Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
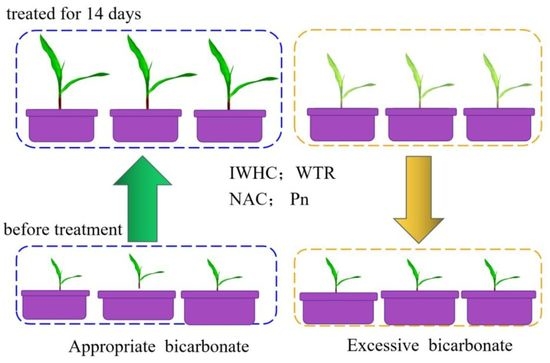
2.2. Sodium Bicarbonate Hydroponic Experiment Using Coix lacryma-jobi L.
2.3. Determination of Biomass Parameters, Electrophysiological and Photosynthetic Characteristics and Chlorophyll Fluorescence
2.4. Statistical Analyses
3. Results
3.1. Responses of the Growth in Coix lacryma-jobi L. to Sodium Bicarbonate
3.2. Responses of Electrophysiological, Intracellular Water Metabolism and Nutrient Transport Parameters of Coix lacryma-jobi L. to Sodium Bicarbonate
3.3. Responses of the Photosynthetic Characteristics of Coix lacryma-jobi L. to Sodium Bicarbonate
3.4. Responses of the Chlorophyll Fluorescence of Coix lacryma-jobi L. to Sodium Bicarbonate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HCO3− | sodium bicarbonate |
| C | capacitance |
| Z | impedance |
| R | resistance |
| F | force |
| d | specific effective thickness |
| Xc | capacitive reactance |
| XL | inductive reactance |
| IC | intrinsic capacitance |
| IR | intrinsic resistance |
| IZ | intrinsic impedance |
| IXc | intrinsic capacitive reactance |
| IXL | intrinsic inductive reactance |
| IWHC | intracellular water-holding capacity |
| IWUE | intracellular water use efficiency |
| IWHT | intracellular water-holding time |
| WTR | water transfer rate |
| UNF | nutrient flux per unit area |
| NAF | active transport flow of nutrient |
| NTR | nutrient transfer rate |
| NTC | nutrient transport capacity |
| NAC | nutrient active transport capacity |
References
- Wu, Y.; Xing, D.; Hang, H.; Zhao, K. Principles and Technology of Determination on Plant’ Adaptation to Karst Environment; Science Press: Beijing, China, 2019; pp. 1–88. [Google Scholar]
- Stokes, T.R.; Griffiths, P.A. An overview of the Karst Areas in British Columbia, Canada. Geosci. Can. 2019, 46, 49–66. [Google Scholar] [CrossRef]
- Luo, L.; Wu, Y.; Li, H.; Xing, D.; Zhou, Y.; Xia, A. Drought Induced Dynamic Traits of Soil Water and Inorganic Carbon in Different Karst Habitats. Water 2022, 14, 3837. [Google Scholar] [CrossRef]
- McCray, J.M.; Matocha, J.E. Effects of soil water levels on solution bicarbonate, chlorosis and growth of sorghum. J. Plant Nutr. 1992, 15, 1877–1890. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, J.; Pu, J.; Yan, J. Bicarbonate daily variations in a karst river: The carbon sink effect of subaquatic vegetation photosynthesis. Acta Geol. Sin.-Engl. 2012, 86, 973–979. [Google Scholar] [CrossRef]
- Hussner, A.; Mettler-Altmann, T.; Weber, A.; Sand-Jense, K. Acclimation of photosynthesis to supersaturated CO2 in aquatic plant bicarbonate users. Freshw. Biol. 2016, 61, 1720–1732. [Google Scholar] [CrossRef]
- Xing, D.; Chen, X.; Wu, Y.; Xu, X.; Chen, Q.; Li, L.; Zhang, C. Rapid prediction of the re-watering time point of Orychophragmus violaceus L. based on the online monitoring of electrophysiological indexes. Sci. Hortic. 2019, 256, 108642. [Google Scholar] [CrossRef]
- Yao, K.; Wu, Y. Rhizospheric bicarbonate improves glucose metabolism and stress tolerance of Broussonetia papyrifera L. seedlings under simulated drought stress. Russ. J. Plant Physl. 2021, 68, 126–135. [Google Scholar] [CrossRef]
- Gong, B.; Wen, D.; Vandenlangenberg, K.; Wei, M.; Yang, F.; Shi, Q.; Wang, X. Comparative effects of NaCl and NaHCO3 stress on photosynthetic parameters, nutrient metabolism, and the antioxidant system in tomato leaves. Sci. Hortic. 2013, 157, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Pu, P.; Tang, Y.; Zhang, L.; Lv, J. C4 photosynthetic enzymes play a key role in wheat spike bracts primary carbon metabolism response under water deficit. Plant Physiol. Biochem. 2019, 142, 163–172. [Google Scholar] [CrossRef]
- Sun, X.; Dong, X.; Li, X.; Chen, L.; Liu, J. Response of growth and physiological characteristics of melon seedlings under alkali stress. North Hortic. 2020, 9, 51–59. [Google Scholar] [CrossRef]
- García, M.J.; García-Mateo, M.J.; Lucena, C.; Romera, F.J.; Rojas, C.L.; Alcántara, E.; Pérez-Vicente, R. Hypoxia and bicarbonate could limit the expression of iron acquisition genes in Strategy I plants by affecting ethylene synthesis and signaling in different ways. Physiol. Plant. 2014, 150, 95–106. [Google Scholar] [CrossRef]
- Ding, W.; Clode, P.L.; Lambers, H. Effects of pH and bicarbonate on the nutrient status and growth of three Lupinus species. Plant Soil. 2020, 447, 9–28. [Google Scholar] [CrossRef]
- Kallala, N.; M’sehli, W.; Jelali, K.; Kais, Z.; Mhadhbi, H. Inoculation with efficient nitrogen fixing and indoleacetic acid producing bacterial microsymbiont enhance tolerance of the model legume Medicago truncatula to iron deficiency. Biomed Res. Int. 2018, 2018, 9134716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, S.; Wu, Y. Root derived bicarbonate assimilation in response to variable water deficit in Camptotheca acuminata seedlings. Photosynth. Res. 2017, 134, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Strategies to increase carbon fixation and sequestration by karst-adaptable plants. Carsol. Sin. 2011, 30, 461–465. [Google Scholar] [CrossRef]
- Tang, Y.; Lian, B. Diversity of endolithic fungal communities in dolomite and limestone rocks from Nanjiang canyon in Guizhou karst area, China. Can. J. Microbiol. 2012, 58, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Wu, Y.; Xing, D.; Hang, H.; Liu, Y.; Zhang, K.; Yan, K. Physiological characteristics and inorganic carbon usage capacity of three biomass plants under simulative karst adversity (bicarbonate stress). Earth Environ. 2015, 43, 21–30. [Google Scholar] [CrossRef]
- Hang, H.; Wu, Y. Quantification of photosynthetic inorganic carbon utilisation via a bidirectional stable carbon isotope tracer. Acta Geochim. 2016, 35, 130–137. [Google Scholar] [CrossRef]
- Wang, R.; Wu, Y.; Xing, D. Biomass production of three biofuel energy plants’use of a new carbon resource by carbonic anhydrase in simulated karst soils: Mechanism and capacity. Energies 2017, 10, 1370. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, G.; Ghosh, I.; Kim, C.J.; Debus, R.J.; Brudvig, G.W. Bicarbonate rescues damaged proton-transfer pathway in photosystem II. BBA-Bioenerg. 2019, 1860, 611–617. [Google Scholar] [CrossRef]
- Zhou, W.; Sui, Z.; Wang, J.; Hu, Y.; Kang, K.H.; Hong, H.R.; Niaz, Z.; Wei, H.; Du, Q.; Peng, C.; et al. Effects of sodium bicarbonate concentration on growth, photosynthesis, and carbonic anhydrase activity of macroalgae Gracilariopsis lemaneiformis, Gracilaria vermiculophylla, and Gracilaria chouae (Gracilariales, Rhodophyta). Photosynth. Res. 2016, 128, 259–270. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, C.; Meng, Q.; Liu, F.; Shen, G.; Zhou, M.; Ao, M. Genetic diversity and structure of Coix lacryma-jobi L. from its world secondary diversity center, southwest China. Int. J. Genom. 2019, 2019, 9815697. [Google Scholar] [CrossRef] [Green Version]
- Miao, G.; Qin, Y.; Guo, J.; Zhang, Q.; Bao, Y. Transcriptome characterization and expression profile of Coix lacryma-jobi L. in response to drought. PLoS ONE 2021, 16, e0256875. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Park, H.J.; Cho, S.J.; Park, S.A.; Kim, M.L. The effect of yulmu (Coix lacryma-jobi L.)-sunsik on obesity and hyperlipidemia in mice fed high-fat diet. J. Korean Soc. Food Sci. Nutr. 2021, 50, 664–671. [Google Scholar] [CrossRef]
- Yu, Q.; Ye, G.; Lei, Z.; Yang, R.; Chen, R.; He, T.; Huang, S. An isolated compound from stems and leaves of Coix lacryma-jobi L. and its anticancer effect. Food Biosci. 2021, 42, 101047. [Google Scholar] [CrossRef]
- Diao, X. Production and genetic improvement of minor cereals in China. Crop. J. 2017, 5, 103–114. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Lu, X.; Pan, H.; Wei, X.; Shi, M.; Su, Y. Research status of germplasm resource and genetic breeding in Coix lacryma-jobi L. Guizhou Agri. Sci. 2022, 50, 8–15. [Google Scholar] [CrossRef]
- Fromm, J.; Lautner, S. Electrical signals and their physiological significance in plants. Plant Cell Environ. 2007, 30, 249–257. [Google Scholar] [CrossRef]
- Volkov, A.G. Plant Electrophysiology; Theory and Methods; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Szechyńska-Hebda, M.; Lewandowska, M.; Karpiński, S. Electrical signaling, photosynthesis and systemic acquired acclimation. Front. Physiol. 2017, 8, 684. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V. Electrical signals as mechanism of photosynthesis regulation in plants. Photosynth. Res. 2016, 130, 373–387. [Google Scholar] [CrossRef]
- Zhang, C.; Su, Y.; Wu, Y.; Li, H.; Zhou, Y.; Xing, D. Comparison on the nutrient plunder capacity of Orychophragmus violaceus and Brassica napus L. Based on electrophysiological properties. Horticulturae 2021, 7, 206. [Google Scholar] [CrossRef]
- Sukhov, V.; Gaspirovich, V.; Mysyagin, S.; Vodeneev, V. High-temperature tolerance of photosynthesis can be linked to local electrical responses in leaves of Pea. Front. Physiol. 2017, 8, 763. [Google Scholar] [CrossRef] [PubMed]
- Sukhov, V.; Surova, L.; Sherstneva, O.; Bushueva, A.; Vodeneev, V. Variation potential induces decreased PSI damage and increased PSII damage under high external temperatures in pea. Funct. Plant Biol. 2015, 42, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Gil, P.M.; Gurovich, L.; Schaffer, B.; Alcayaga, J.; Rey, S.; Iturriaga, R. Root to leaf electrical signaling in avocado in response to light and soil water content. J. Plant Physiol. 2008, 165, 1070–1078. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Xing, D.; Zhao, K.; Yu, R. Rapid measurement of drought resistance in plants based on electrophysiological properties. Trans. ASABE 2015, 58, 1441–1446. [Google Scholar] [CrossRef]
- Harker, F.R.; Dunlop, J. Electrical impedance studies of nectarines during coolstorage and fruit ripening. Postharvest Biol. Technol. 1994, 4, 125–134. [Google Scholar] [CrossRef]
- Ibba, P.; Falco, A.; Abera, B.D.; Cantarella, G.; Petti, L.; Lugli, P. Bio-impedance and circuit parameters: An analysis for tracking fruit ripening. Postharvest Biol. Technol. 2020, 159, 110978. [Google Scholar] [CrossRef]
- Javed, Q.; Wu, Y.; Xing, D.; Azeem, A.; Ullah, I.; Zaman, M. Re-watering: An effective measure to recover growth and photosynthetic characteristics in salt-stressed Brassica napus L. Chil. J. Agric. Res. 2017, 77, 78–86. [Google Scholar] [CrossRef] [Green Version]
- Kertész, Á.; Hlaváčová, Z.; Vozáry, E.; Staroňová, L. Relationship between moisture content and electrical impedance of carrot slices during drying. Int. Agrophys. 2015, 29, 61–66. [Google Scholar] [CrossRef]
- Xing, D.; Chen, X.; Wu, Y.; Zwiazek, J.J. Leaf physiological impedance and elasticity modulus in Orychophragmus violaceus seedlings subjected to repeated osmotic stress. Sci. Hortic. 2021, 276, 109763. [Google Scholar] [CrossRef]
- Xing, D.; Mao, R.; Li, Z.; Wu, Y.; Qin, X.; Fu, W. Leaf intracellular water transport rate based on physiological impedance: A possible role of leaf internal retained water in photosynthesis and growth of tomatoes. Front. Plant Sci. 2022, 13, 845628. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, Y.; Su, Y.; Xing, D.; Dai, Y.; Wu, Y.; Fang, L. A plant’s electrical parameters indicate its physiological state: A study of intracellular water metabolism. Plants 2020, 9, 1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Y.; Su, Y.; Li, H.; Fang, L.; Xing, D. Plant’s electrophysiological properties manifests the composition and nutrient transport characteristics of membrane proteins. Plant Signal. Behav. 2021, 16, e1918867. [Google Scholar] [CrossRef]
- Hajiboland, R.; Yang, X.; Romeld, V. Effects of bicarbonate and high pH on growth of Zn-efficient and Zn-inefficient genotypes of rice, wheat and rye. Plant Soil. 2003, 250, 349–357. [Google Scholar] [CrossRef]
- Haro, R.; Banuelos, M.A.; Quintero, F.J.; Rubio, F.; Rodriguez-Navarro, A. Genetic basis of sodium exclusion and sodium tolerance in yeast, A model for plants. Physiol. Plant. 1993, 89, 868–874. [Google Scholar] [CrossRef]
- Gupta, P.; Seth, C.S. 24-Epibrassinolide Regulates Functional Components of Nitric Oxide Signalling and Antioxidant Defense Pathways to Alleviate Salinity Stress in Brassica juncea L. cv. Varuna. J. Plant Growth Regul. 2023, 42, 183–198. [Google Scholar] [CrossRef]
- Shahsavandi, F.; Eshghi, S.; Gharaghani, A.; Ghasemi-Fasaei, R.; Jafarinia, M. Effects of bicarbonate induced iron chlorosis on photosynthesis apparatus in grapevine. Sci. Hortic. 2020, 270, 109427. [Google Scholar] [CrossRef]
- Li, R.; Shi, F.; Fukuda, K.; Yang, Y. Effects of salt and alkali stresses on germination, growth, photosynthesis and ion accumulation in alfalfa (Medicago sativa L.). Soil Sci. Plant Nutr. 2010, 56, 725–733. [Google Scholar] [CrossRef]
- Wu, Y. Combined effect of bicarbonate and water in photosynthetic oxygen evolution and carbon neutrality. Acta Geochim. 2023, 42, 77–88. [Google Scholar] [CrossRef]
- Tikhonov, K.; Shevela, D.; Klimov, V.V.; Messinger, J. Quantification of bound bicarbonate in photosystem II. Photosynthetica 2017, 56, 210–216. [Google Scholar] [CrossRef]
- Wu, Y. Is bicarbonate directly used as substrate to participate in photosynthetic oxygen evolution. Acta Geochim. 2021, 40, 650–658. [Google Scholar] [CrossRef]
- Warburg, O.; Krippahl, G. Hill-Reaktionen. Z. Naturforsch. 1958, 13, 509–514. [Google Scholar] [CrossRef]
- Agnihotri, A.; Gupta, P.; Dwivedi, A.; Seth, C.S. Counteractive mechanism (s) of salicylic acid in response to lead toxicity in Brassica juncea (L.) Czern. cv. Varuna. Planta 2018, 248, 49–68. [Google Scholar] [CrossRef]
- Singh, D.; Agnihotri, A.; Seth, C.S. Interactive effects of EDTA and oxalic acid on chromium uptake, translocation and photosynthetic attributes in Indian mustard (Brassica juncea L. var. Varuna). Curr. Sci. 2017, 112, 2034–2042. [Google Scholar] [CrossRef]
- Kumar, D.; Dhankher, O.P.; Tripathi, R.D.; Seth, C.S. Titanium dioxide nanoparticles potentially regulate the mechanism (s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L. J. Hazard. Mater. 2023, 454, 131418. [Google Scholar] [CrossRef] [PubMed]
- Giovanna, S.; Francesco, B.; Mario, A.; Federica, C.; Olga, M.; Simona, C. Rapid and positive effect of bicarbonate addition on growth and photosynthetic efficiency of the green microalgae Chlorella Sorokiniana (Chlorophyta, Trebouxiophyceae). Appl. Sci. 2020, 10, 4515. [Google Scholar] [CrossRef]



| [HCO3−] (mmol L−1) | IC (pF) | IR (MΩ) | IZ (MΩ) | IXc (MΩ) | IXL (MΩ) |
|---|---|---|---|---|---|
| 0 | 80.35 ± 22.18 b | 0.79 ± 0.39 a | 0.52 ± 0.20 a | 0.70 ± 0.20 a | 1.26 ± 0.46 a |
| 2.0 | 359.70 ± 44.70 a | 0.07 ± 0.02 b | 0.06 ± 0.01 b | 0.15 ± 0.02 b | 0.19 ± 0.03 b |
| 7.0 | 108.20 ± 13.75 b | 0.41 ± 0.04 ab | 0.31 ± 0.02 ab | 0.50 ± 0.06 ab | 0.77 ± 0.04 ab |
| 12.0 | 97.49 ± 36.47 b | 0.37 ± 0.22 ab | 0.32 ± 0.17 ab | 0.61 ± 0.27 a | 0.83 ± 0.41 ab |
| [HCO3−] (mmol L−1) | d | IWHC | IWUE | IWHT | WTR |
|---|---|---|---|---|---|
| 0 | 153.57 ± 99.08 a | 734.05 ± 298.17 b | 0.27 ± 0.28 a | 38.52 ± 7.86 a | 20.51 ± 12.48 b |
| 2.0 | 407.40 ± 63.75 a | 6848.49 ± 1263.66 a | 0.06 ± 0.01 a | 21.87 ± 1.96 b | 316.87 ± 80.77 a |
| 7.0 | 238.29 ± 176.15 a | 1130.00 ± 217.07 b | 0.20 ± 0.11 a | 33.86 ± 3.24 ab | 33.22 ± 3.83 b |
| 12.0 | 181.56 ± 40.55 a | 997.09 ± 524.67 b | 0.22 ± 0.11 a | 27.80 ± 4.85 ab | 36.93 ± 19.39 b |
| [HCO3−] (mmol L−1) | UNF | NAF | NAC | NTC |
|---|---|---|---|---|
| 0 | 1.70 ± 0.55 a | 0.58 ± 0.12 b | 12.67 ± 10.15 b | 36.39 ± 4.65 b |
| 2.0 | 0.84 ± 0.08 b | 0.77 ± 0.02 a | 245.58 ± 70.30 a | 43.53 ± 0.73 a |
| 7.0 | 1.36 ± 0.20 ab | 0.64 ± 0.06 ab | 21.30 ± 1.70 b | 39.13 ± 2.03 ab |
| 12.0 | 1.03 ± 0.19 ab | 0.75 ± 0.05 ab | 28.07 ± 15.51 b | 42.71 ± 1.64 ab |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Lv, J.; Su, Y.; Wu, Y. Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L. Agronomy 2023, 13, 1790. https://doi.org/10.3390/agronomy13071790
Li H, Lv J, Su Y, Wu Y. Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L. Agronomy. 2023; 13(7):1790. https://doi.org/10.3390/agronomy13071790
Chicago/Turabian StyleLi, Haitao, Jiamei Lv, Yue Su, and Yanyou Wu. 2023. "Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L." Agronomy 13, no. 7: 1790. https://doi.org/10.3390/agronomy13071790
APA StyleLi, H., Lv, J., Su, Y., & Wu, Y. (2023). Appropriate Sodium Bicarbonate Concentration Enhances the Intracellular Water Metabolism, Nutrient Transport and Photosynthesis Capacities of Coix lacryma-jobi L. Agronomy, 13(7), 1790. https://doi.org/10.3390/agronomy13071790