Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea
Abstract
:1. Introduction
2. Materials and Methods
2.1. Arbuscular Mycorrhizal Fungal Inocula and Seed Coating
2.2. Experimental Design
2.3. Plant Measurements
2.4. Crude Protein and Fiber, Fat and Ash Grain Content Analyses
2.5. Mycorrhizal Development
2.6. Statistical Analysis
3. Results
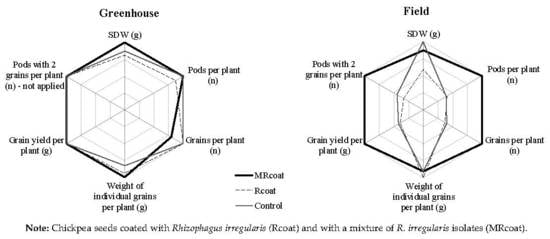
3.1. Growth Parameters
3.2. Grain Quality
3.3. Mycorrhizal Colonization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Iqbal, A.; Ateeq, N.; Khalil, I.A.; Perveen, S.; Saleemullah, S. Physicochemical characteristics and amino acid profile of chickpea cultivars grown in Pakistan. J. Foodserv. 2006, 17, 94–101. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations, Statistics Division. 2017. Available online: http://faostat3.fao.org/ home/E (accessed on 1 March 2019).
- Jukanti, A.K.; Gaur, P.M.; Gowda, C.L.L.; Chibbar, R.N. Nutritional quality and health benefits of chickpea (Cicer arietinum L.): A review. Br. J. Nutr. 2012, 108, S11–S26. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Mishra, A.; Chauhan, P.; Nautiyal, C. Induction of Paenibacillus lentimorbus biofilm by sodium alginate and CaCl2 alleviates drought stress in chickpea. Ann. Appl. Boil. 2011, 159, 372–386. [Google Scholar] [CrossRef]
- Farzaneh, M.; Vierheilig, H.; Lossl, A.; Kaul, H. Arbuscular mycorrhiza enhances nutrient uptake in chickpea. Plant. Soil Environ. 2011, 57, 465–470. [Google Scholar] [CrossRef] [Green Version]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Boil. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Carvalho, P.; Marques, G.; Ferreira, L.; Nunes, M.; Rocha, I.; Ma, Y.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Increased protein content of chickpea (Cicer arietinum L.) inoculated with arbuscular mycorrhizal fungi and nitrogen-fixing bacteria under water deficit conditions. J. Sci. Food Agric. 2017, 97, 4379–4385. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.; Kumar, A.; Al-Dbass, A.M.; Alqarawi, A.A.; Al-Arjani, A.-B.F.; Singh, G.; Farooq, M.; Abd_Allah, E.F. Arbuscular mycorrhizal fungi and biochar improves drought tolerance in chickpea. Saudi J. Boil. Sci. 2018, 26, 614–624. [Google Scholar] [CrossRef]
- Rillig, M.C.; Aguilar-Trigueros, C.A.; Camenzind, T.; Cavagnaro, T.R.; Degrune, F.; Hohmann, P.; Lammel, D.R.; Mansour, I.; Roy, J.; Heijden, M.G.A.; et al. Why farmers should manage the arbuscular mycorrhizal symbiosis. New Phytol. 2019, 222, 1171–1175. [Google Scholar] [CrossRef] [Green Version]
- Kempel, A.; Schmidt, A.K.; Brandl, R.; Schädler, M. Support from the underground: Induced plant resistance depends on arbuscular mycorrhizal fungi. Funct. Ecol. 2010, 24, 293–300. [Google Scholar] [CrossRef]
- Hameed, A.; Wu, Q.-S.; Abd-Allah, E.F.; Hashem, A.; Kumar, A.; Lone, H.A.; Ahmad, P. Role of AM Fungi in Alleviating Drought Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer: New York, NY, USA, 2014; pp. 55–75. [Google Scholar] [CrossRef]
- Kumar, M.; Singh, D.P.; Prabha, R.; Rai, A.K.; Sharma, L. Role of Microbial Inoculants in Nutrient Use Efficiency. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 133–142. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Ma, Y.; Rocha, I.; Carvalho, M.F.; Vosátka, M.; Freitas, H. Arbuscular mycorrhizal fungi are an alternative to the application of chemical fertilizer in the production of the medicinal and aromatic plant Coriandrum sativum L. J. Toxicol. Environ. Health A 2016, 79, 320–328. [Google Scholar] [CrossRef]
- Singh, D.P.; Singh, H.B.; Prabha, R. (Eds.) Microbial Inoculants in Sustainable Agricultural Productivity; Springer: New York, NY, USA, 2016. [Google Scholar]
- Frew, A.; Powell, J.R.; Hiltpold, I.; Allsopp, P.G.; Sallam, N.; Johnson, S.N. Host plant colonisation by arbuscular mycorrhizal fungi stimulates immune function whereas high root silicon concentrations diminish growth in a soil-dwelling herbivore. Soil Boil. Biochem. 2017, 112, 117–126. [Google Scholar] [CrossRef]
- Ryan, M.H.; Graham, J.H. Little evidence that farmers should consider abundance or diversity of arbuscular mycorrhizal fungi when managing crops. New Phytol. 2018, 220, 1092–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Eo, J.-K.; Lee, E.-H.; Park, H.; Eom, A.-H. Effects of Arbuscular Mycorrhizal Fungi and Soil Conditions on Crop Plant Growth. Mycobiology 2017, 45, 20–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frew, A. Arbuscular mycorrhizal fungal diversity increases growth and phosphorus uptake in C3 and C4 crop plants. Soil Boil. Biochem. 2019, 135, 248–250. [Google Scholar] [CrossRef]
- Vosátka, M.; Latr, A.; Gianinazzi, S.; Albrechtova, J. Development of arbuscular mycorrhizal biotechnology and industry: Current achievements and bottlenecks. Symbiosis 2012, 58, 29–37. [Google Scholar] [CrossRef]
- Malusà, E.; Pinzari, F.; Canfora, L. Efficacy of Biofertilizers: Challenges to Improve Crop Production. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 17–40. [Google Scholar] [CrossRef]
- O’Callaghan, M. Microbial inoculation of seed for improved crop performance: Issues and opportunities. Appl. Microbiol. Biotechnol. 2016, 100, 5729–5746. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H. Seed coating with arbuscular mycorrhizal fungi as an ecotechnological approach for sustainable agricultural production of common wheat (Triticum aestivum L.). J. Toxicol. Environ. Health A 2016, 79, 329–337. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Carvalho, M.F.; Magalhães, C.; Janoušková, M.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed coating with inocula of arbuscular mycorrhizal fungi and plant growth promoting rhizobacteria for nutritional enhancement of maize under different fertilisation regimes. Arch. Agron. Soil Sci. 2019, 65, 31–43. [Google Scholar] [CrossRef]
- Rocha, I.; Ma, Y.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Growth and nutrition of cowpea (Vigna unguiculata) under water deficit as influenced by microbial inoculation via seed coating. J. Agron. Crop. Sci. 2019. [Google Scholar] [CrossRef]
- Ma, Y.; Látr, A.; Rocha, I.; Freitas, H.; Vosátka, M.; Oliveira, R.S. Delivery of Inoculum of Rhizophagus irregularis via Seed Coating in Combination with Pseudomonas libanensis for Cowpea Production. Agronomy 2019, 9, 33. [Google Scholar] [CrossRef]
- Cely, M.V.T.; De Oliveira, A.G.; De Freitas, V.F.; De Luca, M.B.; Barazetti, A.R.; Dos Santos, I.M.O.; Gionco, B.; Garcia, G.V.; Prete, C.E.C.; Andrade, G. Inoculant of Arbuscular Mycorrhizal Fungi (Rhizophagus clarus) Increase Yield of Soybean and Cotton under Field Conditions. Front. Microbiol. 2016, 7, 271. [Google Scholar] [CrossRef] [PubMed]
- Lugtenberg, B.; Kamilova, F. Plant-Growth-Promoting Rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef]
- Scott, J.M.; Hill, C.B.; Jessop, R.S. Growth chamber study of phosphorus applied as drilled granules or as seed coatings to wheat sown in soils differing in P-sorption capacity. Fertil. Res. 1991, 29, 281–287. [Google Scholar] [CrossRef]
- Porter, W. The ’most probable number’ method for enumerating infective propagules of vesicular arbuscular mycorrhizal fungi in soil. Soil Res. 1979, 17, 515–519. [Google Scholar] [CrossRef]
- Maâtallah, J.; Berraho, E.B.; Sanjuán, J.; Lluch, C. Phenotypic characterization of rhizobia isolated from chickpea (Cicer arietinum) growing in Moroccan soils. Agron. Sustain. Dev. 2002, 22, 321–329. [Google Scholar] [CrossRef]
- Phillips, J.; Hayman, D. Improved procedures for clearing roots and staining parasitic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161. [Google Scholar] [CrossRef]
- Oliveira, R.S.; Vosátka, M.; Dodd, J.C.; Castro, P.M.L. Studies on the diversity of arbuscular mycorrhizal fungi and the efficacy of two native isolates in a highly alkaline anthropogenic sediment. Mycorrhiza 2005, 16, 23–31. [Google Scholar] [CrossRef]
- Giovannetti, M.; Mosse, B. An evaluation of techniques for measuring vesicular arbuscular mycorrhizal infection in roots. New Phytol. 1980, 84, 489–500. [Google Scholar] [CrossRef]
- Zaidi, A.; Khan, M.; Amil, M. Interactive effect of rhizotrophic microorganisms on yield and nutrient uptake of chickpea (Cicer arietinum L.). Eur. J. Agron. 2003, 19, 15–21. [Google Scholar] [CrossRef]
- Erman, M.; Demir, S.; Ocak, E.; Tüfenkçi, Ş.; Oğuz, F.; Akkopru, A. Effects of Rhizobium, arbuscular mycorrhiza and whey applications on some properties in chickpea (Cicer arietinum L.) under irrigated and rainfed conditions. 1—Yield, yield components, nodulation and AMF colonization. Field Crop. Res. 2011, 122, 14–24. [Google Scholar] [CrossRef]
- Fan, J.; McConkey, B.; Janzen, H.; Townley-Smith, L.; Wang, H. Harvest index–yield relationship for estimating crop residue in cold continental climates. Field Crop. Res. 2017, 204, 153–157. [Google Scholar] [CrossRef]
- Weber, E.; Saxena, M.C.; George, E.; Marschner, H. Role of Vesicular Arbuscular Mycorrhizae in the Mineral Nutrition of Chickpea (Cicer arietinum L.) Grown in Northern Syria. Field Crop. Res. 1993, 32, 115–128. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#data/PP (accessed on 20 June 2019).
- Lekberg, Y.; Koide, R.T. Is plant performance limited by abundance of arbuscular mycorrhizal fungi? A meta-analysis of studies published between 1988 and 2003. New Phytol. 2005, 168, 189–204. [Google Scholar] [CrossRef]
- Lehmann, A.; Barto, E.K.; Powell, J.R.; Rillig, M.C. Mycorrhizal responsiveness trends in annual crop plants and their wild relatives—A meta-analysis on studies from 1981 to 2010. Plant. Soil 2012, 355, 231–250. [Google Scholar] [CrossRef]
- Koide, R.T. Functional complementarity in the arbuscular mycorrhizal symbiosis. New Phytol. 2000, 147, 233–235. [Google Scholar] [CrossRef]
- Jansa, J.; Smith, F.A.; Smith, S.E. Are there benefits of simultaneous root colonization by different arbuscular mycorrhizal fungi? New Phytol. 2008, 177, 779–789. [Google Scholar] [CrossRef]
- Jansa, J.; Thonar, C.; Frossard, E. Enhancement of symbiotic benefits through manipulation of the mycorrhizal community composition. Asp. Appl. Biol. 2009, 98, 9–15. [Google Scholar]
- Hart, M.M.; Klironomos, J.N. Diversity of arbuscular mycorrhizal fungi and ecosystem functioning. In Mycorrhizal Ecology; Springer: Berlin/Heidelberg, Germany, 2003. [Google Scholar] [CrossRef]
- Adewole, M.B.; Ilesanmi, A.O. Effects of soil amendments on the nutritional quality of okra (Abelmoschus esculentus [L.] Moench). J. Soil. Sci. Plant. Nut. 2011, 11, 45–55. [Google Scholar] [CrossRef]
- Masoero, G.; Peiretti, P.G.; Cugnetto, A.; Giovannetti, G. Raw pH fall-out as a sign of a mycorrhizal modifier of Sorghum sudanensis. J. Agron. Res. 2018, 1, 1–11. [Google Scholar] [CrossRef]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Lekberg, Y.; Helgason, T. In situ mycorrhizal function—Knowledge gaps and future directions. New Phytol. 2018, 220, 957–962. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Lehmann, A.; Zheng, W.; You, Z.; Rillig, M.C. Arbuscular mycorrhizal fungi increase grain yields: A meta-analysis. New Phytol. 2019, 222, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Poorter, H.; Bühler, J.; Van Dusschoten, D.; Climent, J.; Postma, J.A. Pot size matters: A meta-analysis of the effects of rooting volume on plant growth. Funct. Plant. Boil. 2012, 39, 839–850. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Bonini, P.; Cardarelli, M. Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int. J. Plant. Prod. 2015, 9, 171–190. [Google Scholar]


| Experimental Scale | Treatment | SDW (g) | Number of Pods Per Plant | Number of Pods with 2 Grains Per Plant | Number of Grains Per Plant | Weight of Individual Grains Per Plant (g) | Grain Yield Per Plant (g) |
|---|---|---|---|---|---|---|---|
| Greenhouse | Control | 1.4 ± 0.1 a | 8 ± 0.6 | NA | 5 ± 0.2 | 0.29 ± 0.0 a | 1.5 ± 0.0 |
| Rcoat | 1.3 ± 0.0 a | 7 ± 0.1 | 5 ± 0.4 | 0.33 ± 0.0 b | 1.5 ± 0.1 | ||
| MRcoat | 1.6 ± 0.1 b | 8 ± 0.9 | 4 ± 0.3 | 0.35 ± 0.0 b | 1.5 ± 0.1 | ||
| Field | Control | 10.2 ± 2.1 | 24 ± 4.1 x | 4 ± 0.6 x | 27 ± 3.8 x | 0.30 ± 0.0 | 8.4 ± 1.4 x |
| Rcoat | 6.0 ± 1.1 | 25 ± 7.6 x | 3 ± 1.4 x | 25 ± 7.9 x | 0.33 ± 0.0 | 7.9 ± 2.5 x | |
| MRcoat | 8.9 ± 0.9 | 62 ± 1.6 y | 9 ± 1.9 y | 68 ± 5.9 y | 0.30 ± 0.0 | 20.1 ± 1.4 y |
| Treatment | Harvest Index (%) | Relative Effectiveness (%) |
|---|---|---|
| Control | 103.7 x | - |
| Rcoat | 147.4 xy | 66.0 |
| MRcoat | 229.8 y | 107.0 |
| Experimental Scale | Treatment | Crude Protein (%) | Fat (%) | Crude Fiber (%) | Ash (%) |
|---|---|---|---|---|---|
| Greenhouse | Control | 19.9 ± 0.4 | 4.2 ± 0.1 | 4.5 ± 0.4 | 2.5 ± 0.1 |
| Rcoat | 19.3 ± 0.3 | 4.6 ± 0.3 | 4.4 ± 0.2 | 2.4 ± 0.1 | |
| MRcoat | 18.4 ± 0.5 | 4.5 ± 0.2 | 4.6 ± 0.6 | 2.3 ± 0.1 | |
| Field | Control | 20.8 ± 0.5 | 3.9 ± 0.1 | 4.1 ± 0.2 y | 3.0 ± 0.2 |
| Rcoat | 20.8 ± 0.2 | 3.9 ± 0.2 | 3.5 ± 0.1 x | 2.9 ± 0.0 | |
| MRcoat | 20.6 ± 0.5 | 3.9 ± 0.3 | 3.1 ± 0.1 x | 2.6 ± 0.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rocha, I.; Duarte, I.; Ma, Y.; Souza-Alonso, P.; Látr, A.; Vosátka, M.; Freitas, H.; Oliveira, R.S. Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy 2019, 9, 471. https://doi.org/10.3390/agronomy9080471
Rocha I, Duarte I, Ma Y, Souza-Alonso P, Látr A, Vosátka M, Freitas H, Oliveira RS. Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy. 2019; 9(8):471. https://doi.org/10.3390/agronomy9080471
Chicago/Turabian StyleRocha, Inês, Isabel Duarte, Ying Ma, Pablo Souza-Alonso, Aleš Látr, Miroslav Vosátka, Helena Freitas, and Rui S. Oliveira. 2019. "Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea" Agronomy 9, no. 8: 471. https://doi.org/10.3390/agronomy9080471
APA StyleRocha, I., Duarte, I., Ma, Y., Souza-Alonso, P., Látr, A., Vosátka, M., Freitas, H., & Oliveira, R. S. (2019). Seed Coating with Arbuscular Mycorrhizal Fungi for Improved Field Production of Chickpea. Agronomy, 9(8), 471. https://doi.org/10.3390/agronomy9080471