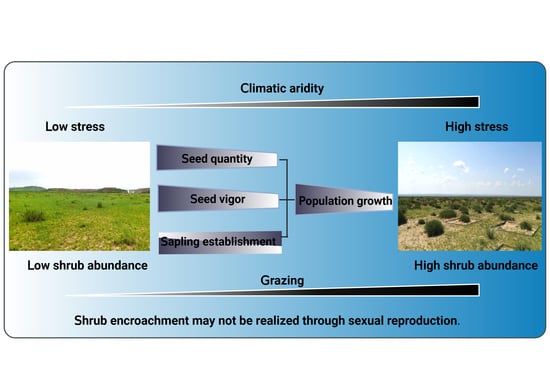
Sexual Reproduction Is Not Responsible for Caragana Shrub Encroachment in Grasslands
Abstract
:1. Introduction
2. Study Area and Methods
2.1. Study Area and Grazing Treatment
2.2. Seed Production Experiment
2.3. Seed Vigor Experiment
2.4. Sapling Establishment Experiment
2.5. Population Growth Rate
2.6. Statistical Analysis
3. Results
3.1. Effects of Climatic Aridity and Grazing on Seed Production
3.2. Effects of Climatic Aridity and Grazing on Seed Vigor
3.3. Effects of Climatic Aridity and Grazing on Sapling Establishment
3.4. Effects of Climatic Aridity and Grazing on Population Growth
4. Discussion
4.1. Effect of Climatic Aridity on Sexual Reproduction
4.2. Effect of Grazing Intensity on Sexual Reproduction
4.3. Interactive Effects between Climatic Aridity and Grazing on Sexual Reproduction
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Eldridge, D.J.; Bowker, M.A.; Maestre, F.T.; Roger, E.; Reynolds, J.F.; Whitford, W.G. Impacts of shrub encroachment on ecosystem structure and functioning: Towards a global synthesis. Ecol. Lett. 2011, 14, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Xiong, X.G. Increased distribution of Caragana microphylla in rangelands and its causes and consequences in Xilin River Basin. Acta. Pratacult. Sin. 2003, 12, 57–62. [Google Scholar]
- van Auken, O.W. Causes and consequences of woody plant encroachment into western North American grasslands. J. Environ. Manag. 2009, 90, 2931–2942. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.N.; Li, Y.C.; Guo, H.Y.; Wang, C.W.; Chen, Q.; He, P.; Ma, C.C. Sandy habitats play an important role in shrub encroachment in grasslands. Agronomy 2022, 12, 2858. [Google Scholar] [CrossRef]
- Ma, C.C.; Zhang, J.H.; Guo, H.Y.; Li, Q.F.; Xie, L.N.; Gao, Y.B. Alterations in canopy size and reproduction of Caragana stenophylla along a climate gradient on the Inner Mongolian Plateau. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 97–103. [Google Scholar] [CrossRef]
- Xie, L.N.; Ma, C.C.; Guo, H.Y.; Li, Q.F.; Gao, Y.B. Distribution pattern of Caragana species under the influence of climate gradient in the Inner Mongolia region, China. J. Arid Land 2014, 6, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L.; Wu, J.B.; Xu, J.S. Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. J. Arid Environ. 2008, 72, 247–254. [Google Scholar] [CrossRef]
- Ma, C.C.; Guo, H.Y.; Wu, J.B.; Wang, J.L.; Qi, S.X.; Wei, Y.R.; Gao, Y.B. Acclimation of photosynthetic traits of Caragana species to desert environment in Inner Mongolian Plateau. Arid Land Res. Manag. 2014, 28, 87–101. [Google Scholar] [CrossRef]
- Guo, H.Y.; Guan, L.J.; Wang, Y.H.; Xie, L.N.; Prather, C.M.; Liu, C.G.; Ma, C.C. Grazing limits natural biological controls of woody encroachment in Inner Mongolia Steppe. Biol. Open 2017, 6, 1569–1574. [Google Scholar] [CrossRef] [Green Version]
- May, F.; Giladi, I.; Ristow, M.; Ziv, Y.; Jeltsch, F. Plant functional traits and community assembly along interacting gradients of productivity and fragmentation. Perspect Plant Ecol. 2013, 15, 304–318. [Google Scholar] [CrossRef]
- Volis, S.; Mendlinger, S.; Ward, D. Differentiation in populations of Hordeum spontaneum along a gradient of environmental productivity and predictability: Life history and local adaptation. Biol. J. Linn. Soc. 2002, 77, 479–490. [Google Scholar] [CrossRef] [Green Version]
- Volis, S.; Mendlinger, S.; Ward, D. Demography and role of the seed bank in Mediterranean and desert populations of wild barley. Basic Appl. Ecol 2004, 5, 53–64. [Google Scholar] [CrossRef] [Green Version]
- Volis, S. Correlated patterns of variation in phenology and seed production in populations of two annual grasses along an aridity gradient. Evol. Ecol. 2007, 21, 381–393. [Google Scholar] [CrossRef]
- Lebrija-Trejos, E.; Lobato, M.C.C.; Sternberg, M. Reproductive traits and seed dynamics at two environmentally contrasting annual plant communities: From fieldwork to theoretical expectations. Isr. J. Ecol. Evol. 2011, 57, 73–90. [Google Scholar] [CrossRef] [Green Version]
- Del Pozo, A.; Ovalle, C.; Aronson, J.; Avendano, J. Ecotypic differentiation in Medicago polymorpha L. along an environmental gradient in central Chile. I. Phenology, biomass production and reproductive patterns. Plant Ecol. 2002, 159, 119–130. [Google Scholar] [CrossRef]
- Traveset, A.; Gulías, J.; Riera, N.; Mus, M. Transition probabilities from pollination to establishment in a rare dioecious shrub species (Rhamnus ludovici-salvatoris) in two habitats. J. Ecol. 2003, 91, 427–437. [Google Scholar] [CrossRef] [Green Version]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Seedling establishment of a boreal tree species (Pinus sylvestris) at its southernmost distribution limit: Consequences of being in a marginal Mediterranean habitat. J. Ecol. 2004, 92, 266–277. Available online: https://www.jstor.org/stable/3599591 (accessed on 7 January 2023). [CrossRef]
- Castro, J.; Zamora, R.; Hódar, J.A.; Gómez, J.M. Alleviation of summer drought boosts establishment success of Pinus sylvestris in a Mediterranean mountain: An experimental approach. Plant Ecol. 2005, 181, 191–202. [Google Scholar] [CrossRef]
- Rysavy, A.; Seifan, M.; Sternberg, M.; Tielbörger, K. Shrub seedling survival under climate change–comparing natural and experimental rainfall gradients. J. Arid Environ. 2014, 111, 14–21. [Google Scholar] [CrossRef]
- Cipriotti, P.A.; Flombaum, P.; Sala, O.E.; Aguiar, M.R. Does drought control emergence and survival of grass seedlings in semi-arid rangelands?: An example with a Patagonian species. J. Arid Environ. 2008, 72, 162–174. [Google Scholar] [CrossRef]
- Hunt, L.P. Low seed availability may limit recruitment in grazed Atriplex vesicaria and contribute to its local extinction. Plant Ecol. 2001, 157, 53–67. Available online: https://www.jstor.org/stable/20051160 (accessed on 7 January 2023). [CrossRef]
- Hickman, K.R.; Hartnett, D.C. Effects of grazing intensity on growth, reproduction, and abundance of three palatable forbs in Kansas tallgrass prairie. Plant Ecol. 2002, 159, 23–33. [Google Scholar] [CrossRef]
- Wan, H.; Bai, Y.; Schönbach, P.; Gierus, M.; Taube, F. Effects of grazing management system on plant community structure and functioning in a semiarid steppe: Scaling from species to community. Plant Soil 2011, 340, 215–226. [Google Scholar] [CrossRef]
- Kratochwil, A.; Fock, S.; Remy, D.; Schwabe, A. Responses of flower phenology and seed production under cattle grazing impact in sandy grasslands. Phytocoenologia 2002, 32, 531–552. [Google Scholar] [CrossRef]
- Liu, D.; An, S.Z.; Kong, Q.G.; Zhang, X.H. Population characters of Ligularia narynensis in Kalajun rangeland. Pratacult. Sci. 2010, 27, 25–29. [Google Scholar]
- Oliva, G.; Collantes, M.; Humano, G. Reproductive effort and seed establishment in grazed tussock grass populations of Patagonia. Rangeland Ecol. Manag. 2013, 66, 164–173. [Google Scholar] [CrossRef]
- Bates, J.D.; Davies, K.W. Cattle grazing and vegetation succession on burned sagebrush steppe. Rangel. Ecol. Manag. 2014, 67, 412–422. [Google Scholar] [CrossRef]
- Farrington, S.J.; Muzika, R.M.; Drees, D.; Knight, T.M. Interactive effects of harvest and deer herbivory on the population dynamics of American ginseng. Conserv. Biol. 2009, 23, 719–728. [Google Scholar] [CrossRef]
- Mayer, R.; Erschbamer, B. Seedling recruitment and seed-/microsite limitation in traditionally grazed plant communities of the alpine zone. Basic. Appl. Ecol. 2011, 12, 10–20. [Google Scholar] [CrossRef]
- Mandle, L.; Ticktin, T. Interactions among fire, grazing, harvest and abiotic conditions shape palm demographic responses to disturbance. J. Ecol. 2012, 100, 997–1008. [Google Scholar] [CrossRef]
- Mandle, L.; Ticktin, T.; Zuidema, P.A. Resilience of palm populations to disturbance is determined by interactive effects of fire, herbivory and harvest. J. Ecol. 2015, 103, 1032–1043. [Google Scholar] [CrossRef]
- Buckley, Y.M.; Bolker, B.M.; Rees, M. Disturbance, invasion and re-invasion: Managing the weed-shaped hole in disturbed ecosystems. Ecol. Lett. 2007, 10, 809–817. [Google Scholar] [CrossRef]
- Li, S.L.; Yu, F.H.; Werger, M.J.; Dong, M.; Ramula, S.; Zuidema, P.A. Understanding the effects of a new grazing policy: The impact of seasonal grazing on shrub demography in the Inner Mongolian steppe. J. Appl. Ecol. 2013, 50, 1377–1386. [Google Scholar] [CrossRef]
- Macias, D.; Mazía, N.; Jacobo, E. Grazing and neighborhood interactions limit woody encroachment in wet subtropical savannas. Basic Appl. Ecol. 2014, 15, 661–668. [Google Scholar] [CrossRef]
- Osem, Y.; Fogel, T.; Moshe, Y.; Brant, S. Managing cattle grazing and overstorey cover for the conversion of pine monocultures into mixed Mediterranean woodlands. Appl. Veg. Sci. 2015, 8, 261–271. [Google Scholar] [CrossRef]
- Wu, G.L.; Du, G.Z.; Liu, Z.H.; Thirgood, S. Effect of fencing and grazing on a Kobresia-dominated meadow in the Qinghai-Tibetan Plateau. Plant Soil 2009, 319, 115–126. [Google Scholar] [CrossRef]
- Khishigjargal, M.; Dulamsuren, C.; Lkhagvadorj, D.; Leuschner, C.; Hauck, M. Contrasting responses of seedling and sapling densities to livestock density in the Mongolian forest-steppe. Plant Ecol. 2013, 214, 1391–1403. [Google Scholar] [CrossRef]
- Zimmermann, H.; Renison, D.; Leyer, I.; Hensen, I. Do we need livestock grazing to promote Polylepis australis tree recruitment in the Central Argentinean Mountains? Ecol. Res. 2009, 24, 1075–1081. [Google Scholar] [CrossRef]
- Forbis, T.A. Seedling demography in an alpine ecosystem. Am. J. Bot. 2003, 90, 1197–1206. [Google Scholar] [CrossRef]
- Wu, G.L.; Li, W.; Li, X.P.; Shi, Z.H. Grazing as a mediator for maintenance of offspring diversity: Sexual and clonal recruitment in alpine grassland communities. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 241–245. [Google Scholar] [CrossRef]
- Bisigato, A.J.; Bertiller, M.B. Temporal and micro-spatial patterning of seedling establishment. Consequences for patch dynamics in the southern Monte, Argentina. Plant Ecol. 2004, 174, 235–246. [Google Scholar] [CrossRef]
- Evju, M.; Halvorsen, R.; Rydgren, K.; Austrheim, G.; Mysterud, A. Effects of sheep grazing and temporal variability on population dynamics of the clonal herb Geranium sylvaticum in an alpine habitat. Plant Ecol. 2011, 212, 1299–1312. [Google Scholar] [CrossRef] [Green Version]
- Faust, C.; Süss, K.; Storm, C.; Schwabe, A. Threatened inland sand vegetation in the temperate zone under different types of abiotic and biotic disturbances during a ten-year period. Flora-Morphol. Distrib. Funct. Ecol. Plants 2011, 206, 611–621. [Google Scholar] [CrossRef]
- Xie, L.N.; Chen, W.Z.; Gabler, C.A.; Han, L.; Guo, H.Y.; Chen, Q.; Ma, C.C.; Gu, S. Effects of grazing intensity on seed production of Caragana stenophylla along a climatic aridity gradient in the Inner Mongolia Steppe, China. J. Arid Land 2016, 8, 890–898. [Google Scholar] [CrossRef] [Green Version]
- Xie, L.N.; Guo, H.Y.; Chen, W.Z.; Liu, Z.; Gu, S.; Ma, C.C. Effects of grazing on population growth characteristics of Caragana stenophylla along a climatic aridity gradient. Rangeland. Ecol. Manag. 2018, 71, 98–105. [Google Scholar] [CrossRef]
- Harel, D.; Holzapfel, C.; Sternberg, M. Seed mass and dormancy of annual plant populations and communities decreases with aridity and rainfall predictability. Basic Appl. Ecol. 2011, 12, 674–684. [Google Scholar] [CrossRef]
- Wang, R.Z.; Gao, Q. Climate-driven changes in shoot density and shoot biomass in Leymus chinensis (Poaceae) on the North-east China Transect (NECT). Global Ecol. Biogeogr. 2003, 12, 249–259. [Google Scholar] [CrossRef]
- Wang, R.Z.; Gao, Q. Morphological responses of Leymus chinensis (Poaceae) to the large-scale climatic gradient along the North-east China Transect (NECT). Divers. Distrib. 2004, 10, 65–73. [Google Scholar] [CrossRef]
- Lotan, A.; Izhaki, I. Could abiotic environment shape fleshy fruit traits? A field study of the desert shrub Ochradenus baccatus. J. Arid. Environ. 2013, 92, 34–41. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Li, X.R.; Fan, H.W.; Wang, X.P.; Tan, H.J.; Li, A.X. Physiological characteristics of several Caragana shrub species under extreme conditions. J. Desert Res. 2005, 25, 182–190. [Google Scholar]
- Fang, X.; Turner, N.C.; Yan, G.; Li, F.; Siddique, K.H. Flower numbers, pod production, pollen viability, and pistil function are reduced and flower and pod abortion increased in chickpea (Cicer arietinum L.) under terminal drought. J. Exp. Bot. 2010, 61, 335–345. [Google Scholar] [CrossRef] [Green Version]
- Salazar-Tortosa, D.; Castro, J.; Saladin, B.; Zimmermann, N.E.; Rubio De Casas, R. Arid environments select for larger seeds in pines (Pinus spp.). Evol. Ecol. 2020, 34, 11–26. [Google Scholar] [CrossRef]
- Sheffer, E.; Batterman, S.A.; Levin, S.A.; Hedin, L.O. Biome-scale nitrogen fixation strategies selected by climatic constraints on nitrogen cycle. Nat. Plants 2015, 1, 15182. [Google Scholar] [CrossRef]
- Adams, M.A.; Turnbull, T.L.; Sprent, J.I.; Buchmann, N. Legumes are different: Leaf nitrogen, photosynthesis, and water use efficiency. Proc. Natl. Acad. Sci. USA 2016, 113, 4098–4103. [Google Scholar] [CrossRef]
- Ouyang, S.; Tian, Y.; Liu, Q.; Zhang, L.; Sun, Y.; Xu, X.; Liu, Y. Symbiotic nitrogen fixation and interspecific transfer by Caragana microphylla in a temperate grassland with 15 N dilution technique. Appl. Soil Ecol. 2016, 108, 221–227. [Google Scholar] [CrossRef]
- Piqueras, J. Herbivory and ramet performance in the clonal herb Trientalis europaea L. J. Ecol. 1999, 87, 450–460. [Google Scholar] [CrossRef]
- Peco, B.; Borghi, C.E.; Malo, J.E.; Acebes, P.; Almirón, M.; Campos, C.M. Effects of bark damage by feral herbivores on columnar cactus Echinopsis (= Trichocereus) terscheckii reproductive output. J. Arid. Environ. 2011, 75, 981–985. [Google Scholar] [CrossRef]
- Becklin, K.M.; Kirkpatrick, H.E. Compensation through rosette formation: The response of scarlet gilia (Ipomopsis aggregata: Polemoniaceae) to mammalian herbivory. Botany 2006, 84, 1298–1303. [Google Scholar] [CrossRef]
- Hayes, C.L.; Talbot, W.A.; Wolf, B.O. Woodrat herbivory influences saguaro (Carnegiea gigantea) reproductive output. J. Arid Environ. 2013, 89, 110–115. [Google Scholar] [CrossRef]
- Boulant, N.; Kunstler, G.; Rambal, S.; Lepart, J. Seed supply, drought, and grazing determine spatio-temporal patterns of recruitment for native and introduced invasive pines in grasslands. Divers. Distrib. 2008, 14, 862–874. [Google Scholar] [CrossRef]
- López-Sánchez, A.; Schroeder, J.; Roig, S.; Sobral, M.; Dirzo, R. Effects of cattle management on oak regeneration in northern Californian Mediterranean oak woodlands. PLoS ONE 2014, 9, e105472. [Google Scholar] [CrossRef] [PubMed]
- Bradley, B.A. Regional analysis of the impacts of climate change on cheatgrass invasion shows potential risk and opportunity. Glob. Chang. Biol. 2009, 15, 196–208. [Google Scholar] [CrossRef]
- Concilio, A.L.; Loik, M.E.; Belnap, J. Global change effects on Bromus tectorum L.(Poaceae) at its high-elevation range margin. Global Chang. Biol. 2013, 19, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.P.; Shao, W.S.; Jin, C.Q.; Song, L.; Gong, S.; Li, G. Effects of enclosure on biomass allocation characteristics of Agropyron mongolicum population in desert steppe. Ecol. Environ. Sci. 2017, 26, 2024–2029. [Google Scholar] [CrossRef]
- Wei, Z.J.; Yan, R.R.; Yun, X.J.; Chu, W.B.; Yang, J. Study on biomass and energy allocation of major plant species in desert steppe under different grazing systems. J. Desert Res. 2011, 31, 1124–1130. [Google Scholar] [CrossRef]
- Devoto, M.; Medan, D. Effects of grazing disturbance on the reproduction of a perennial herb, Cypella herbertii (Lindl.) Herb.(Iridaceae). Plant Syst. Evol. 2004, 243, 165–173. [Google Scholar] [CrossRef]
- Gómez, J.M. Long-term effects of ungulates on performance, abundance, and spatial distribution of two montane herbs. Ecol. Monogr. 2005, 75, 231–258. [Google Scholar] [CrossRef] [Green Version]
- Heckel, C.D.; Bourg, N.A.; McShea, W.J.; Kalisz, S. Nonconsumptive effects of a generalist ungulate herbivore drive decline of unpalatable forest herbs. Ecology 2010, 91, 319–326. [Google Scholar] [CrossRef] [Green Version]
- Warner, P.J.; Cushman, H.J. Influence of herbivores on a perennial plant: Variation with life history stage and herbivore species. Oecologia 2002, 132, 77–85. [Google Scholar] [CrossRef]
- Perevolotsky, A.; Schwartz-Tzachor, R.; Yonathan, R.; Ne’eman, G. Geophytes–herbivore interactions: Reproduction and population dynamics of Anemone coronaria L. Plant Ecol. 2011, 212, 563–571. [Google Scholar] [CrossRef]




| Site | Longitude (°E) | Latitude (°N) | Altitude (m) | Mean Annual Precipitation (mm) | Mean Annual Temperature (°C) | Aridity Index | Aridity Type |
|---|---|---|---|---|---|---|---|
| Xiwu | 117°38′53″ | 44°36′50″ | 1024 | 354 | 2.42 | 28.51 | Subhumid |
| Abaga | 114°58′02″ | 44°04′00″ | 1126 | 245 | 1.87 | 20.64 | Semi-arid |
| Suniteyou | 112°56′39″ | 42°21′00″ | 1144 | 220 | 3.09 | 16.81 | Arid |
| Siziwang | 111°53′22″ | 41°47′28″ | 1492 | 210 | 3.60 | 15.44 | Dry arid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, L.; Li, Y.; Lin, M.; Guo, H.; Wang, Y.; Wang, L.; Ma, C. Sexual Reproduction Is Not Responsible for Caragana Shrub Encroachment in Grasslands. Agronomy 2023, 13, 1848. https://doi.org/10.3390/agronomy13071848
Xie L, Li Y, Lin M, Guo H, Wang Y, Wang L, Ma C. Sexual Reproduction Is Not Responsible for Caragana Shrub Encroachment in Grasslands. Agronomy. 2023; 13(7):1848. https://doi.org/10.3390/agronomy13071848
Chicago/Turabian StyleXie, Lina, Yuchen Li, Mingyan Lin, Hongyu Guo, Yue Wang, Lihong Wang, and Chengcang Ma. 2023. "Sexual Reproduction Is Not Responsible for Caragana Shrub Encroachment in Grasslands" Agronomy 13, no. 7: 1848. https://doi.org/10.3390/agronomy13071848
APA StyleXie, L., Li, Y., Lin, M., Guo, H., Wang, Y., Wang, L., & Ma, C. (2023). Sexual Reproduction Is Not Responsible for Caragana Shrub Encroachment in Grasslands. Agronomy, 13(7), 1848. https://doi.org/10.3390/agronomy13071848