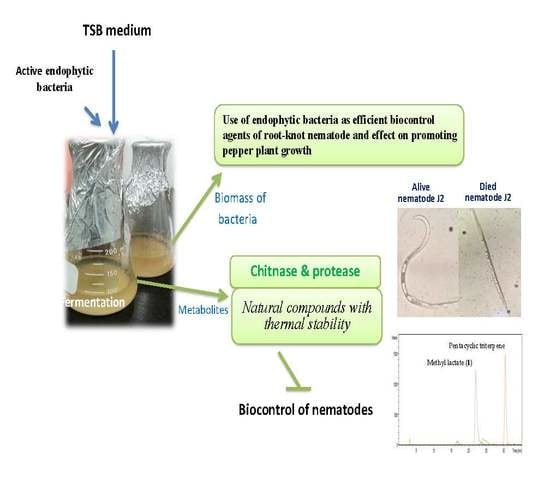
Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Isolation of Endophytic Bacteria
2.3. PCR Amplification, Sequencing, and Phylogenetic Analysis of the 16S rRNA Gene
2.4. Bacterial Strains Preparation
2.5. Anti-Nematode Activity In Vitro Assays
2.6. The Effect of Endophytic Bacteria on Reducing Nematodes and the Plant Promoting Effect for Black Pepper Trees in Plots Experiments
2.7. Enzymic Activity Assays
2.8. Statistical Analysis
3. Results
3.1. Isolation, Evaluation, and Identification of the Active Antinematodes Endophytic Bacteria in the In Vitro Test
3.2. Evaluation of Potent Biocontrol of Meloidogyne sp. by the Selected Endophytic Bacteria under Greenhouse Conditions
3.3. Chitinase, Protease Activities of Fermented Product by Bacillus megaterium DS9
4. Discussion
4.1. Isolation, Evaluation of the Active Antinematodes Endophytic Bacteria
4.2. Primary Determination of Active Components of Fermented Product by Bacillus megaterium DS9
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ravindra, H.; Sehgal, M.; Manu, T.G.; Murali, R.; Latha, M.; Narasimhamurthy, H.B. Incidence of root-knot nematode (Meloidogyne incognita) in black pepper in Karnataka. J. Entomol. Nematol. 2014, 6, 51–55. [Google Scholar]
- Nguyen, V.B.; Wang, S.L.; Nguyen, T.H.; Nguyen, T.H.; Trinh, T.H.T.; Nong, T.T.; Nguyen, T.U.; Nguyen, V.N.; Nguyen, A.D. Reclamation of rhizobacteria newly isolated from black pepper plant roots as potential biocontrol agents of root-knot nematodes. Res. Chem. Intermed. 2019, 45, 5293–5307. [Google Scholar] [CrossRef]
- Pepper (piper spp.), Production/Crops. Food and Agriculture Organization of the United Nations: Statistical Division (FAOSTAT). Available online: http://www.wikiwand.com/en/Black_pepper (accessed on 4 December 2018).
- Thuy, T.T.T.; Yen, N.T.; Tuyet, N.T.A.; Te, L.L.; Waele, D.D. Population dynamics of Meloidogyne incognita on black pepper plants in two agro-ecological regions in Vietnam. Arch. Phytopathol. Plant Protect. 2012, 45, 1527–1537. [Google Scholar]
- Trudgill, D.L.; Blok, V.C. Apomictic, polyphagous root-knot nematodes: Exceptionally successful and damaging biotrophic root pathogens. Annu. Rev. Phytopathol. 2001, 39, 53–77. [Google Scholar] [CrossRef] [PubMed]
- Prabhakaran Nair, K.P. The Agronomy and Economy of Turmeric and Ginger; Elsevier: Amsterdam, The Netherlands, 2013; pp. 139–157. [Google Scholar] [CrossRef]
- Sikora, R.A.; Fernández, E. Nematode parasites of vegetables. In Plant Parasitic Nematodes in Subtropical and Tropical Agriculture, 2nd ed.; Luc, M., Sikora, R.A., Bridge, J., Eds.; CABI Publishing: Wallingford, UK, 2005; pp. 319–376. [Google Scholar]
- Wiratno, M.S.; Ankardiansyah, P.P.; Ahmed, I.A.Y. Biological control of root-knot nematode (meloidogyne spp.) in pepper plants utilizing endophytic bacteria Pseudomonas sp. AND Micrococcus sp. J. Pepper Ind. 2018, 9, 11–22. [Google Scholar]
- Josef, J.; Katarína, K. Nanopesticides: Preparation, Targeting and Controlled Release; Academic Press & Elsevier: London, UK, 2017; Volume 10, pp. 81–127. [Google Scholar]
- Nguyen, V.N.; Kim, Y.J.; Oh, K.T.; Jung, W.J.; Park, R.D. The role of chitinase from Lecanicillium antillanum B-3 in parasitism to root-knot nematode Meloidogyne incognita eggs. Biocontrol. Sci. Technol. 2007, 17, 1047–1105. [Google Scholar] [CrossRef]
- Wheeler, T.A.; Siders, K.T.; Anderson, M.G.; Russell, S.A.; Woodward, J.E.; Mullinix, B.G., Jr. Management of Meloidogyne incognita with chemicals and cultivars in cotton in a semi-arid environment. J. Nematol. 2014, 46, 101–107. [Google Scholar]
- Mokbel, A.A. Impact of some antagonistic organisms in controlling Meloidogyne arenaria infecting tomato plants. JOLST 2013, 1, 69–74. [Google Scholar] [CrossRef]
- Boraha, B.; Ahmed, R.; Hussaina, M.; Phukon, P.; Wann, S.B.; Sarmah, D.K.; Bhau, B.S. Suppression of root-knot disease in Pogostemon cablin caused by Meloidogyne incognita in a rhizobacteria mediated activation of phenylpropanoid pathway. Biol. Control 2018, 119, 43–50. [Google Scholar]
- Mervat, A.A.; Shawky, S.M.; Shaker, G.S. Comparative efficacy of some bioagents, plant oil and plant aqueous extracts in controlling Meloidogyne incognita on growth and yield of grapevines. Ann. Agric. Sci. 2012, 57, 7–18. [Google Scholar] [CrossRef]
- Regaieg, H.; Ciancio, A.; Raouani, N.H.; Grasso, G.; Rosso, L. Effects of culture filtrates from the nematophagous fungus Verticillium leptobactrum on viability of the root-knot nematode Meloidogyne incognita. World J. Microbiol. Biotechnol. 2010, 26, 2285–2289. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Huang, C.C.; Liang, T.W.; Nguyen, V.B.; Pan, P.S.; Wang, S.L. Production and purification of a fungal chitosanase and chitooligomers from Penicillium janthinellum D4 and discovery of the enzyme activators. Carbohydr. Polym. 2014, 108, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.N.; Oh, I.J.; Kim, Y.J.; Kim, K.Y.; Kim, Y.C.; Park, R.D. Purification and characterization of chitinases from Paecilomyces variotii DG-3 parasitizing on Meloidogyne incognita eggs. J. Ind. Microbiol. Biotechnol. 2009, 36, 195–203. [Google Scholar] [CrossRef]
- Vicente, P.C.; Renata, S.C.D.P.; Eduardo, S.F. Volatiles produced by interacting microorganisms potentially useful for the control of plant pathogens. Ciênc. Agrotecnol. Lavras 2010, 34, 525–535. [Google Scholar] [Green Version]
- Abdelnabby, H.M.; Mohamed, H.A.; Abo Aly, H.E. Nematode-antagonistic compounds from certain bacterial species. Egypt. J. Biol. Pest Control 2011, 21, 209–217. [Google Scholar]
- Gao, H.; Qi, G.; Yin, R.; Zhang, H.; Li, C.; Zhao, X. Bacillus cereus strain S2 shows high nematicidal activity against Meloidogyne incognita by producing sphingosine. Sci. Rep. 2016, 24, 28756. [Google Scholar] [CrossRef]
- Elhady, A.; Giné, A.; Topalovic, O.; Jacquiod, S.; Sørensen, S.J.; Sorribas, F.J.; Heuer, H. Microbiomes associated with infective stages of root-knot and lesion nematodes in soil. PLoS ONE 2017, 12, e0177145. [Google Scholar] [CrossRef]
- Basyony, A.G.; Abo-Zaid, G.A. Biocontrol of the root-knot nematode, Meloidogyne incognita, using an eco-friendly formulation from Bacillus subtilis, lab. and greenhouse studies. Egypt. J. Biol. Pest Control 2018, 28, 87. [Google Scholar] [CrossRef]
- Beneduzi, A.; Ambrosini, A.; Passaglia, L.M. Plant growth-promoting rhizobacteria (PGPR): Their potential as antagonists and biocontrol agents. Genet. Mol. Biol. 2012, 35, 1044–1051. [Google Scholar] [CrossRef]
- Khan, Z.; Kim, S.G.; Jeon, Y.H.; Khan, H.U.; Son, S.H.; Kim, Y.H. A plant growth promoting rhizobacterium, Paenibacillus polymyxa strain GBR-1, suppresses root-knot nematode. Bioresour. Technol. 2008, 99, 3016–3023. [Google Scholar] [CrossRef]
- Southey, J.F. Laboratory Methods for Work with Plant and Soil Nematode; Ministry of Agriculture Fisheries and Food, HMSO: London, UK, 1986; p. 202.
- Aravind, R.; Kumar, A.; Eapen, S.J.; Ramana, K.V. Endophytic bacterial flora in root and stem tissues of black pepper (Piper nigrum L.) genotype: Isolation, identification and evaluation against Phytophthora capsici. Lett. Appl. Microbiol. 2009, 48, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Tran, M.D.; Sugimoto, H.; Nguyen, A.D.; Watanabe, T.; Suzuki, K. Identification and characterization of chitinolytic bacteria isolated from a freshwater lake. Biosci. Biotechnol. Biochem. 2018, 82, 343–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Fillipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947. [Google Scholar] [CrossRef]
- Cayrol, J.C.; Djian, C.; Pijarowski, L. Study of the Nematocidal properties of the culture filtrate of the nematophagus fungus Paecilomyces lilacinus. Revue Nematol. 1989, 12, 331–336. [Google Scholar]
- Nguyen, V.B.; Nguyen, Q.V.; Nguyen, A.D.; Wang, S.L. Screening and evaluation of α-glucosidase inhibitors from indigenous medicinal plants in Dak Lak Province, Vietnam. Res. Chem. Intermed. 2017, 43, 3599–3612. [Google Scholar] [CrossRef]
- Tran, T.N.; Doan, C.T.; Nguyen, V.B.; Nguyen, A.D.; Wang, S.L. The isolation of chitinase from Streptomyces thermocarboxydus and its application in the preparation of chitin oligomers. Res. Chem. Intermed. 2019, 45, 727. [Google Scholar] [CrossRef]
- Al-Rehiayani, S.; Hafez, S.L.; Thorton, M.; Sandararaj, P. Effects of Pratylenchus neglectus, Bacillus megaterium, and oil radish or rapeseed green manure on reproductive potential of Meloidogyne chitwoodi on potato. Nematropica 1999, 29, 37–49. [Google Scholar]
- Terefe, M.; Tefera, T.; Sakhuja, P.K. Effect of a formulation of Bacillus firmus on root-knot nematode Meloidogyne incognita infestation and the growth of tomato plants in the greenhouse and nursery. J. Inverteb. Pathol. 2009, 100, 94–99. [Google Scholar] [CrossRef]
- Walker, T.S.; Bais, H.P.; Grotewold, E.; Vivanco, J.M. Root exudation and rhizosphere biology. Plant Physiol. 2003, 132, 44–51. [Google Scholar] [CrossRef]
- Akinrinlola, R.J.; Yuen, G.Y.; Drijber, R.A.; Adesemoye, A.O. Evaluation of Bacillus strains for plant growth promotion and predictability of efficacy by in vitro physiological traits. Int. J. Microbiol. 2018, 2018, 5686874. [Google Scholar] [CrossRef] [PubMed]
- Trinh, T.H.T.; Wang, S.L.; Nguyen, V.B.; Tran, M.D.; Doan, C.T.; Vo, T.P.K.; Huynh, V.Q.; Nguyen, A.D. A potent antifungal rhizobacteria Bacillus velezensis RB.DS29 isolated from black pepper (Piper nigrum L.). Res. Chem. Intermed. 2019, 45, 5309–5323. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Wang, S.L.; Trinh, T.H.T.; Tran, T.N.; Nguyen, V.B.; Doan, C.T.; Huynh, V.Q.; Vo, T.P.K. Plant growth promotion and fungal antagonism of endophytic bacteria for the sustainable production of black pepper (Piper nigrum L.). Res. Chem. Intermed. 2019, 45, 5325–5339. [Google Scholar] [CrossRef]


| Isolates | Mortality (%) of Nematodes | Isolates | Mortality (%) of Nematodes |
|---|---|---|---|
| DS1 | 91.11 c | DR5 | 8.89 jk |
| DS2 | 63.33 e | DR6 | 5.56 kl |
| DS3 | 7.78 jk | DR8 | 7.78 jk |
| DS4 | 6.67 jkl | DR9 | 96.67 ab |
| DS5 | 100 a | DR10 | 100 a |
| DS6 | 57.78 fg | DR11 | 95.56 b |
| DS7 | 95.56 b | DR12 | 57.78 fg |
| DS8 | 100 a | DR13 | 61.11 ef |
| DS9 | 100 a | DM1 | 3.33 ml |
| DS10 | 96.67 ab | DM2 | 5.56 kl |
| DS11 | 91.11 c | DM3 | 15.56 i |
| DS12 | 85.56 d | DM4 | 6.67 jkl |
| DS13 | 96.67 ab | DM5 | 55.56 g |
| DS14 | 51.11 h | DM6 | 10.00 j |
| DR1 | 3.33 ml | DM7 | 95.56 b |
| DR2 | 100 a | DM8 | 0.00 m |
| DR3 | 96.67 ab | Control (no bacterial) | 0.00 m |
| DR4 | 95.56 b |
| No | Bacterial Name | Accession Number |
|---|---|---|
| 1 | Bacillus sp. DR2 | LC496557 |
| 2 | Bacillus sp. DR10 | LC496558 |
| 3 | Bacillus flexus DS5 | LC496559 |
| 4 | Bacillus sp. DS8 | LC496560 |
| 5 | Bacillus megaterium DS9 | LC496561 |
| Treatments | Nematode J2 in Soil (Count) | Reduction of Population Density Ability in Soil (%) | Nematode J2 in Roots (Count) | Reduction of Population Density Ability in Roots (%) | Rate of Buildup (pf/pi) |
|---|---|---|---|---|---|
| DS5 | 69.00 c | 56.33 | 91.67 bc | 38.89 | 0.54 b |
| DS8 | 43.00 d | 72.78 | 35.33 e | 76.44 | 0.26 cd |
| DS9 | 28.67 de | 81.86 | 40.33 e | 73.11 | 0.23 d |
| DR2 | 20.00 e | 87.34 | 77.67 dc | 48.22 | 0.33 c |
| DR10 | 33.67 de | 78.69 | 52.00 de | 65.33 | 0.29 cd |
| Control | 158.00 a | - | 150.00 a | - | 1.03 a |
| LSD | 14.36 | 26.05 | 0.14 | ||
| CV% | 10.13 | 13.93 | 7.47 |
| Treatments | Increased Shoot Length (cm) | formed New Leaves | Increased Root Length (cm) | Chlorophyll a + b (mg/g LFW) |
|---|---|---|---|---|
| DS5 | 25.71 ab | 3.92 a | 6.65 cde | 0.78 d |
| DS8 | 29.38 a | 4.19 a | 8.62 ab | 1.76 a |
| DS9 | 29.81 a | 3.99 a | 8.98 a | 1.52 b |
| DR2 | 28.68 ab | 3.99 a | 7.64 abc | 0.92 c |
| DR10 | 22.64 abc | 3.79 a | 7.2 bcd | 0.75 d |
| Control | 17.25 c | 1.00 b | 5.56 de | 0.57 e |
| CV% | 12.52 | 12.38 | 17.10 | 5.38 |
| LSD | 7.62 | 1.06 | 1.06 | 0.07 |
| Bacterial Strains | Supernatants of Fermented Medium | Enzyme Activity (IU/mL) | Anti-Nematodes Activity (%) | ||
|---|---|---|---|---|---|
| Chitinase | Protease | Nematode J2 Inhibition | Egg Hatching Inhibition | ||
| DS9 | Medium containing casein | - | 0.69 ± 0.06 | 73.33 ± 1.92 | 60.00 ± 8.85 |
| Medium containing chitin | 2.72 ± 0.12 | - | 52.22 ± 4.0 | 71.11 ± 4.0 | |
| Unfermented medium containing casein | - | - | 3.6 ± 0.01 | - | |
| Unfermented medium containing chitin | - | - | 8.1 ± 0.05 | - | |
| Crude Enzymes | Antinematodes Activity (%) of Enzymes Not Treated at 100 °C | Antinematodes Activity (%) Enzymes Treated at 100 °C | ||
|---|---|---|---|---|
| Nematode J2 Inhibition | Egg Hatching Inhibition | Nematode J2 Inhibition | Egg Hatching Inhibition | |
| DS9 protease | 44.58 ± 6.44 | 42.22 ± 3.75 | - | - |
| DS9 chitinase | 15.63 ± 1.74 | 25.56 ± 1.94 | - | - |
| Bacterial Strains | Supernatants of Fermented Medium | Enzyme Activity (IU/mL) | Anti-Nematodes Activity (%) | ||
|---|---|---|---|---|---|
| Chitinase | Protease | Nematode J2 Inhibition | Egg Hatching Inhibition | ||
| DS9 | Medium containing casein | - | - | 30.00 ± 1.92 | 30.00 ± 5.09 |
| Medium containing chitin | - | - | 21.11 ± 2.93 | 37.78 ± 2.22 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.P.H.; Wang, S.-L.; Nguyen, V.B.; Tran, D.M.; Nguyen, D.S.; Nguyen, A.D. Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy 2019, 9, 714. https://doi.org/10.3390/agronomy9110714
Tran TPH, Wang S-L, Nguyen VB, Tran DM, Nguyen DS, Nguyen AD. Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy. 2019; 9(11):714. https://doi.org/10.3390/agronomy9110714
Chicago/Turabian StyleTran, Thi Phuong Hanh, San-Lang Wang, Van Bon Nguyen, Dinh Minh Tran, Dinh Sy Nguyen, and Anh Dzung Nguyen. 2019. "Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam" Agronomy 9, no. 11: 714. https://doi.org/10.3390/agronomy9110714
APA StyleTran, T. P. H., Wang, S.-L., Nguyen, V. B., Tran, D. M., Nguyen, D. S., & Nguyen, A. D. (2019). Study of Novel Endophytic Bacteria for Biocontrol of Black Pepper Root-knot Nematodes in the Central Highlands of Vietnam. Agronomy, 9(11), 714. https://doi.org/10.3390/agronomy9110714