systems. Proc. Natl. Acad. Sci. USA 1931, 17, 311–314. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R.; Collins, S.L.; Delgado-Baquerizo, M.; Hamonts, K.; Pockman, W.T.; Sinsabaugh, R.L.; Smith, M.D.; Knapp, A.K.; Power, S.A. Drought consistently alters the composition of soil fungal and bacterial communities in grasslands from two continents. Glob. Chang. Biol. 2018, 24, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Schimel, J.P. Life in dry soils: Effects of drought on soil microbial communities and processes. Annu. Rev. Evol. Syst. 2018, 49, 409–432. [Google Scholar] [CrossRef]
- Voesenek, L.; Sasidharan, R. Ethylene–and oxygen signalling–drive plant survival during flooding. Plant Biol. 2013, 15, 426–435. [Google Scholar] [CrossRef] [PubMed]
- Gamalero, E.; Glick, B.R. Bacterial modulation of plant ethylene levels. Plant Physiol. 2015, 169, 13–22. [Google Scholar] [CrossRef]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-aminocyclopropane-1-carboxylic acid (acc) deaminase-containing rhizobacteria protect ocimum sanctum plants during waterlogging stress via reduced ethylene generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef]
- Mirmohammadi, S.; Yazdani, J.; Etemadinejad, S.; Asgarinejad, H. A cross-sectional study on work-related musculoskeletal disorders and associated risk factors among hospital health cares. Procedia Manuf. 2015, 3, 4528–4534. [Google Scholar] [CrossRef]
- Hamonts, K.; Clough, T.J.; Stewart, A.; Clinton, P.W.; Richardson, A.E.; Wakelin, S.A.; O’callaghan, M.; Condron, L.M. Effect of nitrogen and waterlogging on denitrifier gene abundance, community structure and activity in the rhizosphere of wheat. FEMS Microbiol. Ecol. 2013, 83, 568–584. [Google Scholar] [CrossRef]
- Laanbroek, H. Bacterial cycling of minerals that affect plant growth in waterlogged soils: A review. Aquat. Bot. 1990, 38, 109–125. [Google Scholar] [CrossRef]
- Noll, M.; Matthies, D.; Frenzel, P.; Derakshani, M.; Liesack, W. Succession of bacterial community structure and diversity in a paddy soil oxygen gradient. Environ. Microbiol. 2005, 7, 382–395. [Google Scholar] [CrossRef]
- Whitman, W.B.; Rainey, F.; Kämpfer, P.; Trujillo, M.; Chun, J.; DeVos, P.; Hedlund, B.; Dedysh, S.; Nedashkovskaya, O. Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons, Ltd.: Chichester, UK, 2016; pp. 1–39. [Google Scholar]
- Kumar, A.; Verma, J.P. Does plant—microbe interaction confer stress tolerance in plants: A review? Microbiol. Res. 2018, 207, 41–52. [Google Scholar] [CrossRef]
- Chirakkara, R.A.; Cameselle, C.; Reddy, K.R. Assessing the applicability of phytoremediation of soils with mixed organic and heavy metal contaminants. Rev. Environ. Sci. Bio/Technol. 2016, 15, 299–326. [Google Scholar] [CrossRef]
- Verma, J.P.; Yadav, J.; Tiwari, K.N.; Kumar, A. Effect of indigenous mesorhizobium spp. And plant growth promoting rhizobacteria on yields and nutrients uptake of chickpea (Cicer arietinum L.) under sustainable agriculture. Ecol. Eng. 2013, 51, 282–286. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, L.-H.; Yang, J.-C.; Liu, H.; Dai, J.-L. Health risk to residents and stimulation to inherent bacteria of various heavy metals in soil. Sci. Total Environ. 2015, 508, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Fatnassi, I.C.; Chiboub, M.; Saadani, O.; Jebara, M.; Jebara, S.H. Impact of dual inoculation with rhizobium and pgpr on growth and antioxidant status of Vicia faba L. under copper stress. C. R. Biol. 2015, 338, 241–254. [Google Scholar] [CrossRef]
- Alam, M.A.; Seetharam, K.; Zaidi, P.H.; Dinesh, A.; Vinayan, M.T.; Nath, U.K. Dissecting heat stress tolerance in tropical maize (Zea mays L.). Field Crops Res. 2017, 204, 110–119. [Google Scholar] [CrossRef]
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Yadav, J.; Verma, J.P.; Jaiswal, D.K.; Kumar, A. Evaluation of pgpr and different concentration of phosphorus level on plant growth, yield and nutrient content of rice (Oryza sativa). Ecol. Eng. 2014, 62, 123–128. [Google Scholar]
- Jiang, Q.-Y.; Zhuo, F.; Long, S.-H.; Zhao, H.-D.; Yang, D.-J.; Ye, Z.-H.; Li, S.-S.; Jing, Y.-X. Can arbuscular mycorrhizal fungi reduce cd uptake and alleviate cd toxicity of lonicera japonica grown in cd-added soils? Sci. Rep. 2016, 6, 21805. [Google Scholar] [CrossRef]
- Hilker, M.; Schwachtje, J.; Baier, M.; Balazadeh, S.; Bäurle, I.; Geiselhardt, S.; Hincha, D.K.; Kunze, R.; Mueller-Roeber, B.; Rillig, M.C. Priming and memory of stress responses in organisms lacking a nervous system. Biol. Rev. 2016, 91, 1118–1133. [Google Scholar] [CrossRef]
- Xu, Z.Z.; Zhou, G.S. Combined effects of water stress and high temperature on photosynthesis, nitrogen metabolism and lipid peroxidation of a perennial grass leymus chinensis. Planta 2006, 224, 1080–1090. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Jia, B.; Zhou, G. Elevated-co2 response of stomata and its dependence on environmental factors. Front. Plant Sci. 2016, 7, 657. [Google Scholar] [CrossRef] [PubMed]
- Reise, S.P.; Waller, N.G. Item response theory and clinical measurement. Annu. Rev. Clin. Psychol. 2009, 5, 27–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.; Maiti, S.K. Natural mycorrhizal colonization in tree species growing on the reclaimed coalmine overburden dumps: Case study from jharia coalfields, india. Energy Dev. 2010, 3, 761–770. [Google Scholar]
- Thomashow, M.F. Molecular basis of plant cold acclimation: Insights gained from studying the cbf cold response pathway. Plant Physiol. 2010, 154, 571–577. [Google Scholar] [CrossRef]
- Prasch, C.M.; Sonnewald, U. Simultaneous application of heat, drought, and virus to arabidopsis plants reveals significant shifts in signaling networks. Plant Physiol. 2013, 162, 1849–1866. [Google Scholar] [CrossRef]
- Meena, K.K.; Sorty, A.M.; Bitla, U.M.; Choudhary, K.; Gupta, P.; Pareek, A.; Singh, D.P.; Prabha, R.; Sahu, P.K.; Gupta, V.K. Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Front. Plant Sci. 2017, 8, 172. [Google Scholar] [CrossRef]
- Glick, B.R.; Gamalero, E. Recent developments in the study of plant microbiomes. Microorganisms 2021, 9, 1533. [Google Scholar] [CrossRef]
- Wei, F.; Feng, H.; Zhang, D.; Feng, Z.; Zhao, L.; Zhang, Y.; Deakin, G.; Peng, J.; Zhu, H.; Xu, X. Composition of rhizosphere microbial communities associated with healthy and verticillium wilt diseased cotton plants. Front. Microbiol. 2021, 12, 618169. [Google Scholar] [CrossRef]
- Lazcano, C.; Boyd, E.; Holmes, G.; Hewavitharana, S.; Pasulka, A.; Ivors, K. The rhizosphere microbiome plays a role in the resistance to soil-borne pathogens and nutrient uptake of strawberry cultivars under field conditions. Sci. Rep. 2021, 11, 3188. [Google Scholar] [CrossRef]
- Mavrodi, D.V.; Mavrodi, O.V.; Elbourne, L.D.; Tetu, S.; Bonsall, R.F.; Parejko, J.; Yang, M.; Paulsen, I.T.; Weller, D.M.; Thomashow, L.S. Long-term irrigation affects the dynamics and activity of the wheat rhizosphere microbiome. Front. Plant Sci. 2018, 9, 345. [Google Scholar] [CrossRef]
- Epihov, D.Z.; Saltonstall, K.; Batterman, S.A.; Hedin, L.O.; Hall, J.S.; van Breugel, M.; Leake, J.R.; Beerling, D.J. Legume–microbiome interactions unlock mineral nutrients in regrowing tropical forests. Proc. Natl. Acad. Sci. USA 2021, 118, e2022241118. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, P.A.; Rothballer, M.; Chowdhury, S.P.; Nussbaumer, T.; Gutjahr, C.; Falter-Braun, P. Systems biology of plant-microbiome interactions. Mol. Plant 2019, 12, 804–821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vorholt, J. Microbial life in the phyllosphere. Nature reviews microbiology. Seccondary Microbial life in the phyllosphere. Nat. Rev. Microbiol. 2012, 10, 820–840. [Google Scholar]
- Zarraonaindia, I.; Owens, S.M.; Weisenhorn, P.; West, K.; Hampton-Marcell, J.; Lax, S.; Bokulich, N.A.; Mills, D.A.; Martin, G.; Taghavi, S. The soil microbiome influences grapevine-associated microbiota. mBio 2015, 6, e02527-14. [Google Scholar] [CrossRef] [PubMed]
- Zgadzaj, R.; Thiergart, T.; Bozsóki, Z.; Garrido-Oter, R.; Radutoiu, S.; Schulze-Lefert, P. Lotus japonicus symbiosis signaling genes and their role in the establishment of root-associated bacterial and fungal communities. BioRxiv 2019, 1, 547687. [Google Scholar]
- Schlaeppi, K.; Dombrowski, N.; Oter, R.G.; van Themaat, E.V.L.; Schulze-Lefert, P. Quantitative divergence of the bacterial root microbiota in arabidopsis thaliana relatives. Proc. Natl. Acad. Sci. USA 2014, 111, 585–592. [Google Scholar] [CrossRef]
- Peiffer, J.A.; Spor, A.; Koren, O.; Jin, Z.; Tringe, S.G.; Dangl, J.L.; Buckler, E.S.; Ley, R.E. Diversity and heritability of the maize rhizosphere microbiome under field conditions. Proc. Natl. Acad. Sci. USA 2013, 110, 6548–6553. [Google Scholar] [CrossRef]
- Walters, W.A.; Jin, Z.; Youngblut, N.; Wallace, J.G.; Sutter, J.; Zhang, W.; González-Peña, A.; Peiffer, J.; Koren, O.; Shi, Q. Large-scale replicated field study of maize rhizosphere identifies heritable microbes. Proc. Natl. Acad. Sci. USA 2018, 115, 7368–7373. [Google Scholar] [CrossRef]
- Haney, C.H.; Samuel, B.S.; Bush, J.; Ausubel, F.M. Associations with rhizosphere bacteria can confer an adaptive advantage to plants. Nat. Plants 2015, 1, 15051. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Sorty, A.M.; Meena, K.K.; Choudhary, K.; Bitla, U.M.; Minhas, P.; Krishnani, K. Effect of plant growth promoting bacteria associated with halophytic weed (Psoralea corylifolia L.) on germination and seedling growth of wheat under saline conditions. Appl. Biochem. Biotechnol. 2016, 180, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Mrowka, M.; Jöbges, M.; Berding, G.; Schimke, N.; Shing, M.; Odin, P. Computerized movement analysis and beta-cit-spect in patients with restless legs syndrome. J. Neural Transm. 2005, 112, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Omar, M.; Osman, M.; Kasim, W.; Abd El-Daim, I. Salinity and Water Stress; Springer: Berlin/Heidelberg, Germany, 2009; pp. 133–147. [Google Scholar]
- Tittabutr, P.; Piromyou, P.; Longtonglang, A.; Noisa-Ngiam, R.; Boonkerd, N.; Teaumroong, N. Alleviation of the effect of environmental stresses using co-inoculation of mungbean by bradyrhizobium and rhizobacteria containing stress-induced acc deaminase enzyme. Soil Sci. Plant Nutr. 2013, 59, 559–571. [Google Scholar] [CrossRef]
- Meena, K.K.; Kumar, M.; Kalyuzhnaya, M.G.; Yandigeri, M.S.; Singh, D.P.; Saxena, A.K.; Arora, D.K. Epiphytic pink-pigmented methylotrophic bacteria enhance germination and seedling growth of wheat (Triticum aestivum) by producing phytohormone. Antonie Van Leeuwenhoek 2012, 101, 777–786. [Google Scholar] [CrossRef]
- Oliveira, C.; Alves, V.; Marriel, I.; Gomes, E.; Scotti, M.; Carneiro, N.; Guimaraes, C.; Schaffert, R.; Sá, N. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the brazilian cerrado biome. Soil Biol. Biochem. 2009, 41, 1782–1787. [Google Scholar] [CrossRef]
- Kucera, J.; Bouchal, P.; Cerna, H.; Potesil, D.; Janiczek, O.; Zdrahal, Z.; Mandl, M. Kinetics of anaerobic elemental sulfur oxidation by ferric iron in acidithiobacillus ferrooxidans and protein identification by comparative 2-de-ms/ms. Antonie Van Leeuwenhoek 2012, 101, 561–573. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A. Dose-dependent response of trichoderma harzianum in improving drought tolerance in rice genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef]
- Ahmad, F.; Ahmad, I.; Khan, M.S. Indole acetic acid production by the indigenous isolates of azotobacter and fluorescent pseudomonas in the presence and absence of tryptophan. Turk. J. Biol. 2005, 29, 29–34. [Google Scholar]
- Brotman, Y.; Landau, U.; Cuadros-Inostroza, Á.; Takayuki, T.; Fernie, A.R.; Chet, I.; Viterbo, A.; Willmitzer, L. Trichoderma-plant root colonization: Escaping early plant defense responses and activation of the antioxidant machinery for saline stress tolerance. PLoS Pathog. 2013, 9, e1003221. [Google Scholar] [CrossRef]
- Chang, P.; Gerhardt, K.E.; Huang, X.-D.; Yu, X.-M.; Glick, B.R.; Gerwing, P.D.; Greenberg, B.M. Plant growth-promoting bacteria facilitate the growth of barley and oats in salt-impacted soil: Implications for phytoremediation of saline soils. Int. J. Phytoremediat. 2014, 16, 1133–1147. [Google Scholar] [CrossRef] [PubMed]
- Simmons, C.; Reddy, A.; Simmons, B.; Singer, S.; VanderGheynst, J. Effect of inoculum source on the enrichment of microbial communities on two lignocellulosic bioenergy crops under thermophilic and high-solids conditions. J. Appl. Microbiol. 2014, 117, 1025–1034. [Google Scholar] [CrossRef] [PubMed]
- Naveed, M.; Hussain, M.B. Zahir a. Zahir, birgit mitter & angela sessitsch. Plant Growth Regul. 2014, 73, 121–131. [Google Scholar]
- Kaushal, M.; Wani, S.P. Plant-growth-promoting rhizobacteria: Drought stress alleviators to ameliorate crop production in drylands. Ann. Microbiol. 2016, 66, 35–42. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Baltruschat, H.; Fodor, J.; Harrach, B.D.; Niemczyk, E.; Barna, B.; Gullner, G.; Janeczko, A.; Kogel, K.H.; Schäfer, P.; Schwarczinger, I. Salt tolerance of barley induced by the root endophyte piriformospora indica is associated with a strong increase in antioxidants. New Phytol. 2008, 180, 501–510. [Google Scholar] [CrossRef]
- Suarez, C.; Cardinale, M.; Ratering, S.; Steffens, D.; Jung, S.; Montoya, A.M.Z.; Geissler-Plaum, R.; Schnell, S. Plant growth-promoting effects of hartmannibacter diazotrophicus on summer barley (Hordeum vulgare L.) under salt stress. Appl. Soil Ecol. 2015, 95, 23–30. [Google Scholar] [CrossRef]
- Vinayarani, G.; Prakash, H. Growth promoting rhizospheric and endophytic bacteria from Curcuma longa L. as biocontrol agents against rhizome rot and leaf blight diseases. Plant Pathol. J. 2018, 34, 218. [Google Scholar] [CrossRef]
- Vandana, U.K.; Rajkumari, J.; Singha, L.P.; Satish, L.; Alavilli, H.; Sudheer, P.D.; Chauhan, S.; Ratnala, R.; Satturu, V.; Mazumder, P.B. The endophytic microbiome as a hotspot of synergistic interactions, with prospects of plant growth promotion. Biology 2021, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.; Qin, C.; Ahanger, M.A.; Raza, S.; Khan, M.I.; Ashraf, M.; Ahmed, N.; Zhang, L. Role of arbuscular mycorrhizal fungi in plant growth regulation: Implications in abiotic stress tolerance. Front. Plant Sci. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Zhang, H.; Kim, M.-S.; Sun, Y.; Dowd, S.E.; Shi, H.; Paré, P.W. Soil bacteria confer plant salt tolerance by tissue-specific regulation of the sodium transporter hkt1. Mol. Plant-Microbe Interact. 2008, 21, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, A.; Kumari, S.; Jain, S.; Varma, A.; Tuteja, N.; Choudhary, D.K. Pgpr-mediated expression of salt tolerance gene in soybean through volatiles under sodium nitroprusside. J. Basic Microbiol. 2016, 56, 1274–1288. [Google Scholar] [CrossRef] [PubMed]
- Jha, Y.; Sablok, G.; Subbarao, N.; Sudhakar, R.; Fazil, M.T.; Subramanian, R.; Squartini, A.; Kumar, S. Bacterial-induced expression of rab18 protein in orzya sativa salinity stress and insights into molecular interaction with gtp ligand. J. Mol. Recognit. 2014, 27, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Singh, S. A review on possible elicitor molecules of cyanobacteria: Their role in improving plant growth and providing tolerance against biotic or abiotic stress. J. Appl. Microbiol. 2014, 117, 1221–1244. [Google Scholar] [CrossRef] [PubMed]
- Marulanda, A.; Azcón, R.; Chaumont, F.; Ruiz-Lozano, J.M.; Aroca, R. Regulation of plasma membrane aquaporins by inoculation with a bacillus megaterium strain in maize (Zea mays L.) plants under unstressed and salt-stressed conditions. Planta 2010, 232, 533–543. [Google Scholar] [CrossRef] [PubMed]
- Ait Barka, E.; Nowak, J.; Clément, C. Enhancement of chilling resistance of inoculated grapevine plantlets with a plant growth-promoting rhizobacterium, burkholderia phytofirmans strain psjn. Appl. Environ. Microbiol. 2006, 72, 7246–7252. [Google Scholar] [CrossRef] [PubMed]
- Del Amor, F.M.; Cuadra-Crespo, P. Plant growth-promoting bacteria as a tool to improve salinity tolerance in sweet pepper. Funct. Plant Biol. 2011, 39, 82–90. [Google Scholar] [CrossRef]
- Dardanelli, M.S.; de Cordoba, F.J.F.; Espuny, M.R.; Carvajal, M.A.R.; Díaz, M.E.S.; Serrano, A.M.G.; Okon, Y.; Megías, M. Effect of azospirillum brasilense coinoculated with rhizobium on phaseolus vulgaris flavonoids and nod factor production under salt stress. Soil Biol. Biochem. 2008, 40, 2713–2721. [Google Scholar] [CrossRef]
- Naveed, M.; Mitter, B.; Reichenauer, T.G.; Wieczorek, K.; Sessitsch, A. Increased drought stress resilience of maize through endophytic colonization by Burkholderia phytofirmans psjn and Enterobacter sp. FD17. Environ. Exp. Bot. 2014, 97, 30–39. [Google Scholar] [CrossRef]
- Cho, S.M.; Kang, B.R.; Han, S.H.; Anderson, A.J.; Park, J.-Y.; Lee, Y.-H.; Cho, B.H.; Yang, K.-Y.; Ryu, C.-M.; Kim, Y.C. 2r, 3r-butanediol, a bacterial volatile produced by pseudomonas chlororaphis o6, is involved in induction of systemic tolerance to drought in arabidopsis thaliana. Mol. Plant-Microbe Interact. 2008, 21, 1067–1075. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; el-daim-Allah, E.F.; Alqarawi, A.; John, R.; Egamberdieva, D.; Gucel, S. Role of trichoderma harzianum in mitigating NaCl stress in indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Jin, C.; Yang, J.; Liu, Q.; Wu, S.; Li, D.; Guan, Y.; Cai, Y. Prenatal and lactational lead exposure enhanced oxidative stress and altered apoptosis status in offspring rats’ hippocampus. Biol. Trace Elem. Res. 2013, 151, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Kloepper, J.W.; Schroth, M.N. Relationship of in vitro antibiosis of plant growth promoting rhizobacteria to plant growth and the displacement of root microflora. Phytopathology 1981, 71, 1020–1024. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, Q.; Ali, S.; Arif, M.S.; Hussain, S.; Rizvi, H. Influence of pseudomonas aeruginosa as pgpr on oxidative stress tolerance in wheat under zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef]
- Raymond, J.; Siefert, J.L.; Staples, C.R.; Blankenship, R.E. The natural history of nitrogen fixation. Mol. Biol. Evol. 2004, 21, 541–554. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (pgpr): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef] [PubMed]
- Giordano, W.; Hirsch, A.M. The expression of maexp1, a melilotus alba expansin gene, is upregulated during the sweetclover-sinorhizobium meliloti interaction. Mol. Plant-Microbe Interact. 2004, 17, 613–622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruto, M.; Prigent-Combaret, C.; Muller, D.; Moënne-Loccoz, Y. Analysis of genes contributing to plant-beneficial functions in plant growth-promoting rhizobacteria and related proteobacteria. Sci. Rep. 2014, 4, 6261. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A.; Oves, M. Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ. Chem. Lett. 2009, 7, 1–19. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.; Ahemad, M.; Oves, M. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol. Immunol. Hung. 2009, 56, 263–284. [Google Scholar] [CrossRef]
- Guo, J.K.; Ding, Y.Z.; Feng, R.W.; Wang, R.G.; Xu, Y.M.; Chen, C.; Wei, X.L.; Chen, W.M. Burkholderia metalliresistens sp. Nov. a multiple metal-resistant and phosphate-solubilising species isolated from heavy metal-polluted soil in southeast china. Antonie Van Leeuwenhoek 2015, 107, 1591–1598. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, U.D.; Saha, C.; Maiti, M.; Lahiri, S.; Ghosh, S.; Seal, A.; MitraGhosh, M. Root associated iron oxidizing bacteria increase phosphate nutrition and influence root to shoot partitioning of iron in tolerant plant typha angustifolia. Plant Soil 2014, 381, 279–295. [Google Scholar] [CrossRef]
- El-Tarabily, K.A.; ElBaghdady, K.Z.; AlKhajeh, A.S.; Ayyash, M.M.; Aljneibi, R.S.; El-Keblawy, A.; AbuQamar, S.F. Polyamine-producing actinobacteria enhance biomass production and seed yield in Salicornia bigelovii. Biol. Fertil. Soils 2020, 56, 499–519. [Google Scholar] [CrossRef]
- Neubauer, U.; Furrer, G.; Schulin, R. Heavy metal sorption on soil minerals affected by the siderophore desferrioxamine b: The role of fe (iii)(hydr) oxides and dissolved fe (iii). Eur. J. Soil Sci. 2002, 53, 45–55. [Google Scholar] [CrossRef]
- Mathew, B.T.; Torky, Y.; Amin, A.; Mourad, A.H.; Ayyash, M.M.; El-Keblawy, A.; Hilal-Alnaqbi, A.; AbuQamar, S.F.; El-Tarabily, K.A. Halotolerant marine rhizosphere-competent actinobacteria promote Salicornia bigelovii growth and seed production using seawater irrigation. Front. Microbiol. 2020, 11, 552. [Google Scholar] [CrossRef]
- Patten, C.L.; Glick, B.R. Bacterial biosynthesis of indole-3-acetic acid. Can. J. Microbiol. 1996, 42, 207–220. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth-promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 963401. [Google Scholar] [CrossRef] [Green Version]
- Camerini, S.; Senatore, B.; Lonardo, E.; Imperlini, E.; Bianco, C.; Moschetti, G.; Rotino, G.L.; Campion, B.; Defez, R. Introduction of a novel pathway for iaa biosynthesis to rhizobia alters vetch root nodule development. Arch. Microbiol. 2008, 190, 67–77. [Google Scholar] [CrossRef]
- Arshad, M.; Saleem, M.; Hussain, S. Perspectives of bacterial acc deaminase in phytoremediation. TRENDS Biotechnol. 2007, 25, 356–362. [Google Scholar] [CrossRef]
- Kapoor, R.; Evelin, H.; Mathur, P.; Giri, B. Plant Acclimation to Environmental Stress; Springer: Berlin/Heidelberg, Germany, 2013; pp. 359–401. [Google Scholar]
- Balestrini, R.; Lumini, E. Focus on mycorrhizal symbioses. Appl. Soil Ecol. 2018, 123, 299–304. [Google Scholar] [CrossRef]
- Posta, K.; Duc, N.H. Benefits of arbuscular mycorrhizal fungi application to crop production under water scarcity. In Drought Detect Solut; InTechOpen: Rijeka, Croatia, 2020. [Google Scholar]
- Ouledali, S.; Ennajeh, M.; Ferrandino, A.; Khemira, H.; Schubert, A.; Secchi, F. Influence of arbuscular mycorrhizal fungi inoculation on the control of stomata functioning by abscisic acid (aba) in drought-stressed olive plants. S. Afr. J. Bot. 2019, 121, 152–158. [Google Scholar] [CrossRef]
- Laxa, M.; Liebthal, M.; Telman, W.; Chibani, K.; Dietz, K.-J. The role of the plant antioxidant system in drought tolerance. Antioxidants 2019, 8, 94. [Google Scholar] [CrossRef]
- Bao, X.; Wang, Y.; Olsson, P.A. Arbuscular mycorrhiza under water-carbon-phosphorus exchange between rice and arbuscular mycorrhizal fungi under different flooding regimes. Soil Biol. Biochem. 2019, 129, 169–177. [Google Scholar] [CrossRef]
- Wang, Y.; Qiu, Q.; Yang, Z.; Hu, Z.; Tam, N.F.-Y.; Xin, G. Arbuscular mycorrhizal fungi in two mangroves in south china. Plant Soil 2010, 331, 181–191. [Google Scholar] [CrossRef]
- Estrada, B.; Aroca, R.; Maathuis, F.J.; Barea, J.M.; Ruiz-Lozano, J.M. Arbuscular mycorrhizal fungi native from a m editerranean saline area enhance maize tolerance to salinity through improved ion homeostasis. Plant Cell Environ. 2013, 36, 1771–1782. [Google Scholar] [CrossRef]
- Al-Karaki, G.N. Nursery inoculation of tomato with arbuscular mycorrhizal fungi and subsequent performance under irrigation with saline water. Sci. Hortic. 2006, 109, 1–7. [Google Scholar] [CrossRef]
- Talaat, N.B.; Shawky, B.T. Protective effects of arbuscular mycorrhizal fungi on wheat (Triticum aestivum L.) plants exposed to salinity. Environ. Exp. Bot. 2014, 98, 20–31. [Google Scholar] [CrossRef]
- Vurukonda, S.S.K.P.; Vardharajula, S.; Shrivastava, M.; SkZ, A. Enhancement of drought stress tolerance in crops by plant growth promoting rhizobacteria. Microbiol. Res. 2016, 184, 13–24. [Google Scholar] [CrossRef]
- Miliute, I.; Buzaite, O.; Baniulis, D.; Stanys, V. Bacterial endophytes in agricultural crops and their role in stress tolerance: A review. Zemdirbyste-Agriculture 2015, 102, 465–478. [Google Scholar] [CrossRef]
- Mitter, B.; Petric, A.; Shin, M.W.; Chain, P.S.; Hauberg-Lotte, L.; Reinhold-Hurek, B.; Nowak, J.; Sessitsch, A. Comparative genome analysis of burkholderia phytofirmans psjn reveals a wide spectrum of endophytic lifestyles based on interaction strategies with host plants. Front. Plant Sci. 2013, 4, 120. [Google Scholar] [CrossRef] [PubMed]
- Sgroy, V.; Cassán, F.; Masciarelli, O.; Del Papa, M.F.; Lagares, A.; Luna, V. Isolation and characterization of endophytic plant growth-promoting (pgpb) or stress homeostasis-regulating (pshb) bacteria associated to the halophyte prosopis strombulifera. Appl. Microbiol. Biotechnol. 2009, 85, 371–381. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.L.; Hussain, J.; Al-Harrasi, A.; Al-Rawahi, A.; Lee, I.-J. Endophytic fungi: Resource for gibberellins and crop abiotic stress resistance. Crit. Rev. Biotechnol. 2015, 35, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Singh, L.P.; Gill, S.S.; Tuteja, N. Unraveling the role of fungal symbionts in plant abiotic stress tolerance. Plant Signal. Behav. 2011, 6, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.J.; Henson, J.; Van Volkenburgh, E.; Hoy, M.; Wright, L.; Beckwith, F.; Kim, Y.-O.; Redman, R.S. Stress tolerance in plants via habitat-adapted symbiosis. ISME J. 2008, 2, 404–416. [Google Scholar] [CrossRef] [PubMed]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Sicher, R.C.; Kim, M.S.; Kim, S.-H.; Strem, M.D.; Melnick, R.L.; Bailey, B.A. The beneficial endophyte trichoderma hamatum isolate dis 219b promotes growth and delays the onset of the drought response in theobroma cacao. J. Exp. Bot. 2009, 60, 3279–3295. [Google Scholar] [CrossRef] [PubMed]
- Mastouri, F.; Björkman, T.; Harman, G.E. Seed treatment with trichoderma harzianum alleviates biotic, abiotic, and physiological stresses in germinating seeds and seedlings. Phytopathology 2010, 100, 1213–1221. [Google Scholar] [CrossRef] [Green Version]
- Sziderics, A.; Rasche, F.; Trognitz, F.; Sessitsch, A.; Wilhelm, E. Bacterial endophytes contribute to abiotic stress adaptation in pepper plants (Capsicum annuum L.). Can. J. Microbiol. 2007, 53, 1195–1202. [Google Scholar] [CrossRef]
- Mathew, D.C.; Ho, Y.-N.; Gicana, R.G.; Mathew, G.M.; Chien, M.-C.; Huang, C.-C. A rhizosphere-associated symbiont, photobacterium spp. Strain meld1, and its targeted synergistic activity for phytoprotection against mercury. PLoS ONE 2015, 10, e0121178. [Google Scholar]
- Babu, A.G.; Shea, P.J.; Sudhakar, D.; Jung, I.-B.; Oh, B.-T. Potential use of pseudomonas koreensis agb-1 in association with miscanthus sinensis to remediate heavy metal (loid)-contaminated mining site soil. J. Environ. Manag. 2015, 151, 160–166. [Google Scholar] [CrossRef]
- Shen, F.-T.; Yen, J.-H.; Liao, C.-S.; Chen, W.-C.; Chao, Y.-T. Screening of rice endophytic biofertilizers with fungicide tolerance and plant growth-promoting characteristics. Sustainability 2019, 11, 1133. [Google Scholar] [CrossRef]
- Meneses, C.H.; Rouws, L.F.; Simões-Araújo, J.L.; Vidal, M.S.; Baldani, J.I. Exopolysaccharide production is required for biofilm formation and plant colonization by the nitrogen-fixing endophyte gluconacetobacter diazotrophicus. Mol. Plant-Microbe Interact. 2011, 24, 1448–1458. [Google Scholar] [CrossRef]
- Sun, Z.; Song, J.; Xin, X.A.; Xie, X.; Zhao, B. Arbuscular mycorrhizal fungal 14-3-3 proteins are involved in arbuscule formation and responses to abiotic stresses during am symbiosis. Front. Microbiol. 2018, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Moon, Y.-S.; Ali, S. A fruitful decade of bacterial acc deaminase biotechnology: A pragmatic approach towards abiotic stress relief in plants. Theor. Exp. Plant Physiol. 2022, 34, 109–129. [Google Scholar] [CrossRef]
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Al-Harrasi, A.; Lee, I.-J. Indole acetic acid and acc deaminase from endophytic bacteria improves the growth of solanum lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Gupta, G.; Panwar, J.; Jha, P.N. Natural occurrence of pseudomonas aeruginosa, a dominant cultivable diazotrophic endophytic bacterium colonizing Pennisetum glaucum (L.) R. Br. Appl. Soil Ecol. 2013, 64, 252–261. [Google Scholar] [CrossRef]
- Ali, S.; Moon, Y.-S.; Hamayun, M.; Khan, M.A.; Bibi, K.; Lee, I.-J. Pragmatic role of microbial plant biostimulants in abiotic stress relief in crop plants. J. Plant Interact. 2022, 17, 705–718. [Google Scholar] [CrossRef]
- Zaidi, S.; Usmani, S.; Singh, B.R.; Musarrat, J. Significance of bacillus subtilis strain sj-101 as a bioinoculant for concurrent plant growth promotion and nickel accumulation in brassica juncea. Chemosphere 2006, 64, 991–997. [Google Scholar] [CrossRef] [PubMed]
- Shoebitz, M.; Ribaudo, C.M.; Pardo, M.A.; Cantore, M.L.; Ciampi, L.; Cura, J.A. Plant growth promoting properties of a strain of enterobacter ludwigii isolated from lolium perenne rhizosphere. Soil Biol. Biochem. 2009, 41, 1768–1774. [Google Scholar] [CrossRef]
- Cocking, E.C. Endophytic colonization of plant roots by nitrogen-fixing bacteria. Plant Soil 2003, 252, 169–175. [Google Scholar] [CrossRef]
- Gray, E.; Smith, D. Intracellular and extracellular pgpr: Commonalities and distinctions in the plant–bacterium signaling processes. Soil Biol. Biochem. 2005, 37, 395–412. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Lavanya, N.; Muthumeena, B.; Raguchander, T.; Suresh, S.; Samiyappan, R. Pseudomonas fluorescens enhances resistance and natural enemy population in rice plants against leaffolder pest. J. Appl. Entomol. 2008, 132, 469–479. [Google Scholar] [CrossRef]
- Dey, R.; Pal, K.; Bhatt, D.; Chauhan, S. Growth promotion and yield enhancement of peanut (Arachis hypogaea L.) by application of plant growth-promoting rhizobacteria. Microbiol. Res. 2004, 159, 371–394. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Chen, J.; Shim, H.; Bai, Z. New advances in plant growth-promoting rhizobacteria for bioremediation. Environ. Int. 2007, 33, 406–413. [Google Scholar] [CrossRef]
- Emenecker, R.J.; Strader, L.C. Auxin-abscisic acid interactions in plant growth and development. Biomolecules 2020, 10, 281. [Google Scholar] [CrossRef] [PubMed]
- Zahir, Z.A.; Abbas, S.A.; Khalid, M.; Arshad, M. Substrate dependent microbially derived plant hormones for improving growth of maize seedlings. Pak. J. Biol. Sci. 2000, 3, 289–291. [Google Scholar] [CrossRef]
- Burd, G.I.; Dixon, D.G.; Glick, B.R. Plant growth-promoting bacteria that decrease heavy metal toxicity in plants. Can. J. Microbiol. 2000, 46, 237–245. [Google Scholar] [CrossRef]
- Thakuria, D.; Talukdar, N.; Goswami, C.; Hazarika, S.; Boro, R.; Khan, M. Characterization and screening of bacteria from rhizosphere of rice grown in acidic soils of assam. Curr. Sci. 2004, 86, 978–985. [Google Scholar]
- Chandra, S.; Choure, K.; Dubey, R.C.; Maheshwari, D.K. Rhizosphere competent mesorhizobiumloti mp6 induces root hair curling, inhibits sclerotinia sclerotiorum and enhances growth of indian mustard (Brassica campestris). Braz. J. Microbiol. 2007, 38, 124–130. [Google Scholar] [CrossRef]
- Muratova, A.Y.; Turkovskaya, O.; Antonyuk, L.; Makarov, O.; Pozdnyakova, L.; Ignatov, V. Oil-oxidizing potential of associative rhizobacteria of the genus azospirillum. Microbiology 2005, 74, 210–215. [Google Scholar] [CrossRef]
- Beneduzi, A.; Peres, D.; Vargas, L.K.; Bodanese-Zanettini, M.H.; Passaglia, L.M.P. Evaluation of genetic diversity and plant growth promoting activities of nitrogen-fixing bacilli isolated from rice fields in south brazil. Appl. Soil Ecol. 2008, 39, 311–320. [Google Scholar] [CrossRef]
- Roesti, D.; Gaur, R.; Johri, B.; Imfeld, G.; Sharma, S.; Kawaljeet, K.; Aragno, M. Plant growth stage, fertiliser management and bio-inoculation of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria affect the rhizobacterial community structure in rain-fed wheat fields. Soil Biol. Biochem. 2006, 38, 1111–1120. [Google Scholar]
- Dimkpa, C.; Svatoš, A.; Merten, D.; Büchel, G.; Kothe, E. Hydroxamate siderophores produced by streptomyces acidiscabies e13 bind nickel and promote growth in cowpea (Vigna unguiculata L.) under nickel stress. Can. J. Microbiol. 2008, 54, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bano, A.; Ali, S.; Babar, M.A. Crosstalk amongst phytohormones from planta and pgpr under biotic and abiotic stresses. Plant Growth Regul. 2020, 90, 189–203. [Google Scholar] [CrossRef]
- Mathimaran, N.; Jegan, S.; Thimmegowda, M.N.; Prabavathy, V.R.; Yuvaraj, P.; Kathiravan, R.; Sivakumar, M.N.; Manjunatha, B.N.; Bhavitha, N.C.; Sathish, A. Intercropping transplanted pigeon pea with finger millet: Arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria boost yield while reducing fertilizer input. Front. Sustain. Food Syst. 2020, 4, 88. [Google Scholar] [CrossRef]
- Bakhshandeh, E.; Gholamhosseini, M.; Yaghoubian, Y.; Pirdashti, H. Plant growth promoting microorganisms can improve germination, seedling growth and potassium uptake of soybean under drought and salt stress. Plant Growth Regul. 2020, 90, 123–136. [Google Scholar] [CrossRef]
- Ndeddy Aka, R.J.; Babalola, O.O. Effect of bacterial inoculation of strains of Pseudomonas aeruginosa, Alcaligenes feacalis and Bacillus subtilis on germination, growth and heavy metal (cd, cr, and ni) uptake of Brassica juncea. Int. J. Phytoremediat. 2016, 18, 200–209. [Google Scholar] [CrossRef]
- Kim, A.-Y.; Shahzad, R.; Kang, S.-M.; Seo, C.-W.; Park, Y.-G.; Park, H.-J.; Lee, I.-J. Iaa-producing klebsiella variicola ay13 reprograms soybean growth during flooding stress. J. Crop Sci. Biotechnol. 2017, 20, 235–242. [Google Scholar] [CrossRef]
- Asghari, B.; Khademian, R.; Sedaghati, B. Plant growth promoting rhizobacteria (pgpr) confer drought resistance and stimulate biosynthesis of secondary metabolites in pennyroyal (Mentha pulegium L.) under water shortage condition. Sci. Hortic. 2020, 263, 109132. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Kavino, M.; Raguchander, T.; Subbian, P.; Samiyappan, R. Plant growth promoting bacteria enhance water stress resistance in green gram plants. Acta Physiol. Plant. 2011, 33, 203–209. [Google Scholar] [CrossRef]
- Samaddar, S.; Chatterjee, P.; Choudhury, A.R.; Ahmed, S.; Sa, T. Interactions between pseudomonas spp. and their role in improving the red pepper plant growth under salinity stress. Microbiol. Res. 2019, 219, 66–73. [Google Scholar] [PubMed]
- Lopes, M.; Dias-Filho, M.; Castro, T.; Silva, G. Light and plant growth-promoting rhizobacteria effects on brachiaria brizantha growth and phenotypic plasticity to shade. Grass Forage Sci. 2018, 73, 493–499. [Google Scholar] [CrossRef]
- Czarnes, S.; Mercier, P.E.; Lemoine, D.G.; Hamzaoui, J.; Legendre, L. Impact of soil water content on maize responses to the plant growth-promoting rhizobacterium azospirillum lipoferum crt1. J. Agron. Crop Sci. 2020, 206, 505–516. [Google Scholar] [CrossRef]
- Fu, J.; Luo, Y.; Sun, P.; Gao, J.; Zhao, D.; Yang, P.; Hu, T. Effects of shade stress on turfgrasses morphophysiology and rhizosphere soil bacterial communities. BMC Plant Biol. 2020, 20, 92. [Google Scholar] [CrossRef]
- Müller, D.B.; Vogel, C.; Bai, Y.; Vorholt, J.A. The plant microbiota: Systems-level insights and perspectives. Annu. Rev. Genet. 2016, 50, 211–234. [Google Scholar] [CrossRef]
- Vickers, N.J. Animal communication: When I’m calling you, will you answer too? Curr. Biol. 2017, 27, 713–715. [Google Scholar] [CrossRef]
- Morton, A.G. History of Botanical Science. An Account of the Development of Botany from Ancient Times to the Present Day; Academic Press: Cambridge, MA, USA, 1981. [Google Scholar]
- Brown, M.E. Seed and root bacterization. Annu. Rev. Phytopathol. 1974, 12, 181–197. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—current knowledge and future directions. Front. Plant Sci. 2017, 8, 1617–1635. [Google Scholar] [CrossRef]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Gyaneshwar, P.; Hirsch, A.M.; Moulin, L.; Chen, W.-M.; Elliott, G.N.; Bontemps, C.; Estrada-de Los Santos, P.; Gross, E.; dos Reis Jr, F.B.; Sprent, J.I. Legume-nodulating betaproteobacteria: Diversity, host range, and future prospects. Mol. Plant-Microbe Interact. 2011, 24, 1276–1288. [Google Scholar] [CrossRef] [PubMed]
- Mus, F.; Crook, M.B.; Garcia, K.; Garcia Costas, A.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.; Poole, P.S. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Plant growth-promoting rhizobacteria allow reduced application rates of chemical fertilizers. Microb. Ecol. 2009, 58, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Adesemoye, A.; Torbert, H.; Kloepper, J. Increased plant uptake of nitrogen from 15n-depleted fertilizer using plant growth-promoting rhizobacteria. Appl. Soil Ecol. 2010, 46, 54–58. [Google Scholar] [CrossRef]
- White, J.F.; Chen, Q.; Torres, M.S.; Mattera, R.; Irizarry, I.; Tadych, M.; Bergen, M. Collaboration between grass seedlings and rhizobacteria to scavenge organic nitrogen in soils. AoB Plants 2015, 7, plu093. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Cameron, D.D.; Hodge, A. Resolving the ‘nitrogen paradox’of arbuscular mycorrhizas: Fertilization with organic matter brings considerable benefits for plant nutrition and growth. Plant Cell Environ. 2016, 39, 1683–1690. [Google Scholar] [CrossRef]
- Kertesz, M.A.; Mirleau, P. The role of soil microbes in plant sulphur nutrition. J. Exp. Bot. 2004, 55, 1939–1945. [Google Scholar] [CrossRef]
- Xia, Y.; DeBolt, S.; Dreyer, J.; Scott, D.; Williams, M.A. Characterization of culturable bacterial endophytes and their capacity to promote plant growth from plants grown using organic or conventional practices. Front. Plant Sci. 2015, 6, 490. [Google Scholar] [CrossRef]
- Gupta, M.; Bisht, S.; Singh, B.; Gulati, A.; Tewari, R. Enhanced biomass and steviol glycosides in stevia rebaudiana treated with phosphate-solubilizing bacteria and rock phosphate. Plant Growth Regul. 2011, 65, 449–457. [Google Scholar] [CrossRef]
- da Costa, E.M.; de Lima, W.; Oliveira-Longatti, S.M.; de Souza, F.M. Phosphate-solubilising bacteria enhance oryza sativa growth and nutrient accumulation in an oxisol fertilized with rock phosphate. Ecol. Eng. 2015, 83, 380–385. [Google Scholar] [CrossRef]
- Borgi, M.A.; Saidi, I.; Moula, A.; Rhimi, S.; Rhimi, M. The attractive serratia plymuthica bma1 strain with high rock phosphate-solubilizing activity and its effect on the growth and phosphorus uptake by Vicia faba L. Plants Geomicrobiol. J. 2020, 37, 437–445. [Google Scholar] [CrossRef]
- Selvakumar, G.; Joshi, P.; Suyal, P.; Mishra, P.K.; Joshi, G.K.; Venugopalan, R.; Bisht, J.K.; Bhatt, J.C.; Gupta, H.S. Rock phosphate solubilization by psychrotolerant Pseudomonas spp. And their effect on lentil growth and nutrient uptake under polyhouse conditions. Ann. Microbiol. 2013, 63, 1353–1362. [Google Scholar] [CrossRef]
- Ben Zineb, A.; Trabelsi, D.; Ayachi, I.; Barhoumi, F.; Aroca, R.; Mhamdi, R. Inoculation with elite strains of phosphate-solubilizing bacteria enhances the effectiveness of fertilization with rock phosphates. Geomicrobiol. J. 2020, 37, 22–30. [Google Scholar] [CrossRef]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef]
- Shayanthan, A.; Ordoñez, P.A.C.; Oresnik, I.J. The role of synthetic microbial communities (syncom) in sustainable agriculture. Front. Agron. 2022, 58, 896307. [Google Scholar] [CrossRef]
- Vassilev, N.; Malusà, E.; Neri, D.; Xu, X. Plant Root Interaction with Associated Microbiomes to Improve Plant Resiliency and Crop Biodiversity. Front. Plant Sci. 2021. Available online: https://www.frontiersin.org/research-topics/11355/plant-root-interaction-with-associated-microbiomes-to-improve-plant-resiliency-and-crop-biodiversity (accessed on 12 July 2022). [CrossRef]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef]
- de Souza, R.S.C.; Okura, V.K.; Armanhi, J.S.L.; Jorrín, B.; Lozano, N.; Da Silva, M.J.; González-Guerrero, M.; de Araújo, L.M.; Verza, N.C.; Bagheri, H.C. Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci. Rep. 2016, 6, 28774. [Google Scholar] [CrossRef] [Green Version]
- Mahmud, A.A.; Upadhyay, S.K.; Srivastava, A.K.; Bhojiya, A.A. Biofertilizers: A nexus between soil fertility and crop productivity under abiotic stress. Curr. Res. Environ. Sustain. 2021, 3, 100063. [Google Scholar] [CrossRef]
- Fasusi, O.A.; Cruz, C.; Babalola, O.O. Agricultural sustainability: Microbial biofertilizers in rhizosphere management. Agriculture 2021, 11, 163. [Google Scholar] [CrossRef]
- Roberts, K.G.; Gloy, B.A.; Joseph, S.; Scott, N.R.; Lehmann, J. Life cycle assessment of biochar systems: Estimating the energetic, economic, and climate change potential. Environ. Sci. Technol. 2010, 44, 827–833. [Google Scholar] [CrossRef]
- Dong, W.; Ma, F.; Li, C.; Fu, Z.; Huang, Y.; Liu, J. Evaluation of anti-aging performance of biochar modified asphalt binder. Coatings 2020, 10, 1037. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, A.; Ji, C.; Joseph, S.; Bian, R.; Li, L.; Pan, G.; Paz-Ferreiro, J. Biochar’s effect on crop productivity and the dependence on experimental conditions—a meta-analysis of literature data. Plant Soil 2013, 373, 583–594. [Google Scholar] [CrossRef]
- Guo, X.-X.; Liu, H.-T.; Zhang, J. The role of biochar in organic waste composting and soil improvement: A review. Waste Manag. 2020, 102, 884–899. [Google Scholar] [CrossRef]
- Glaser, B.; Wiedner, K.; Seelig, S.; Schmidt, H.-P.; Gerber, H. Biochar organic fertilizers from natural resources as substitute for mineral fertilizers. Agron. Sustain. Dev. 2015, 35, 667–678. [Google Scholar] [CrossRef]
- Faloye, O.; Alatise, M.; Ajayi, A.; Ewulo, B. Effects of biochar and inorganic fertiliser applications on growth, yield and water use efficiency of maize under deficit irrigation. Agric. Water Manag. 2019, 217, 165–178. [Google Scholar] [CrossRef]
- Kammann, C.I.; Schmidt, H.-P.; Messerschmidt, N.; Linsel, S.; Steffens, D.; Müller, C.; Koyro, H.-W.; Conte, P.; Joseph, S. Plant growth improvement mediated by nitrate capture in co-composted biochar. Sci. Rep. 2015, 5, 11080. [Google Scholar] [CrossRef]
- Kwak, M.-J.; Jeong, H.; Madhaiyan, M.; Lee, Y.; Sa, T.-M.; Oh, T.K.; Kim, J.F. Genome information of methylobacterium oryzae, a plant-probiotic methylotroph in the phyllosphere. PLoS ONE 2014, 9, e106704. [Google Scholar]
- Raoul des Essarts, Y.; Cigna, J.; Quêtu-Laurent, A.; Caron, A.; Munier, E.; Beury-Cirou, A.; Hélias, V.; Faure, D. Biocontrol of the potato blackleg and soft rot diseases caused by dickeya dianthicola. Appl. Environ. Microbiol. 2016, 82, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juan, Z.; Ting, L.; Liu, W.-C.; Zhang, D.-P.; Dan, D.; Wu, H.-L.; Zhang, T.-T.; Liu, D.-W. Transcriptomic insights into growth promotion effect of trichoderma afroharzianum tm2-4 microbial agent on tomato plants. J. Integr. Agric. 2021, 20, 1266–1276. [Google Scholar]
- Gu, S.; Lian, F.; Yang, H.; Han, Y.; Zhang, W.; Yang, F.; Gao, J. Synergic effect of microorganism and colloidal biochar-based organic fertilizer on the growth and fruit quality of tomato. Coatings 2021, 11, 1453. [Google Scholar] [CrossRef]
- Chaparro, J.M.; Badri, D.V.; Vivanco, J.M. Rhizosphere microbiome assemblage is affected by plant development. ISME J. 2014, 8, 790–803. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Bardgett, R.D.; Smith, P.; Reay, D.S. Microorganisms and climate change: Terrestrial feedbacks and mitigation options. Nat. Rev. Microbiol. 2010, 8, 779–790. [Google Scholar] [CrossRef]
- Nadeem, S.M.; Ahmad, M.; Zahir, Z.A.; Javaid, A.; Ashraf, M. The role of mycorrhizae and plant growth promoting rhizobacteria (pgpr) in improving crop productivity under stressful environments. Biotechnol. Adv. 2014, 32, 429–448. [Google Scholar] [CrossRef]
- Bakker, M.G.; Manter, D.K.; Sheflin, A.M.; Weir, T.L.; Vivanco, J.M. Harnessing the rhizosphere microbiome through plant breeding and agricultural management. Plant Soil 2012, 360, 1–13. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.-T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef]
- Ali, S.; Khan, N. Delineation of mechanistic approaches employed by plant growth promoting microorganisms for improving drought stress tolerance in plants. Microbiol. Res. 2021, 249, 126771. [Google Scholar] [CrossRef]
- Cochard, B.; Giroud, B.; Crovadore, J.; Chablais, R.; Arminjon, L.; Lefort, F. Endophytic pgpr from tomato roots: Isolation, in vitro characterization and in vivo evaluation of treated tomatoes (Solanum lycopersicum L). Microorganisms 2022, 10, 765. [Google Scholar] [CrossRef]
- del Carmen Orozco-Mosqueda, M.; Glick, B.R.; Santoyo, G. Acc deaminase in plant growth-promoting bacteria (pgpb): An efficient mechanism to counter salt stress in crops. Microbiol. Res. 2020, 235, 126439. [Google Scholar] [CrossRef] [PubMed]
- Etesami, H.; Glick, B.R. Halotolerant plant growth–promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot. 2020, 178, 104124. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O.; Santoyo, G.; Perazzolli, M. The potential role of microbial biostimulants in the amelioration of climate change-associated abiotic stresses on crops. Front. Microbiol. 2022, 12, 4392. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Parvin, K.; Bardhan, K.; Nahar, K.; Anee, T.I.; Masud, A.A.C.; Fotopoulos, V. Biostimulants for the regulation of reactive oxygen species metabolism in plants under abiotic stress. Cells 2021, 10, 2537. [Google Scholar] [CrossRef] [PubMed]
- Igiehon, N.O.; Babalola, O.O.; Cheseto, X.; Torto, B. Effects of rhizobia and arbuscular mycorrhizal fungi on yield, size distribution and fatty acid of soybean seeds grown under drought stress. Microbiol. Res. 2021, 242, 126640. [Google Scholar] [CrossRef]
- Koza, N.A.; Adedayo, A.A.; Babalola, O.O.; Kappo, A.P. Microorganisms in plant growth and development: Roles in abiotic stress tolerance and secondary metabolites secretion. Microorganisms 2022, 10, 1528. [Google Scholar] [CrossRef]
- Kumar, A.; Maleva, M.; Bruno, L.B.; Rajkumar, M. Synergistic effect of acc deaminase producing pseudomonas sp. Tr15a and siderophore producing bacillus aerophilus tr15c for enhanced growth and copper accumulation in Helianthus annuus L. Chemosphere 2021, 276, 130038. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Bhardwaj, A.K.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Bacterial endophyte mediated plant tolerance to salinity: Growth responses and mechanisms of action. World J. Microbiol. Biotechnol. 2020, 36, 26. [Google Scholar] [CrossRef]
- Lau, S.-E.; Teo, W.F.A.; Teoh, E.Y.; Tan, B.C. Microbiome engineering and plant biostimulants for sustainable crop improvement and mitigation of biotic and abiotic stresses. Discov. Food 2022, 2, 9. [Google Scholar] [CrossRef]
- Mohammadi, M.A.; Cheng, Y.; Aslam, M.; Jakada, B.H.; Wai, M.H.; Ye, K.; He, X.; Luo, T.; Ye, L.; Dong, C. Ros and oxidative response systems in plants under biotic and abiotic stresses: Revisiting the crucial role of phosphite triggered plants defense response. Front. Microbiol. 2021, 12, 631318. [Google Scholar] [CrossRef]
- Muzhinji, N.; Ntuli, V. Genetically modified organisms and food security in southern africa: Conundrum and discourse. GM Crops Food 2021, 12, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Omotayo, O.P.; Babalola, O.O. Resident rhizosphere microbiome’s ecological dynamics and conservation: Towards achieving the envisioned sustainable development goals, a review. Int. Soil Water Conserv. Res. 2021, 9, 127–142. [Google Scholar] [CrossRef]
- Popp, J.; Kovács, S.; Oláh, J.; Divéki, Z.; Balázs, E. Bioeconomy: Biomass and biomass-based energy supply and demand. New Biotechnol. 2021, 60, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Leach, J.E.; Tringe, S.G.; Sa, T.; Singh, B.K. Plant–microbiome interactions: From community assembly to plant health. Nat. Rev. Microbiol. 2020, 18, 607–621. [Google Scholar] [CrossRef]
- Zhao, L.; Lu, L.; Wang, A.; Zhang, H.; Huang, M.; Wu, H.; Xing, B.; Wang, Z.; Ji, R. Nano-biotechnology in agriculture: Use of nanomaterials to promote plant growth and stress tolerance. J. Agric. Food Chem. 2020, 68, 1935–1947. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Sharma, A.; Salwan, R. Molecular Aspects of Plant Beneficial Microbes in Agriculture; Elsevier: Amsterdam, The Netherlands, 2020; pp. 1–18. [Google Scholar]
- Lorena, B.-B.; Javiera, O.; Franco, C.J. Microbial Management of Plant Stresses; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–12. [Google Scholar]
- Bertola, M.; Ferrarini, A.; Visioli, G. Improvement of soil microbial diversity through sustainable agricultural practices and its evaluation by-omics approaches: A perspective for the environment, food quality and human safety. Microorganisms 2021, 9, 1400. [Google Scholar] [CrossRef] [PubMed]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.-C.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]




| Plants | Abiotic Stress | Microbial Inoculation | Tolerance Method | References |
|---|---|---|---|---|
| Arabidopsis thaliana | Salt | Bacillus subtilis GB03 | Tissue-specific regulation of sodium transporter HKT1. | [126] |
| Glycine max | Salt | Pseudomonas simiae | 4-nitroguaiacol and quinolone promote soybean-seed germination. | [61] |
| Oryza sativa | Salt | Root-associated growth-promoting rhizobacteria | Expression of salt-stress-related RAB18 plant gene. | [127] |
| Oryza sativa, Triticum aestivum, Zea mays, Gossypium hirsutum | Salt | Cyanobacteria and cyanobacterial extracts | Phytohormones as elicitor molecule. | [128] |
| Zea mays | Osmotic stress | Bacillus megaterium | High hydraulic conductance and increased root expression and ZmPIP isoforms. | [129] |
| Vitis vinifera, Capsicum annuum | Salt | Burkholderia, Arthrobacter Bacillus | Increased accumulation of proline. | [130] |
| Capsicum annuum | Salinity | Azospirillum brasilense and Pantoea dispersa (Co-inoculation) | High stomatal conductance and Photosynthesis. | [131] |
| Arabidopsis | Salinity | Bacillus subtilis | Decreased root transcriptional expression of a high-affinity K+ transporter (AtHKT1) decreasing root Na+ import. | [126] |
| Phaseolus vulgaris | Salinity | Azospirillum brasilense strain Cd | Stimulation of persistent exudation of flavonoids. | [132] |
| Zea mays | Drought | Burkholderia phytofirmans Enterobacter sp. FD17 | Increased photosynthesis, root and shoot biomass under drought conditions. | [133] |
| Arabidopsis thaliana | Drought | Pseudomonas chlororaphis O6 | Production of 2R,3R butanediol- a volatile compound. | [134] |
| Triticum aestivum | Heat | Bacillus amyloliquefaciens, Azospirillum brasilence | Reduced regeneration of reactive oxygen species, pre-activation of heat shock transcription factors, and changes in metabolome. | [135] |
| Brassica juncea | Arsenic toxicity | Staphylococcus arlettae | Increased dehydrogenase, phosphatase, and available phosphorus in soil. | [136] |
| Triticum aestivum | Zn toxicity | Pseudomonas aeruginosa | Improved biomass, N and P uptake, and soluble protein. | [137] |
| Phragmites australis | Hg toxicity | Photobacterium spp. | IAA and mercury reductase activity. | [138] |
| Miscanthus sinensis | Cd, AS, Cu, Pb and Zn toxicity | Pseudomonas koreensis AGB-1 | ACC deaminase and IAA production. | [139] |
| Endophyte | Mechanism | Plant | Stress | References |
|---|---|---|---|---|
| Piriformospora indica | Mutualism mechanism, drought-related genes regulation | Barley | Salinity | [169] |
| B. phytofirmans PsJN | Initiation of host stress responses | Vegetables | Abiotic stress | [167] |
| Curvularia protuberate | Metabolite synthesis and improves plant physiology | Tomato and rice | Heat and drought | [170] |
| C. protuberata | Entophytic colonization | Wheat | Heat and salinity | [171] |
| P. indica | Increased ascorbate concentration | Barley | Salt stress | [172] |
| Chaetomium globosum LK4 | Increased plat biomass | Capsicum annum | Drought | [170] |
| Trichoderma | Enhanced growth of roots | Cacao | Drought | [173] |
| Trichoderma harzianum strain T22 | Low synthesis of lipid peroxides | Cacao | [174] |
| Crops | PGPR | PGR | Responses | References |
|---|---|---|---|---|
| Maize | Azotobacter sp. | Indole-3-acetic acid | Inoculation with strain enhances IAA and growth-promoting effects on maize. | [191] |
| Canola, tomato | Kluyvera ascorbate SUD 165 | Siderophores, indole-3-acetic acid | Both strains decreased plant-growth inhibition by heavy metals (nickel, lead, and zinc). | [192] |
| Groundnut | Pseudomonas fluorescens | Siderophores, indole-3-acetic acid | Involvement of ACC deaminase and siderophore production promoted nodulation and yield of groundnut. | [193] |
| Rice | Rhizobium leguminosarum | Indole-3-acetic acid | Growth-promoting effects upon inoculation on axenically grown rice seedlings were observed. | [93] |
| Rice | Azospirillum brasilense A3, A4, A7, A10, CDJA | Indole-3-acetic acid, | Bacterial strains increased rice grain yield. | [194] |
| Brassica | Mesorhizobium loti MP6 | Chrom-azurol, siderophore (CAS), hydrocyanic acid (HCN), indole-3-acetic acid | Mesorhizobium loti MP6–coated seeds enhanced seed germination, early vegetative growth, and grain yield. | [195] |
| Wheat | Azospirillum lipoferum strains 15 | Promoted development of wheat root system, even under crude-oil contamination. | [196] | |
| Sesbenia, Mung bean | Azotobacter sp. and Pseudomonas sp. | Indole-3-acetic acid | Increasing concentration of tryptophane from 1 to 5 mg mL−1 resulted in decreased growth. | [66] |
| Rice | Bacillus sp. and Paenibacillus sp. | Indole-3-acetic acid | The isolate SVPR 30, i.e., strain of Bacillus sp., proved to be efficient in promoting a significant increase in the root and shoot parts of rice plants. | [197] |
| Wheat | Pseudomonas sp. | Indole-3-acetic acid | A combined bio-inoculation of diacetyl-phloreglucinol-producing PGPRs and AMFs and improved the nutritional quality of wheat grain. | [198] |
| Cowpea | Streptomyces acidiscabies E13 | Hydroxamate siderophores | S. acidiscabies promoted cowpea growth under nickel stress. | [199] |
| Plants | Abiotic Factor | PGPM | Inoculation | Effect | Reference |
|---|---|---|---|---|---|
| Cajanus cajan, Eleusine coracana | Soil | Pseudomonas | Seed | Increase growth and improve nutrient-deficient soil. | [201] |
| Glycine max | Soil | Trichoderma sp. | Seed | Improve germination, growth, and K uptake under drought and salts tress. | [202] |
| Brassica juncea, aeruginosa, Alcaligenes feacalis | Soil | Pseudomonas | Seed | Increase growth, metal tolerance, and phytoextraction efficiency. | [203] |
| Glycine max | Water | Klebsiella variicola | Soil | Improve plant growth and flood tolerance by inducing adventitious root. | [204] |
| Mentha pulegium L. | Water | Azotobacter Chroococcm Azospirillum brasilense | Seed | Improve physiological and phytochemical parameters and drought tolerance. | [205] |
| Vigna radiata | Water | Pseudomonas fluorescens | Seed | Increase vigor, biomass, activity of catalase and peroxidase, accumulation of proline, and water-stress tolerance. | [206] |
| Capsicum annuum | Soil | Pseudomonas sp. | Seed | Increase growth by increasing ACC deaminase and reduce ethylene under salinity stress. | [207] |
| Brachiaria brizantha | Light | Burkholderia Pseudomonass | Soil | Increase plant growth and shade tolerance. | [208] |
| Zea mays | Water | Azospirillum sp. | Seed | Increase growth and drought and flood tolerance. | [209] |
| Ophiopogon japonicus | Light | Kaistobacter sp. Pseudomonas | Soil | Increase growth and shade tolerance. | [210] |
| Plants | Mineral Source | Bacterial Species | Outcomes | References |
|---|---|---|---|---|
| Stevia rebaudiana | Mussoorie rock phosphate | Burkholderia gladioli MTCC 10216; B. gladioli MTCC10217, Enterobacter aerogenes MTCC 10208, and Serratia marcescens MTCC 10238 | Enhanced growth and stevioside and rebaudioside-A metabolites. Increased amount of P in soil. | [226] |
| Rice | Bayóvar rock phosphate | Burkholderia sp. UFLA 04-21; Paenibacillus kribbensis UFLA 03-10; Enterobacter sp. UFPI B5-6; and Pseudomonas sp. UFPI B5-8A | Increased biomass, tillers and nutrients accumulation | [227] |
| Vicia faba | Rock phosphate | Serratia plymuthica BMA1 | Increased plant growth and P uptake | [228] |
| Lentil (Lens culinaris Medik. cv. VL Masoor 507) | Udaipur rock phosphate | Pseudomonas sp. RT5RP2 and Pseudomonas sp. RT6RP | Increased P uptake by plants | [229] |
| Medicago truncatula Gaertn. | Tunisian rock phosphate | Pseudomonas corrugata SP77, Pseudomonas koreensis LT62 and Pseudomonas | Enhanced shoot dry weight and nodule fresh weigh | [230] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munir, N.; Hanif, M.; Abideen, Z.; Sohail, M.; El-Keblawy, A.; Radicetti, E.; Mancinelli, R.; Haider, G. Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy 2022, 12, 2069. https://doi.org/10.3390/agronomy12092069
Munir N, Hanif M, Abideen Z, Sohail M, El-Keblawy A, Radicetti E, Mancinelli R, Haider G. Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy. 2022; 12(9):2069. https://doi.org/10.3390/agronomy12092069
Chicago/Turabian StyleMunir, Neelma, Maria Hanif, Zainul Abideen, Muhammed Sohail, Ali El-Keblawy, Emanuele Radicetti, Roberto Mancinelli, and Ghulam Haider. 2022. "Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses" Agronomy 12, no. 9: 2069. https://doi.org/10.3390/agronomy12092069
APA StyleMunir, N., Hanif, M., Abideen, Z., Sohail, M., El-Keblawy, A., Radicetti, E., Mancinelli, R., & Haider, G. (2022). Mechanisms and Strategies of Plant Microbiome Interactions to Mitigate Abiotic Stresses. Agronomy, 12(9), 2069. https://doi.org/10.3390/agronomy12092069