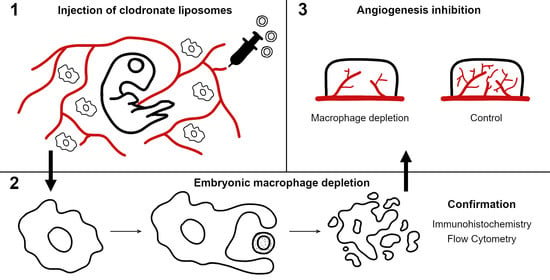
Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ex ovo CAM assay
2.2. Depletion of Macrophages by Clodronate Liposome Injection
2.3. Immunohistochemistry
2.4. Flow Cytometry
2.5. CAM Angiogenesis Assay
2.6. Embryo Viability after Injection with Clodronate Liposomes and Free Clodronate
2.7. Statistics
3. Results
3.1. Clodronate Liposome Injection Depletes Macrophages in the Chicken Embryo
3.2. Injection of Clodronate Liposomes but not Free Clodronate Reduces Viability of the Chicken Embryo
3.3. Depletion of Macrophages Induces a Reduced Angiogenic Response
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Corliss, B.A.; Azimi, M.S.; Munson, J.M.; Peirce, S.M.; Murfee, W.L. Macrophages: An inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation 2016, 23, 95–121. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P. Mechanisms of angiogenesis and arteriogenesis. Nat. Med. 2000, 6, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Du Cheyne, C.; Tay, H.; De Spiegelaere, W. The complex TIE between macrophages and angiogenesis. Anat. Histol. Embryol. 2019, 49, 585–596. [Google Scholar] [CrossRef] [PubMed]
- Bahramsoltani, M.; De Spiegelaere, W.; Janczyk, P.; Hiebl, B.; Cornillie, P.; Plendl, J. Quantitation of angiogenesis in vitro induced by VEGF-A and FGF-2 in two different human endothelial cultures—An all-in-one assay. Clin. Hemorheol. Microcirc. 2010, 46, 189–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak-Sliwinska, P.; Alitalo, K.; Allen, E.; Anisimov, A.; Aplin, A.C.; Auerbach, R.; Augustin, H.G.; Bates, D.O.; van Beijnum, J.R.; Bender, R.H.F.; et al. Consensus guidelines for the use and interpretation of angiogenesis assays. Angiogenesis 2018, 21, 425–532. [Google Scholar] [CrossRef] [Green Version]
- Petrovova, E.; Giretova, M.; Kvasilova, A.; Benada, O.; Danko, J.; Medvecky, L.; Sedmera, D. Preclinical alternative model for analysis of porous scaffold biocompatibility applicable in bone tissue engineering. Altex Altern. Anim. Exp. 2019, 36, 121–130. [Google Scholar]
- Ribatti, D. The chick embryo chorioallantoic membrane (CAM). A multifaceted experimental model. Mech. Dev. 2016, 141, 70–77. [Google Scholar] [CrossRef]
- Aleksandrowicz, E.; Herr, I. Ethical euthanasia and short-term anesthesia of the chick embryo. Altex Altern. Anim. Exp. 2015, 32, 143–147. [Google Scholar]
- Schlatter, P.; Konig, M.F.; Karlsson, L.M.; Burri, P.H. Quantitative study of intussusceptive capillary growth in the chorioallantoic membrane (CAM) of the chicken embryo. Microvasc. Res. 1997, 54, 65–73. [Google Scholar] [CrossRef]
- Auerbach, R.; Kubai, L.; Knighton, D.; Folkman, J. A simple procedure for the long-term cultivation of chicken embryos. Dev. Biol. 1974, 41, 391–394. [Google Scholar] [CrossRef]
- Dohle, D.S.; Pasa, S.D.; Gustmann, S.; Laub, M.; Wissler, J.H.; Jennissen, H.P.; Dunker, N. Chick ex ovo culture and ex ovo CAM assay: How it really works. J. Vis. Exp. 2009, 33, e1620. [Google Scholar]
- Zajac, E.; Schweighofer, B.; Kupriyanova, T.A.; Juncker-Jensen, A.; Minder, P.; Quigley, J.P.; Deryugina, E.I. Angiogenic capacity of M1- and M2-polarized macrophages is determined by the levels of TIMP-1 complexed with their secreted proMMP-9. Blood 2013, 122, 4054–4067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowak-Sliwinska, P.; Segura, T.; Iruela-Arispe, M.L. The chicken chorioallantoic membrane model in biology, medicine and bioengineering. Angiogenesis 2014, 17, 779–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nucera, S.; Biziato, D.; De Palma, M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 2011, 55, 495–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fantin, A.; Vieira, J.M.; Gestri, G.; Denti, L.; Schwarz, Q.; Prykhozhij, S.; Peri, F.; Wilson, S.W.; Ruhrberg, C. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 2010, 116, 829–840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFalco, T.; Bhattacharya, I.; Williams, A.V.; Sams, D.M.; Capel, B. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc. Natl. Acad. Sci. USA 2014, 111, E2384–E2393. [Google Scholar] [CrossRef] [Green Version]
- Zijlstra, A.; Aimes, R.T.; Zhu, D.; Regazzoni, K.; Kupriyanova, T.; Seandel, M.; Deryugina, E.I.; Quigley, J.P. Collagenolysis-dependent angiogenesis mediated by matrix metalloproteinase-13 (collagenase-3). J. Biol. Chem. 2004, 279, 27633–27645. [Google Scholar] [CrossRef] [Green Version]
- Balic, A.; Garcia-Morales, C.; Vervelde, L.; Gilhooley, H.; Sherman, A.; Garceau, V.; Gutowska, M.W.; Burt, D.W.; Kaiser, P.; Hume, D.A.; et al. Visualisation of chicken macrophages using transgenic reporter genes: Insights into the development of the avian macrophage lineage. Development 2014, 141, 3255–3265. [Google Scholar] [CrossRef] [Green Version]
- Van Rooijen, N.; van Kesteren-Hendrikx, E. Clodronate liposomes: Perspectives in research and therapeutics. J. Liposome Res. 2002, 12, 81–94. [Google Scholar] [CrossRef]
- Lehenkari, P.P.; Kellinsalmi, M.; Napankangas, J.P.; Ylitalo, K.V.; Monkkonen, J.; Rogers, M.J.; Azhayev, A.; Vaananen, H.K.; Hassinen, I.E. Further insight into mechanism of action of clodronate: Inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol. Pharmacol. 2002, 61, 1255–1262. [Google Scholar] [CrossRef] [Green Version]
- Zeisberger, S.M.; Odermatt, B.; Marty, C.; Zehnder-Fjallman, A.H.M.; Ballmer-Hofer, K.; Schwendener, R.A. Clodronate-liposome-mediated depletion of tumour-associated macrophages: A new and highly effective antiangiogenic therapy approach. Br. J. Cancer 2006, 95, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Reusser, N.M.; Dalton, H.J.; Pradeep, S.; Gonzalez-Villasana, V.; Jennings, N.B.; Vasquez, H.G.; Wen, Y.F.; Rupaimoole, R.; Nagaraja, A.S.; Gharpure, K.; et al. Clodronate inhibits tumor angiogenesis in mouse models of ovarian cancer. Cancer Biol. Ther. 2014, 15, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Morales, R.A.; Allende, M.L. Peripheral macrophages promote tissue regeneration in zebrafish by fine-tuning the inflammatory response. Front. Immunol. 2019, 10, 253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zijlstra, A.; Seandel, M.; Kupriyanova, T.A.; Partridge, J.J.; Madsen, M.A.; Hahn-Dantona, E.A.; Quigley, J.P.; Deryugina, E.I. Proangiogenic role of neutrophil-like inflammatory heterophils during neovascularization induced by growth factors and human tumor cells. Blood 2006, 107, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, M.; Shing, Y.; Folkman, J. Quantitation of angiogenesis and antiangiogenesis in the chick-embryo chorioallantoic membrane. Microvasc. Res. 1994, 47, 31–40. [Google Scholar] [CrossRef]
- Wood, W.; Turmaine, M.; Weber, R.; Camp, V.; Maki, R.A.; McKercher, S.R.; Martin, P. Mesenchymal cells engulf and clear apoptotic footplate cells in macrophageless PU.1 null mouse embryos. Development 2000, 127, 5245–5252. [Google Scholar]
- MacDonald, K.P.; Palmer, J.S.; Cronau, S.; Seppanen, E.; Olver, S.; Raffelt, N.C.; Kuns, R.; Pettit, A.R.; Clouston, A.; Wainwright, B.; et al. An antibody against the colony-stimulating factor 1 receptor depletes the resident subset of monocytes and tissue- and tumor-associated macrophages but does not inhibit inflammation. Blood 2010, 116, 3955–3963. [Google Scholar] [CrossRef] [Green Version]
- Hamburger, V.; Hamilton, H.L. A series of normal stages in the development of the chick embryo. J. Morphol. 1951, 88, 49–92. [Google Scholar] [CrossRef]
- Guth, A.M.; Hafeman, S.D.; Elmslie, R.E.; Dow, S.W. Liposomal clodronate treatment for tumour macrophage depletion in dogs with soft-tissue sarcoma. Vet. Comp. Oncol. 2013, 11, 296–305. [Google Scholar] [CrossRef] [Green Version]
- Kameka, A.M.; Haddadi, S.; Jamaldeen, F.J.; Moinul, P.; He, X.T.; Nawazdeen, F.H.P.; Bonfield, S.; Sharif, S.; van Rooijen, N.; Abdul-Careem, M.F. Clodronate treatment significantly depletes macrophages in chickens. Can. J. Vet. Res. 2014, 78, 274–282. [Google Scholar]
- Galletti, G.; Scielzo, C.; Barbaglio, F.; Rodriguez, T.V.; Riba, M.; Lazarevic, D.; Cittaro, D.; Simonetti, G.; Ranghetti, P.; Scarfo, L.; et al. Targeting macrophages sensitizes chronic lymphocytic leukemia to apoptosis and Inhibits disease progression. Cell Rep. 2016, 14, 1748–1760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawanishi, N.; Mizokami, T.; Niihara, H.; Yada, K.; Suzuki, K. Macrophage depletion by clodronate liposome attenuates muscle injury and inflammation following exhaustive exercise. Biochem. Biophys. Rep. 2016, 5, 146–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Epelman, S.; Lavine, K.J.; Randolph, G.J. Origin and functions of tissue macrophages. Immunity 2014, 41, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, Q.; Wang, Y.; Cheng, S.; Lian, C.; Tang, B.; Wang, F.; Lu, Z.; Ji, Y.; Zhao, R.; Zhang, W.; et al. Site-directed genome knockout in chicken cell line and embryos can use CRISPR/Cas gene editing technology. G3 (Bethesda) 2016, 6, 1787–1792. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Hua, L.; Harmer, D.; Li, P.; Ren, G. Cre driver mice targeting macrophages. Methods Mol. Biol. 2018, 1784, 263–275. [Google Scholar]
- Hume, D.A.; MacDonald, K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef]
- Lin, W.Y.; Xu, D.Q.; Austin, C.D.; Caplazi, P.; Senger, K.; Sun, Y.L.; Jeet, S.; Young, J.; Delarosa, D.; Suto, E.; et al. Function of CSF1 and IL34 in macrophage homeostasis, inflammation, and cancer. Front. Immunol. 2019, 10. [Google Scholar] [CrossRef]
- Wu, Z.; Harne, R.; Chintoan-Uta, C.; Hu, T.J.; Wallace, R.; MacCallum, A.; Stevens, M.P.; Kaiser, P.; Balic, A.; Hume, D.A. Regulation and function of macrophage colony-stimulating factor (CSF1) in the chicken immune system. Dev. Comp. Immunol. 2020, 105, 103586. [Google Scholar] [CrossRef]
- Bader, J.E.; Enos, R.T.; Velazquez, K.T.; Carson, M.S.; Nagarkatti, M.; Nagarkatti, P.S.; Chatzistamou, I.; Davis, J.M.; Carson, J.A.; Robinson, C.M.; et al. Macrophage depletion using clodronate liposomes decreases tumorigenesis and alters gut microbiota in the AOM/DSS mouse model of colon cancer. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 314, G22–G31. [Google Scholar] [CrossRef]
- Claassen, I.; Van Rooijen, N.; Claassen, E. A new method for removal of mononuclear phagocytes from heterogeneous cell populations in vitro, using the liposome-mediated macrophage ‘suicide’ technique. J. Immunol. Methods 1990, 134, 153–161. [Google Scholar] [CrossRef]
- Qian, Q.; Jutila, M.A.; Van Rooijen, N.; Cutler, J.E. Elimination of mouse splenic macrophages correlates with increased susceptibility to experimental disseminated candidiasis. J. Immunol. 1994, 152, 5000–5008. [Google Scholar] [PubMed]
- Rosowski, E.E. Determining macrophage versus neutrophil contributions to innate immunity using larval zebrafish. Dis. Models Mech. 2020, 13, dmm041889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosef, N.; Vadakkan, T.J.; Park, J.H.; Poche, R.A.; Thomas, J.L.; Dickinson, M.E. The phenotypic and functional properties of mouse yolk-sac-derived embryonic macrophages. Dev. Biol. 2018, 442, 138–154. [Google Scholar] [CrossRef]
- Misra, R.M.; Bajaj, M.S.; Kale, V.P. Vasculogenic mimicry of HT1080 tumour cells in vivo: Critical role of HIF-1alpha-neuropilin-1 axis. PLoS ONE 2012, 7, e50153. [Google Scholar] [CrossRef]
- Ribatti, D.; Maruotti, N.; Nico, B.; Longo, V.; Mangierv, D.; Vacca, A.; Cantatore, F.P. Clodronate inhibits angiogenesis in vitro and in vivo. Oncol. Rep. 2008, 19, 1109–1112. [Google Scholar] [CrossRef] [Green Version]
- Walter, C.; Klein, M.O.; Pabst, A.; Al-Nawas, B.; Duschner, H.; Ziebart, T. Influence of bisphosphonates on endothelial cells, fibroblasts, and osteogenic cells. Clin. Oral Investig. 2010, 14, 35–41. [Google Scholar] [CrossRef]
- Danenberg, H.D.; Fishbein, I.; Gao, J.C.; Monkkonen, J.; Reich, R.; Gati, I.; Moerman, E.; Golomb, G. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation 2002, 106, 599–605. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tay, H.; Du Cheyne, C.; Demeyere, K.; De Craene, J.; De Bels, L.; Meyer, E.; Zijlstra, A.; Spiegelaere, W.D. Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay. Cells 2021, 10, 5. https://doi.org/10.3390/cells10010005
Tay H, Du Cheyne C, Demeyere K, De Craene J, De Bels L, Meyer E, Zijlstra A, Spiegelaere WD. Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay. Cells. 2021; 10(1):5. https://doi.org/10.3390/cells10010005
Chicago/Turabian StyleTay, Hanna, Charis Du Cheyne, Kristel Demeyere, Jurgen De Craene, Lobke De Bels, Evelyne Meyer, Andries Zijlstra, and Ward De Spiegelaere. 2021. "Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay" Cells 10, no. 1: 5. https://doi.org/10.3390/cells10010005
APA StyleTay, H., Du Cheyne, C., Demeyere, K., De Craene, J., De Bels, L., Meyer, E., Zijlstra, A., & Spiegelaere, W. D. (2021). Depletion of Embryonic Macrophages Leads to a Reduction in Angiogenesis in the Ex Ovo Chick Chorioallantoic Membrane Assay. Cells, 10(1), 5. https://doi.org/10.3390/cells10010005