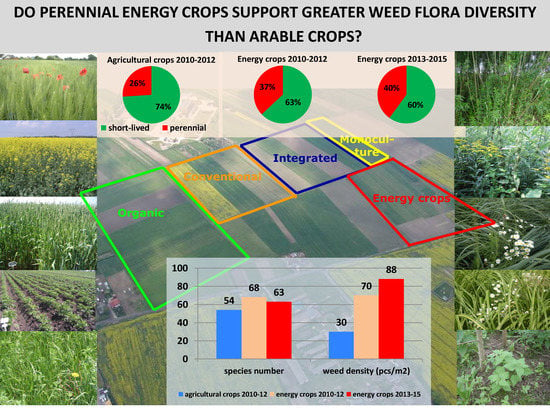
Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of Experimental Plots with Perennial Energy Crops
2.2. Characteristics of the Experimental Plots with Annual Crops Cultivated under Different Farming Systems
2.3. Methodology Used for Flora Diversity Assessments
2.4. Statistical Analyses
2.4.1. Diversity Indicators
2.4.2. Hierarchical Classification
2.4.3. Ordination Method
2.4.4. Assessment of the Significance of Differences
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pedroli, B.; Elbersen, B.; Frederiksen, P.; Grandin, U.; Heikkila, R.; Krogh, P.H.; Izakovicova’, Z.; Johansen, A.; Meiresonne, L.; Spijker, J. Is energy cropping in Europe compatible with biodiversity? Opportunities and threats to biodiversity from land-based production of biomass for bioenergy purposes. Biomass Bioenergy 2013, 55, 73–86. [Google Scholar] [CrossRef]
- Fletcher, R.J., Jr.; Robertson, B.A.; Evans, L.; Doran, P.J.; Alavalapati, J.R.R.; Schemske, D.W. Biodiversity conservation in the era of biofuels, risks and opportunities. Front. Ecol. Environ. 2011, 3, 161–168. [Google Scholar] [CrossRef]
- Dauber, J.; Jones, M.B.; Stout, J.C. The impact of biomass crop cultivation on temperate biodiversity. GCB Bioenergy 2010, 2, 289–309. [Google Scholar] [CrossRef]
- Perttu, K.L. Ecological, biological balances and conservation. Biomass Bioenergy 1995, 9, 107–116. [Google Scholar] [CrossRef]
- Britt, C. Methodologies for Ecological Monitoring in Bioenergy Crops; A review and recommendations, Defra Project NF0408; ADAS Contract Report for the Department for Environment, Food and Rural Affairs: Oxford, UK, 2003; p. 63. [Google Scholar]
- European Environmental Agency. How much Bioenergy Can Europe Produce without Harming the Environment; EEA Report 7/2006; European Environmental Agency: Copenhagen, Denmark, 2006; p. 67. [Google Scholar]
- Börjesson, P. Environmental effects of energy crop cultivation in Sweden—I: Identification and quantification. Biomass Bioenergy 1999, 16, 137–154. [Google Scholar] [CrossRef]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Buhler, D.D.; Netzer, D.A.; Riemenschneider, D.E.; Hartzler, R.G. Weed management in short rotation poplar and herbaceous perennial crops grown for biofuel production. Biomass Bioenergy 1998, 14, 385–394. [Google Scholar] [CrossRef]
- Hartman, J.C.; Nippert, J.B.; Orozco, R.A.; Springer, C.J. Potential ecological impacts of switchgrass (Panicum virgatum L.) biofuel cultivation in the Central Great Plains, USA. Biomass Bioenergy 2011, 35, 3415–3421. [Google Scholar] [CrossRef]
- Sage, R.B. Short rotation coppice for energy: Towards ecological guidelines. Biomass Bioenergy 1998, 15, 39–47. [Google Scholar] [CrossRef]
- Cunningham, M.D.; Bishop, J.D.; McKay, H.V.; Sage, R.B. ARBRE Monitoring-Ecology of Short Rotation Coppice; URN 04/961; DTI: London, UK, 2004; p. 157. [Google Scholar]
- Fry, D.A.; Slater, F.M.; Reboud, X. The effect on plant communities and associated taxa of planting short rotation willow coppice in Wales. Asp. Appl. Biol. 2008, 90, 287–293. [Google Scholar]
- Rowe, R.L.; Street, N.R.; Taylor, G. Identifying potential environmental impacts of large-scale deployment of dedicated bioenergy crops in the UK. Renew. Sustain. Energy Rev. 2009, 13, 271–290. [Google Scholar] [CrossRef]
- Tuomisto, H.L.; Hodge, I.D.; Riordan, P.; Macdonald, D.W. Comparing energy balances, greenhouse gas balances and biodiversity impacts of contrasting farming systems with alternative land uses. Agric. Syst. 2012, 108, 42–49. [Google Scholar] [CrossRef]
- Sage, R.B.; Robertson, P.A.; Poulson, J.G. Enhancing the Conservation Value of Short Rotation Biomass Coppice: Phase 1 the Identification of Wildlife Conservation Potential; ETSU B/W5/0027/REP; DTI: London, UK, 1994; p. 119. [Google Scholar]
- Mola-Yudego, B.; Díaz-Yáñez, O.; Dimitriou, I. How Much Yield Should We Expect from Fast-Growing Plantations for Energy? Divergences Between Experiments and Commercial Willow Plantations. Bioenergy Res. 2015, 8, 1769–1777. [Google Scholar] [CrossRef]
- Dauber, J.; Bolte, A. Bioenergy: Challenge or support for the conservation of biodiversity? GCB Bioenergy 2014, 6, 180–182. [Google Scholar] [CrossRef] [Green Version]
- Tokarska-Guzik, B. The Establishment and Spread of Alien Plant Species (Kenophytes) in the Flora of Poland; University of Silesia: Katowice, Poland, 2005; Volume 2372, p. 192. [Google Scholar]
- Anioł-Kwiatkowska, J.; Śliwiński, M. Energy plants of alien origin-threat to the native flora of Poland. Pam. Puł. 2009, 150, 35–44. (In Polish) [Google Scholar]
- Buddenhagen, C.E.; Chimera, C.; Clifford, P. Assessing biofuel crop invasiveness: A case study. PLoS ONE 2009, 4, e5261. [Google Scholar] [CrossRef]
- Tokarska-Guzik, B.; Dajdok, Z.; Zając, M.; Zając, A.; Urbisz, A.; Danielewicz, W.; Hołdyński, C. The Plants of Foreign Origin in Poland, with Particular Regard to Invasive Species; Generalna Dyrekcja Ochrony Środowiska: Warsaw, Poland, 2012; p. 197. [Google Scholar]
- Verdade, L.M.; Piña, C.I.; Rosalino, L.M. Biofuels and biodiversity: Challenges and opportunities. Environ. Dev. 2015, 15, 64–78. [Google Scholar] [CrossRef]
- Kovacs-Lang, E.; Simpson, I.C. Biodiversity Measurements and Indicators for Long-Term Integrated Monitoring; No LIMITS, Report No. 6; Institute of Ecology and Botany of the Hungarian Academy of Sciences, Vácrátót & Centre for Ecology and Hydrology: Grange-over-Sands, UK, 2000; p. 24. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources, 2nd ed.; World Soil Resources Reports No. 103; FAO: Rome, Italy, 2006; p. 132. [Google Scholar]
- Kuś, J.; Faber, A.; Stasiak, M.; Kawalec, A. Productivity of Selected Plant Species Cultivated for Energy Purposes in Different Habitats; Studia i Raporty IUNG-PIB: Puławy, Poland, 2008; Volume 11, pp. 67–80. (In Polish) [Google Scholar]
- Available online: https://www.agrofagi.com.pl/352,programmes-for-integrated-pest-management.html (accessed on 11 October 2019).
- Rutkowski, L. Key to Identification of Vascular Plants in Lowland Poland; PWN: Warsaw, Poland, 2007; p. 822. [Google Scholar]
- Shannon, C.E. A mathematical theory of communications. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of diversity. Nature 1949, 168, 668. [Google Scholar] [CrossRef]
- Kovach, W.L. MVSP version 3; Kovach Computing Services: Anglesey, UK, 2011; p. 112. [Google Scholar]
- Jongman, R.H.G.; ter Braak, C.J.H.; Van Tongeren, D.F.R. Data Analysis in Community and Landscape Ecology; Pudoc.: Wageningen, The Netherlands, 1987; p. 295. [Google Scholar]
- Kent, M.; Coker, P. Vegetation Description and Analysis: A Practical Approach; Belhaven Press: London, UK, 1992; p. 363. [Google Scholar]
- Magurran, A.E. Ecological Diversity and Its Measurement; Princeton University Press: Princeton, NJ, USA, 1988; p. 179. [Google Scholar]
- Piernik, A. The Use of Numerical Methods in Ecology; Nicolaus Copernicus University: Toruń, Poland, 2012; p. 113. [Google Scholar]
- Lepš, J.; Šmilauer, P. Multivariate Analysis of Ecological Data Using CANOCO; Cambridge University Press: Cambridge, UK, 2003; p. 269. [Google Scholar]
- Ter Braak, C.J.F.; Smilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide: Software for Canonical Community Ordination, version 4.5; Microcomputer Power: Ithaca, NY, USA, 2002; p. 55. [Google Scholar]
- Van der Maarel, E. Multivariate analysis in plant ecology. In Numerical Methods in Studying the Structure and Functioning of Vegetation; Kaźmierczak, E., Ed.; V School and XLVI Geobotanical Seminar of the Polish Botanical Society; University Nicholas Copernicus in Torun: Torun, Poland, 1998; pp. 65–108. [Google Scholar]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. PAST Paleontological Statistics Version 3.14: Reference Manual; University of Oslo: Oslo, Norway, 2016; p. 259. [Google Scholar]
- Semere, T.; Slater, F.M. Ground flora, small mammal and bird species diversity in miscanthus (Miscanthus×giganteus) and reed canary grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Rola, J.; Sekutowski, T.; Rola, H.; Badowski, M. Weed problems on the new Miscanthus gigantheus plantations. Pam. Puł. 2009, 150, 233–246. [Google Scholar]
- Rola, J.; Sekutowski, T.; Rola, H.; Badowski, M. Biodiversity of weed communities on willow (Salix viminalis L.) plantation in the dolnoslaskie and opolskie voivodeship. Pam. Puł. 2007, 145, 165–175. [Google Scholar]
- Trąba, C.; Majda, J.; Wolański, P. Plant communities accompanying plantations of Salix cordata „Americana” Hort. and Salix viminalis L. in Podkarpackie voivodeship. Pam. Puł. 2007, 145, 221–231. [Google Scholar]
- Kościk, B.; Ziemińska-Smyk, M. Weed communities in perennial energy plants. Pam. Puł. 2009, 150, 171–180. [Google Scholar]
- Bourke, D.; Stanley, D.; O’Rourke, E.; Thompson, R.; Carnus, T.; Dauber, J.; Emmerson, M.; Whelan, P.; Heco, F.; Flynn, E.; et al. Response of farmland biodiversity to the introduction of bioenergy crops: Effects of local factors and surrounding landscape context. GCB Bioenergy 2014, 6, 275–289. [Google Scholar] [CrossRef]
- Sekutowski, T.; Bortniak, M. Usage of microbiotest Phytotoxkit™ in detecting of allelopathic potential of Phalaris arundinacea. J. Res. Appl. Agric. Eng. 2009, 54, 88–93. [Google Scholar]
- Sobisz, Z.; Ratuszniak, I. Vascular flora of Salix viminalis L., Helianthus tuberosus L. and Rosa multiflora Thunb. crops on Central Pomerania. Pam. Puł. 2009, 150, 307–322. [Google Scholar]
- Zanin, G.; Otto, S.; Riello, L.; Borin, M. Ecological interpretation of weed flora dynamics under different tillage systems. Agric. Ecosyst. Environ. 1997, 66, 177–188. [Google Scholar] [CrossRef]
- Hyvönen, T.; Ketoja, E.; Salonen, J.; Jalli, H.; Tiainen, J. Weed species diversity and community composition in organic and conventional cropping of spring cereals. Agric. Ecosyst. Environ. 2003, 97, 131–149. [Google Scholar] [CrossRef]
- Armengot, L.; José-María, L.; Chamorro, L.; Sans, F.X. Weed harrowing in organically grown cereal crops avoids yield losses without reducing weed diversity. Agron. Sustain. Dev. 2013, 33, 405–411. [Google Scholar] [CrossRef]
- Baessler, C.; Klotz, S. Effects of changes in agricultural land-use on landscape structure and arable weed vegetation over the last 50 years. Agric. Ecosyst. Environ. 2006, 115, 43–50. [Google Scholar] [CrossRef]
- Krawczyk, R. Trends in changes of weed infestation-hope and risk. Prog. Plant Prot. 2005, 45, 233–241. [Google Scholar]
- Smeets, E.M.W.; Lewandowski, I.; Andre, M.; Faaij, P.C. The economical and environmental performance of miscanthus and switchgrass production and supply chains in a European setting. Renew. Sustain. Energy Rev. 2009, 13, 1230–1245. [Google Scholar] [CrossRef]
- Weih, M.; Karacic, A.; Munkert, H.; Verwijst, T.; Diekmann, M. Influence of young poplar stands on floristic diversity in agricultural landscapes (Sweden). Basic Appl Ecol. 2003, 4, 149–156. [Google Scholar] [CrossRef]
- Archaux, F.; Chevaliera, R.; Berthelot, A. Towards practices favourable to plant diversity in hybrid poplar plantations. For. Ecol. Manag. 2010, 259, 2410–2417. [Google Scholar] [CrossRef]
- Anioł-Kwiatkowska, J.; Kącki, Z.; Śliwiński, M. A comparison of species composition of three energy willow crops. Pam. Puł. 2009, 150, 19–34. [Google Scholar]
- Korniak, T.; Hołdyński, C.; Wąsowicz, K. Changes in the weed flora of willow plantations in north-eastern Poland. Pam. Puł. 2009, 150, 159–170. [Google Scholar]
- Wojciechowski, W.; Sowinski, J.; Zawieja, J. The effect of age of willow plantation on weed infestation in the Sudety Mountains. Pam. Puł. 2009, 150, 351–358. [Google Scholar]
- Rowe, R.L.; Goulson, D.; Doncaster, C.P.; Clarke, D.J.; Taylor, G.; Hanley, M.E. Evaluating ecosystem processes in willow short rotation coppice bioenergy plantations. GCB Bioenergy 2013, 5, 257–266. [Google Scholar] [CrossRef]
- Whittingham, M.J. The future of agri-environment schemes: Biodiversity gains and ecosystem service delivery? J. Appl. Ecol. 2011, 48, 509–513. [Google Scholar] [CrossRef]
- Manning, P.; Taylor, G.; Hanley, M.E. Bioenergy, Food Production and Biodiversity—An Unlikely Alliance? GCB Bioenergy 2015, 7, 570–576. [Google Scholar] [CrossRef]











| Type of Crop for Energy Purposes | Crop Species | Type of Management Adopted | Year of Plantation Establishment | Plant Density (pl. ha −1)/Sowing Rate (Seeds ha−1) | Yield (t DM* ha−1 Year−1) |
|---|---|---|---|---|---|
| Trees and bushes (T) | willow (w_1) | harvested every year | 2004 | 40,000 cuttings ha−1 | 16.3 |
| willow (w_3) | harvested every 3 years | 2004 | 40,000 cuttings ha−1 | 26.4 | |
| poplar (po) | harvested every year | 2008 | 8,000 cuttings ha−1 | 13.4 | |
| false acacia (a) | harvested every year | 2008 | 17,000 cuttings ha−1 | 9.0 | |
| Perennial dicotyledonous plants (D) | Virginia mallow cultivated from seedlings (v) | harvested every year | 2004 | 20,000 plants ha−1 | 17.7 |
| Virginia mallow cultivated from seeds (v_s) | harvested every year | 2004 | 1.5 kg seeds ha−1 | 14.5 | |
| Jerusalem artichoke (j) | harvested every year | 2004 | 20,000 tubers ha−1 | 12.7 | |
| Perennial grasses (G) | miscanthus (m) | harvested every year, usually before winter | 2004 | 15,000 cuttings ha−1 | 18.9 |
| reed canary grass (r) | harvested every year | 2004 | 20 kg seeds ha−1 | 9.6 | |
| switchgrass (s) | harvested every year | 2008 | 22,000 plants ha−1 | 14.6 | |
| big bluestem (b) | harvested every year | 2008 | 22,000 plants ha−1 | 12.8 | |
| prairie cordgrass (pr) | harvested every year | 2008 | 11,000 plants ha−1 | 23.2 |
| Items | Organic (O) | Integrated (I) | Conventional (C) | Monoculture (M) |
|---|---|---|---|---|
| Crop rotation | potato spring wheat + undersown crop clovers and grasses (1st year) clovers and grasses (2nd year) winter wheat + catch crop | potato spring wheat + catch crop fava bean or blue lupine winter wheat + catch crop | winter rape winter wheat spring wheat | winter wheat |
| Organic fertilization | compost (30 t ha−1) under potato + catch crop | compost (30 t ha−1) under potato + 2× catch crop | rape straw, winter wheat straw | wheat straw (every two years) |
| Mineral fertilization (kg ha−1) | according to the results of soil analysis, use of P and K fertilizers in the form of natural rock | NPK (85 + 55 + 65) | NPK (140 + 60 + 80) | |
| Retardants | - | 1–2× | 2× | |
| Fungicides | - | 2× | 2–3× | |
| Weed control | weeder harrow 2–3× | weeder harrow 1× herbicides 1–2× | herbicides 2–3× | |
| Agricultural Crops 2010–2012 | Energy Crops 2010–2012 | Energy Crops 2013–2015 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No | Species | Density (plants m−2) | Dr* | Species | Density (plants m−2) | Dr* | Species | Density (plants m−2) | Dr* |
| 1. | Vio.arv | 5.818 | S | Con.can | 11.429 | S | Gal.apa | 10.324 | S |
| 2. | Chen.alb | 5.456 | S | Vio.arv | 8.827 | S | Vio.arv | 9.059 | S |
| 3. | Ste.med | 2.496 | S | Ste.med | 5.763 | S | Cap.bur | 8.199 | S |
| 4. | Gal.apa | 1.558 | S | Chen.alb | 4.859 | S | Ste.med | 7.875 | S |
| 5. | Ech.cru | 1.512 | S | Sen.vul (2) | 4.782 | S | Ech.cru | 6.743 | S |
| 6. | Cap.bur | 1.373 | S | Lac.ser | 4.500 | S | Sol.gig | 6.294 | P |
| 7. | Fal.con | 1.353 | S | Tar.off | 3.795 | P | Gal.par | 5.706 | S |
| 8. | Tri.ino | 1.205 | S | Poa.ann | 2.821 | S | Ely.rep | 4.941 | P |
| 9. | Lam.pur | 1.004 | S | Gal.apa | 2.513 | S | Che.alb | 4.037 | S |
| 10. | Fum.off | 0.927 | S | Cap.bur | 2.147 | S | Tar.off | 3.603 | P |
| 11. | Ape.spi | 0.903 | S | Sol.gig | 1.859 | P | Poa.pra (3) | 2.596 | P |
| 12. | Lap.com | 0.772 | S | Tri.ino | 1.808 | S | Ara.tha (2) | 1.779 | S |
| 13. | Equ.arv | 0.747 | P | Ely.rep | 1.603 | P | Con.can | 1.765 | S |
| 14. | Cir.arv | 0.713 | P | Jun.buf (2) | 1.359 | S | Son.arv | 1.750 | P |
| 15. | Pap.rho | 0.577 | S | Cer.arv | 1.141 | P | Sen.vul (2) | 1.662 | S |
| 16. | Vic.hir (1) | 0.548 | S | Ech.cru | 1.064 | S | Tri.ino | 1.544 | S |
| 17. | Ely.rep | 0.412 | P | Ape.spi | 0.910 | S | Lac.ser | 1.250 | S |
| 18. | Tar.off | 0.292 | P | Epi.par (2) | 0.859 | P | Art.vul (2) | 1.074 | P |
| 19. | Ger.pus (1) | 0.269 | S | Equ.arv | 0.667 | P | Geu.urb (2) | 1.015 | P |
| 20. | Ver.per | 0.255 | S | Arr.ela (2) | 0.538 | P | Pol.per | 0.735 | S |
| 21. | Lyc.arv | 0.155 | S | Urt.dio (2) | 0.474 | P | Cer.arv | 0.662 | P |
| 22. | Myo.arv | 0.151 | S | Fal.con | 0.436 | S | Equ.arv | 0.618 | P |
| 23. | Mel.alb | 0.118 | S | Ara.tha (2) | 0.397 | S | Urt.dio (2) | 0.559 | P |
| 24. | Pla.mai | 0.113 | P | Gal.par | 0.385 | S | Lap.com | 0.515 | S |
| 25. | Pol.avi | 0.103 | S | Cre.tec (2) | 0.385 | S | Ape.spi | 0.456 | S |
| 26. | Pol.per | 0.094 | S | Son.arv | 0.346 | P | Ger.dis | 0.441 | S |
| 27. | Cen.cya | 0.077 | S | Ger.dis | 0.333 | S | Sis.loe (2) | 0.309 | S |
| 28. | Ero.cic | 0.064 | S | Fum.off | 0.308 | S | Pol.avi | 0.250 | S |
| 29. | Gal.par | 0.062 | S | Mel.alb | 0.295 | S | Eri.ann (2) | 0.235 | S |
| 30. | Con.can | 0.056 | S | Myo.min | 0.269 | S | Cir.arv | 0.176 | P |
| 31. | Ama.ret | 0.054 | S | Pol.avi | 0.269 | S | Con.arv | 0.176 | S |
| 32. | Son.arv | 0.046 | P | Agr.cap (2) | 0.256 | P | Rum.ace | 0.176 | P |
| 33. | Bra.nap | 0.044 | S | Cir.arv | 0.256 | P | Epi.par (2) | 0.176 | P |
| 34. | Tri.rep | 0.038 | S | Pla.mai (2) | 0.250 | P | Che.pol (3) | 0.132 | S |
| 35. | Myo.min | 0.035 | S | Ant.arv | 0.218 | S | Spe.arv (2) | 0.132 | S |
| 36. | Ger.dis | 0.032 | S | Spe.arv (2) | 0.179 | S | Myo.min | 0.118 | S |
| 37. | Lam.amp (1) | 0.031 | S | Art.vul (2) | 0.173 | P | Mel.alb | 0.103 | S |
| 38. | Poa.ann | 0.031 | S | Geu.urb (2) | 0.154 | P | Pap.rho | 0.103 | S |
| 39. | Cer.arv | 0.023 | P | Rum.ace | 0.154 | P | Poa.ann | 0.103 | S |
| 40. | Gal.tet | 0.021 | S | Ver.per | 0.115 | S | Cre.tec (2) | 0.074 | S |
| 41. | Pla.lan | 0.019 | P | Ero.cic | 0.103 | S | Lol.per (3) | 0.067 | P |
| 42. | Rap.rap (1) | 0.018 | S | Eri.ann (2) | 0.090 | S | Pla.mai | 0.059 | P |
| 43. | Ant.arv | 0.017 | S | Pol.per | 0.077 | S | Lam.pur | 0.059 | S |
| 44. | Vic.cra | 0.015 | P | Ver.hed (2) | 0.077 | S | Myo.arv | 0.059 | S |
| 45. | Con.arv | 0.009 | P | Vic.cra | 0.051 | P | Rum. acetosa (3) | 0.059 | P |
| 46. | Ach.mil | 0.009 | P | Lam.pur | 0.038 | S | Dau.car | 0.044 | P |
| 47. | Con.reg (1) | 0.009 | S | Lyc.arv | 0.038 | S | Arr.ela (2) | 0.044 | P |
| 48. | Eup.hel | 0.008 | P | Eup.hel | 0.038 | P | Bro.ine (2) | 0.044 | P |
| 49. | Rum.ace | 0.008 | P | Fes.ovi (2) | 0.038 | P | Ama.ret | 0.044 | S |
| 50. | Lac.ser | 0.008 | S | Cen.cya | 0.038 | S | Thl.arv | 0.044 | S |
| 51. | Sin.arv (1) | 0.003 | S | Hie.pil (2) | 0.038 | P | Son.ole | 0.037 | P |
| 52. | Sol.gig | 0.003 | P | Sam.nig (2) | 0.026 | S | Pla.lan | 0.029 | P |
| 53. | Pot.ans (1) | 0.003 | P | Tri.rep | 0.026 | S | Tri.rep | 0.029 | P |
| 54. | Thl.arv | 0.003 | S | Ach.mil | 0.026 | P | Ach.mil | 0.029 | P |
| 55. | Des.sop (2) | 0.026 | S | Sid.her (2) | 0.029 | P | |||
| 56. | Gal.tet | 0.026 | S | Vic.cra | 0.029 | P | |||
| 57. | Sis.loe (2) | 0.019 | S | Gal.tet | 0.022 | S | |||
| 58. | Leo.his (2) | 0.019 | P | Bra.nap | 0.016 | S | |||
| 59. | Cam.pat (2) | 0.019 | P | Ero.cic | 0.015 | S | |||
| 60. | Aeg.pod (2) | 0.019 | P | Con.arv | 0.015 | P | |||
| 61. | Con.arv | 0.013 | P | Pol.lap (3) | 0.015 | S | |||
| 62. | Bro.ine (2) | 0.013 | P | Ant.arv | 0.015 | S | |||
| 63. | Vio.tri (2) | 0.013 | S | Gna.uli (2) | 0.015 | S | |||
| 64. | Myo.arv | 0.013 | S | ||||||
| 65. | Sol.nig (2) | 0.013 | S | ||||||
| 66. | Gna.uli (2) | 0.013 | S | ||||||
| 67. | Gna.sil (2) | 0.013 | S | ||||||
| 68. | Sid.her (2) | 0.013 | P | ||||||
| Total | 29.667 | Total | 69.833 | Total | 88.272 | ||||
| Variable | Marginal Effects | Conditional Effects | ||
|---|---|---|---|---|
| Lambda | Lambda | P-level of Significance | F-Ratio | |
| Grasses 2010–2012 (G1) | 0.09 | 0.09 | 0.002 | 3.55 |
| Organic system (ORG) | 0.09 | 0.08 | 0.002 | 3.58 |
| Integrated system (INT) | 0.07 | 0.07 | 0.002 | 3.19 |
| Trees and bushes 2013–2015 (T2) | 0.06 | 0.04 | 0.004 | 2.20 |
| Wheat monoculture (MONO) | 0.06 | 0.06 | 0.002 | 2.83 |
| Grasses 2013–2015 (G2) | 0.05 | 0.05 | 0.002 | 2.66 |
| Trees and bushes 2010–2012 (T1) | 0.05 | 0.04 | 0.004 | 2.10 |
| Conventional system (CONW) | 0.04 | 0.05 | 0.002 | 2.83 |
| Dicotyledons 2013–2015 (D2) | 0.04 | - | - | - |
| Dicotyledons 2010–2012 (D1) | 0.02 | 0.02 | 0.358 | 1.10 |
| Test of RDA canonical axes significance: | first canonical axis | 0.002 | 6.881 | |
| all canonical axes | 0.002 | 3.197 | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity. Agronomy 2019, 9, 695. https://doi.org/10.3390/agronomy9110695
Feledyn-Szewczyk B, Matyka M, Staniak M. Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity. Agronomy. 2019; 9(11):695. https://doi.org/10.3390/agronomy9110695
Chicago/Turabian StyleFeledyn-Szewczyk, Beata, Mariusz Matyka, and Mariola Staniak. 2019. "Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity" Agronomy 9, no. 11: 695. https://doi.org/10.3390/agronomy9110695
APA StyleFeledyn-Szewczyk, B., Matyka, M., & Staniak, M. (2019). Comparison of the Effect of Perennial Energy Crops and Agricultural Crops on Weed Flora Diversity. Agronomy, 9(11), 695. https://doi.org/10.3390/agronomy9110695