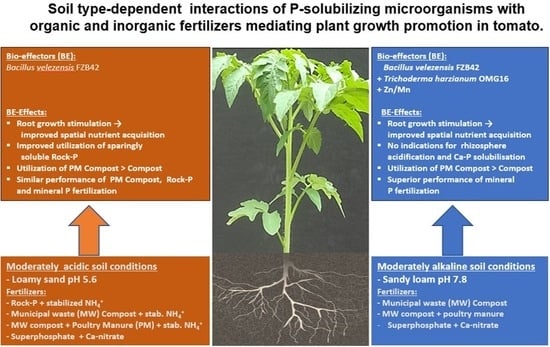
Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Properties
2.2. Test Plant
2.3. Culture Conditions
2.4. Fertilization
2.4.1. Alkaline Soil (Experiment 1)
2.4.2. Acidic Soil (Experiment 2)
2.5. Bioeffectors (BEs)
2.5.1. Alkaline Soil
2.5.2. Acidic Soil
2.6. Plant Biomass and Root Length
2.7. Shoot N, P, K, and Mg Concentration and Content
2.8. Rhizosphere Soil pH
2.9. Phosphorous Recovery Efficiency
2.10. Experimental Setup and Data Analysis
3. Results
3.1. Experiment 1, Alkaline Soil (pH 7.8)
3.1.1. Plant Growth and Rhizosphere pH
3.1.2. Plant Nutritional Status
3.2. Experiment 2, Acidic Soil (pH 5.6)
3.2.1. Plant Growth and Development
3.2.2. Plant Nutritional Status
4. Discussion
4.1. Fertilizer Effects
4.2. PGPM Effects
4.2.1. Alkaline Soil
4.2.2. Acidic Soil
4.3. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, W.J.; Zhang, X.Y. A forecast analysis on fertilizers consumption worldwide. Environ. Monit. Assess. 2007, 133, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tilman, D.; Fargione, J.; Wolff, B.; D’Antonio, C.; Dobson, A. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [PubMed]
- Stoffella, P.J.; He, Z.I.; Wilson, S.B.; Ozores-Hampton, M.; Roe, B.E. Compost Utilization in Subtropical Horticultural Cropping Systems. In I International Symposium on Organic Matter Management and Compost in Horticulture; Biala, J., Prange, R., Raviv, M., Eds.; Acta Horticulturae: Adelaide, Australia, 2014; pp. 95–108. [Google Scholar]
- Zhou, X.; Qiao, M.; Wang, F.H.; Zhu, Y.G. Use of commercial organic fertilizer increases the abundance of antibiotic resistance genes and antibiotics in soil. Environ. Sci. Pollut. Res. 2017, 24, 701–710. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Sharma, B.S.; Riyaz, Z.; Sayed, M.H.T.; Thivakaran, A.G. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. Springer Plus 2013, 2, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sheraz, M.S.; Hassan, G.I.; Samoon, S.A.; Rather, H.A.; Dar, S.A.; Zehra, B. Bio-fertilizers in Organic Agriculture. J. Phytol. 2010, 2, 42–54. [Google Scholar]
- Verbon, E.; Liberman, L.M. Microbes affect endogenous mechanisms controlling root development. Trends Plant Sci. 2016, 21, 218–229. [Google Scholar] [CrossRef] [PubMed]
- Menzies, N.; Harbison, D.; Dart, P. Soil chemistry-facts and fiction and their influence on the fertilizer decision making process. In Proceedings of the 26th Annual Conference of the Grassland Society of NSW, Bathurst, Australia, 26–28 July 2011; pp. 49–63. [Google Scholar]
- Schütz, L.; Gattinger, A.; Meier, M.; Muller, A.; Boller, T.; Mäder, P.; Mathimaran, N. Improving crop yield and nutrient use efficiency via biofertilization–A global meta-analysis. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Thonar, C.; Lekfeldt, J.D.S.; Cozzolino, V.; Kundel, D.; Kulhánek, M.; Mosimann, C.; Neumann, G.; Piccolo, A.; Rex, M.; Symanczik, S.; et al. Potential of three microbial bio-effectors to promote maize growth and nutrient acquisition from alternative phosphorous fertilizers in contrasting soils. Chem. Biol. Technol. Agric. 2017, 4, 7. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Weinmann, M.; Müller, T. Improving fertilizer-depot exploitation and maize growth by inoculation with plant growth-promoting bacteria: From lab to field. Chem. Biol. Technol. Agric. 2016, 3, 15. [Google Scholar] [CrossRef]
- Nkebiwe, P.M.; Neumann, G.; Müller, T. Densely rooted rhizosphere hotspots induced around subsurface NH4+-fertilizer depots: A home for soil PGPMs? Chem. Biol. Technol. Agric. 2017, 4, 29. [Google Scholar] [CrossRef]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Handbuch der Landwirtschaftlichen Versuchs- und Untersuchungsmethodik Methodenbuch Band I Die Untersuchung von Böden, 4th ed.; VDLUFA Verlag: Darmsatdt, Germany, 1991. [Google Scholar]
- Bai, Z.; Li, H.; Yang, X.; Zhou, B.; Shi, X.; Wang, B.; Li, D.; Shen, J.; Chen, Q.; Qin, Q.; et al. The critical soil P levels for crop yield, soil fertility and environmental safety in different soil types. Plant Soil 2013, 372, 27–37. [Google Scholar] [CrossRef]
- VDLUFA (Verband Deutscher Landwirtschaftlicher Untersuchungs-und Forschungsanstalten e.V. Speyer, Germany). Standpunkt. Phosphordüngung nach Bodenuntersuchung und Pflanzenbedarf; VDLUFA Verlag: Darmsatdt, Germany, 2018. [Google Scholar]
- Landwirtschaftskammer Niedersachsen. Richtwerte für die Düngung in Niedersachsen. In Auszug aus den Düngungsrichtlinien, Stand März 2008, Mikronährstoffe Bor, Mangan, Kupfer und Zink. Landwirtschaftskammer Niedersachsen; Fachbereich Pflanzenbau: Hannover, Germany, 2008. [Google Scholar]
- Gericke, S.; Kurmis, B. Die kolorimetrische Phophorsäurebestimmung mit Ammonium-Vanadat-Molybdat und ihre Anwendung in der Pflanzenanalyse. Z. Pflanzenernaehr. Bodenkd 1952, 59, 235–247. [Google Scholar]
- Pilbeam, D.J.; Cakmak, I.; Marschner, H.; Kirkby, E.A. Effect of withdrawal of phosphorus on nitrate assimilation and PEP carboxylase activity in tomato. Plant Soil 1993, 154, 111–117. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root excretion of carboxylic acids and protons in phosphorus-deficient plants. Plant Soil 1999, 211, 121–130. [Google Scholar] [CrossRef]
- Campbell, R.C. Reference sufficiency ranges for plant analysis in the southern region of the United States. In Southern Cooperative Series Bulletin #394; North Carolina Department of Agriculture and Consumer Services Agronomic Division: Raleigh, NC, USA, 2009; p. 79. [Google Scholar]
- Abbasi, M.K.; Musa, N.; Manzoor, M. Mineralization of soluble P fertilizers and insoluble rock phosphate in response to phosphate-solubilizing bacteria and poultry manure and their effect on the growth and P utilization efficiency of chilli (Capsicum annuum L.). Biogeosciences 2015, 12, 4607–4619. [Google Scholar] [CrossRef]
- Kanabo, A.I.; Gilkes, R. The role of soil pH in the dissolution of phosphate rock fertilizers. Fert. Res. 1987, 12, 165–173. [Google Scholar] [CrossRef]
- Neumann, G.; Römheld, V. Root-induced changes in the availability of nutrients in the rhizosphere. In Plant Roots the Hidden Half, 3rd ed.; Waisel, Y., Eshel, A., Kafkafi, U., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 617–649. [Google Scholar]
- Cakmak, I. Possible role of Zn in protecting plant cells from damage by reactive oxygen species. New Phytol. 2000, 146, 185–205. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Elmer, W.H.; Huber, D.M. Mineral Nutrition and Plant Disease; American Phytopathological Society: St. Paul, AK, USA, 2007. [Google Scholar]
- Von Rad, U.; Klein, I.; Dobrev, P.I.; Kottova, J.; Zazimolova, E.; Fekete, A.; Hartmann, A.; Schmitt-Kopplin, P.; Durner, J. Response of Arabidopsis thaliana to N-hexanoyl-DL-homoserine-lactone, a bacterial quorum sensing molecule produce in the rhizosphere. Planta 2008, 229, 73–85. [Google Scholar] [CrossRef] [PubMed]
- Soni, R.; Yadav, S.K.; Rajput, A.S. ACC-Deaminase Producing Rhizobacteria: Prospects and Application as Stress Busters for Stressed Agriculture. Microorg. Green Revolut. 2018, 7, 161–175. [Google Scholar]
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J.; Lee, I.J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Electron. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G. The role of ethylene in plant adaptations for phosphate acquisition in soils—A review. Front. Plant Sci. 2016, 6, 1224. [Google Scholar] [CrossRef] [PubMed]
- Borriss, R. Bacillus, a plant beneficial bacterium. In Principles of Plant Microbe Interactions; Lugtenberg, B., Ed.; Springer International Publishing: Cham, Switzerland, 2015; pp. 379–389. [Google Scholar]
- Meng, Q. Characterization of Bacillus Amyloliquefaciens Strain BAC03 in Disease Control and Plant Growth Promotion; ProQuest Dissertations Publishing: East Lansing, MI, USA, 2014. [Google Scholar]
- Saber, W.I.A.; Ghoneem, K.M.; Rashad, Y.M.; Al-Askar, A.A. Trichoderma harzianum WKY1: An indole acetic acid producer for growth improvement and anthracnose disease control in sorghum. Biocontrol Sci. Technol. 2017, 27, 654–676. [Google Scholar] [CrossRef]
- Yusran, Y.; Roemheld, V.; Mueller, T. Effects of Pseudomonas sp. “Proradix” and Bacillus amyloliquefaciens FZB42 on the establishment of AMF infection, nutrient acquisition and growth of tomato affected by Fusarium oxysporum Schlecht f.sp. radicis-lycopersici Jarvis and shoemaker. In Proceedings of the International Plant Nutrition Colloquium XVI, Sacramento, CA, USA, 26–30 August 2009; p. 1106. [Google Scholar]
- Saleem, M.; Law, A.D.; Sahib, M.R.; Pervaiz, Z.H.; Zhang, Q. Impact of root system architecture on rhizosphere and root microbiome. Rhizosphere 2018, 6, 47–51. [Google Scholar] [CrossRef]
- Suen, P.K.; Zhang, S.; Sun, S.S.M. Molecular characterization of a tomato purple acid phosphatase during seed germination and seedling growth under phosphate stress. Plant Cell Rep. 2015, 34, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Lekfeldt, J.D.S.; Rex, M.; Mercl, F.; Magid, K.; Tlustoš, P.; Magid, J. Effect of bioeffectors and recycled P-fertiliser products on the growth of spring wheat. Chem. Biol. Technol. Agric. 2016, 3, 22. [Google Scholar] [CrossRef]
- Rosen, C.J.; Bierman, P.M. Using Manure and Compost as Nutrient Sources for Fruit and Vegetable Crops. Available online: https://www.plantgrower.org/uploads/6/5/5/4/65545169/manure-and-compost.pdf (accessed on 29 May 2018).
- Ögüt, M.; Er, F.; Neumann, G. Increased proton extrusion of wheat roots by inoculation with phosphorus solubilising microorganisms. Plant Soil 2011, 339, 285–297. [Google Scholar] [CrossRef]
- Dobermann, A.R. Nitrogen Use Efficiency–State of the Art; Agronomy—Faculty Publications: Lincoln, NE, USA, 2005; pp. 1–16. [Google Scholar]








| Soil Properties | Soil Origin | |
|---|---|---|
| Atebubu | Dormaa Ahenkro | |
| Soil pH (CaCl2) | 5.6 | 7.8 |
| Total Nitrogen [%] | 0.05 | 0.30 |
| NO3 -N [mg kg−1 soil] | 2.4 | 44.2 |
| Plant available P [mg kg−1 soil] | 7.22 (P CAL) | 2.22 (P Olsen) |
| Total P (ICP-OES) [mg kg−1 soil] | 90 | 473 |
| K (CAL extract) [mg kg−1 soil] | 33.2 | 357 |
| Mg (CaCl2) [mg kg−1 soil] | 110 | 250 |
| Total Ca [mg kg−1 soil] | 632 | 10,523 |
| Fe (CAT extract) [mg kg−1 soil] | 56.5 | 29.0 |
| Zn (CAT extract) [mg kg−1 soil] | <1 | 4.0 |
| Mn (CAT extract) [mg kg−1 soil] | 188.0 | 27.3 |
| Cu (CAT extract) [mg kg−1 soil] | 0.54 | 1.14 |
| Total Carbon [%] | 0.75 | 4.82 |
| Humus [%] | 1.23 | 7.89 |
| Sand (63–2000 µm) % | 66.4 | 44.4 |
| Silt (2–63 µm) % | 28.6 | 38.3 |
| Clay (<2 µm) % | 5.0 | 17.3 |
| Shoot Mineral Content (mg Plant−1) | |||||
|---|---|---|---|---|---|
| N | P | K | Mg | Ca | |
| No fertilization | 66.3 e | 2.7 d | 102.9 d | 18.0 e | 62.0 e |
| CFB | 94.2 d * | 7.5 b * | 169.2 c * | 25.1 cd * | 102.9 cd * |
| Compost | 86.6 d | 6.3 bc | 157.6 c | 23.5 d | 85.5 d |
| Compost_CFB | 107.3 d * | 7.4 b | 192.3 bc * | 28.3 cd * | 110.7 c * |
| PM Compost | 148.4 c | 5.8 bc | 181.2 c | 31.2 bc | 108.6 cd |
| PM Compost_CFB | 213.6 b * | 8.7 b * | 232.2 b * | 37.8 b * | 143.2 b * |
| NP_ | 289.1 a | 37.0 a | 405.1 a | 57.7 a | 233.7 a |
| Shoot Minerals Content (mg Plant−1) | |||||
|---|---|---|---|---|---|
| N | P | K | Mg | Ca | |
| Unfertilized | 71.2 d | 3.4 f | 108.9 e | 22.2 c | 46.6 d |
| Compost _NH4+ | 108.9 d | 6.5 ef | 159.9 de | 28.5 bc | 71.0 c |
| Compost _NH4+_FZB42 | 106.2 d | 7.5 ef | 194.9 cd* | 30.3 bc | 81.0 bc |
| PM Compost_NH4+ | 228.3 c | 9.7 de | 199.9 c | 33.7 b | 79.6 bc |
| PM Compost_NH4+_FZB42 | 247.7 c | 13.2 d | 268.9 a* | 49.3 a * | 111.0 a * |
| Rock P_NH4+ | 314.8 b | 30.7 c | 214.1 bc | 34.8 b | 65.0 c |
| Rock P_NH4+_FZB42 | 394.1 a * | 37.3 b | 297 a | 50.0 a * | 94.7 b * |
| TSP_NO3− (NP) | 373.8 a | 66.9 a | 253.5 ab | 35.5 b | 80.4 bc |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mpanga, I.K.; Dapaah, H.K.; Geistlinger, J.; Ludewig, U.; Neumann, G. Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato. Agronomy 2018, 8, 213. https://doi.org/10.3390/agronomy8100213
Mpanga IK, Dapaah HK, Geistlinger J, Ludewig U, Neumann G. Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato. Agronomy. 2018; 8(10):213. https://doi.org/10.3390/agronomy8100213
Chicago/Turabian StyleMpanga, Isaac Kwadwo, Harrison Kwame Dapaah, Joerg Geistlinger, Uwe Ludewig, and Günter Neumann. 2018. "Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato" Agronomy 8, no. 10: 213. https://doi.org/10.3390/agronomy8100213
APA StyleMpanga, I. K., Dapaah, H. K., Geistlinger, J., Ludewig, U., & Neumann, G. (2018). Soil Type-Dependent Interactions of P-Solubilizing Microorganisms with Organic and Inorganic Fertilizers Mediate Plant Growth Promotion in Tomato. Agronomy, 8(10), 213. https://doi.org/10.3390/agronomy8100213