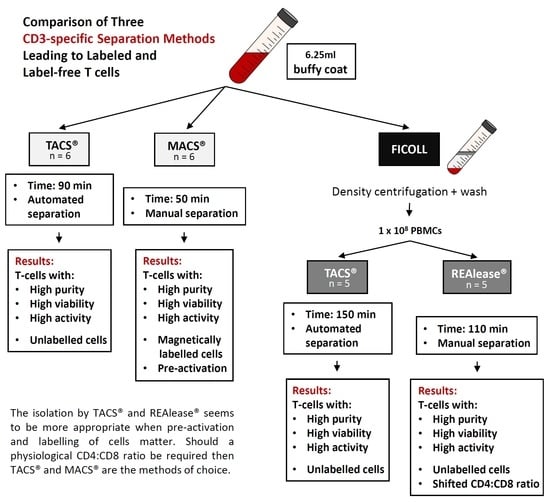
Comparison of Three CD3-Specific Separation Methods Leading to Labeled and Label-Free T Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Separation
2.2. Cell Separation from Buffy Coat Using MACS® and TACS® System
2.3. Cell Separation from PBMCs Using TACS® and REAlease® System
2.4. Cell Proliferation Assay
2.4.1. Microscopy-Based Proliferation Assay
2.4.2. VPD450-Based Proliferation Assay
2.5. Detection of Cytokines
2.6. Annexin-V Assay
2.7. Cell Viability and Receptor Detection
- Whole mononuclear white cell population: aCD45-PerCP (2D1; BioLegend, San Diego, CA, USA), aCD3-V450 (UCHT1; Becton Dickinson, Heidelberg, Germany), aCD19-PE (SJ25C1; Becton Dickinson, Heidelberg, Germany), aCD14-APC (M5E2; BioLegend, San Diego, CA, USA), aCD16-FITC (B73.1; BioLegend, San Diego, CA, USA), and aCD56-FITC (HCD56; BioLegend, San Diego, CA, USA).
- Erythrocyte contamination: aCD235a-FITC (HI264; BioLegend, San Diego, CA, USA), aCD45-PerCP (2D1; BioLegend, San Diego, CA, USA), and aCD81-APC (1D6; Thermo Fisher Scientific, Waltham, MA, USA).
- T cell markers: aCD3-V450 (UCHT1, Becton Dickinson, Heidelberg, Germany), aCD4-PE (OKT4; BioLegend, San Diego, CA, USA), aCD8-FITC (SK1; BioLegend, San Diego, CA, USA), and aCD45-PerCP (2D1; BioLegend, San Diego, CA, USA).
- Proliferation assay: aCD3-FITC (SK7; BioLegend, San Diego, CA, USA), aCD8-APC (SK1; BioLegend, San Diego, CA, USA).
3. Results
3.1. Yield and Viability of CD3-Positive Cells
3.2. Purity and Characterization of CD3 Isolated Cells
3.3. Functional Activity of CD3 Isolated Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grützkau, A.; Radbruch, A. Small but mighty: How the MACS-technology based on nanosized superparamagnetic particles has helped to analyze the immune system within the last 20 years. Cytom. A 2010, 77, 643–647. [Google Scholar] [CrossRef]
- Miltenyi, S.; Müller, W.; Weichel, W.; Radbruch, A. High gradient magnetic cell separation with MACS. Cytometry 1990, 11, 231–238. [Google Scholar] [CrossRef]
- Mohr, F.; Przibilla, S.; Leonhardt, F.; Stemberger, C.; Dreher, S.; Müller, T.R.; Fräßle, S.P.; Schmidt, G.P.; Kiene, M.-L.; Stadler, H.; et al. Efficient immunoaffinity chromatography of lymphocytes directly from whole blood. Sci. Rep. 2018, 8, 16731. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.; Gerdes, W.; Leonhardt, F.; Berthold, R.; Sack, U.; Grahnert, A. A comparative study of two separation methods to isolate monocytes. Cytom. A 2019, 95, 234–241. [Google Scholar] [CrossRef] [Green Version]
- Miltenyi Biotec. REAlease™ Immunomagnetic Separation Technology, Reversible Cell Labeling for Maximal Flexibility. Available online: https://www.miltenyibiotec.com/_Resources/Persistent/1eaf2fd9af660a3c37b9b19791f955fe442a712c/REAlease%20Immunomagnetic%20Separation%20Technology%20brochure.pdf (accessed on 19 October 2021).
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aranda, F.; Buqué, A.; Bloy, N.; Castoldi, F.; Eggermont, A.; Cremer, I.; Fridman, W.H.; Fucikova, J.; Galon, J.; Spisek, R.; et al. Trial Watch: Adoptive cell transfer for oncological indications. Oncoimmunology 2015, 4, e1046673. [Google Scholar] [CrossRef] [Green Version]
- Zhang, R.; Deng, Q.; Jiang, Y.-Y.; Zhu, H.-B.; Wang, J.; Zhao, M.-F. Effect and changes in PD-1 expression of CD19 CAR-T cells from T cells highly expressing PD-1 combined with reduced-dose PD-1 inhibitor. Oncol. Rep. 2019, 41, 3455–3463. [Google Scholar] [CrossRef] [PubMed]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef]
- Seimetz, D.; Heller, K.; Richter, J. Approval of First CAR-Ts: Have we solved all Hurdles for ATMPs? Cell Med. 2019, 11, 2155179018822781. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Assesment Report Kymriah EMA/485563/2018. 2018. Available online: https://www.ema.europa.eu/en/documents/assessment-report/kymriah-epar-public-assessment-report_en.pdf (accessed on 7 May 2021).
- Schultz, K.L.W. Summary Basis for Regulatory Action Template Breyanzi FDA, BLA STN#: 125714/0. 2021. Available online: https://fda.report/media/146242/Summary+Basis+for+Regulatory+Action+-+BREYANZI.pdf (accessed on 7 May 2021).
- European Medicines Agency. CHMP Assessment Report Tecartus EMA/588798/2020. 2020. Available online: https://www.ema.europa.eu/en/documents/assessment-report/tecartus-epar-public-assessment-report_en.pdf (accessed on 7 May 2021).
- Price Graeme. Summary Basis for Regulatory Action Tecartus FDA, BLA STN#:125703. 2020. Available online: https://www.fda.gov/media/141093/download (accessed on 7 May 2021).
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Goh, R.-Y.; Kim, S.-H.; Han, J.-Y. Lineage-specific chimerism analysis in nucleated cells, T cells and natural killer cells after myeloablative allogeneic hematopoietic stem cell transplantation. Korean J. Hematol. 2011, 46, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Platzbecker, U.; Wermke, M.; Radke, J.; Oelschlaegel, U.; Seltmann, F.; Kiani, A.; Klut, I.-M.; Knoth, H.; Röllig, C.; Schetelig, J.; et al. Azacitidine for treatment of imminent relapse in MDS or AML patients after allogeneic HSCT: Results of the RELAZA trial. Leukemia 2012, 26, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Lange, T.; Hubmann, M.; Burkhardt, R.; Franke, G.-N.; Cross, M.; Scholz, M.; Leiblein, S.; Al-Ali, H.K.; Edelmann, J.; Thiery, J.; et al. Monitoring of WT1 expression in PB and CD34(+) donor chimerism of BM predicts early relapse in AML and MDS patients after hematopoietic cell transplantation with reduced-intensity conditioning. Leukemia 2011, 25, 498–505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR-T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sommermeyer, D.; Hudecek, M.; Kosasih, P.L.; Gogishvili, T.; Maloney, D.G.; Turtle, C.J.; Riddell, S.R. Chimeric antigen receptor-modified T cells derived from defined CD8+ and CD4+ subsets confer superior antitumor reactivity in vivo. Leukemia 2016, 30, 492–500. [Google Scholar] [CrossRef] [Green Version]
- Howard, R.R.; Fasano, C.S.; Frey, L.; Miller, C.H. Reference intervals of CD3, CD4, CD8, CD4/CD8, and absolute CD4 values in Asian and Non-Asian populations. Cytometry 1996, 26, 231–232. [Google Scholar] [CrossRef]
- Amadori, A.; Zamarchi, R.; De Silvestro, G.; Forza, G.; Cavatton, G.; Danieli, G.A.; Clementi, M.; Chieco-Bianchi, L. Genetic control of the CD4/CD8 T-cell ratio in humans. Nat. Med. 1995, 1, 1279–1283. [Google Scholar] [CrossRef]
- Pierzchalski, A.; Mittag, A.; Bocsi, J.; Tarnok, A. An innovative cascade system for simultaneous separation of multiple cell types. PLoS ONE 2013, 8, e74745. [Google Scholar] [CrossRef] [Green Version]
- Stock, S.; Schmitt, M.; Sellner, L. Optimizing Manufacturing Protocols of Chimeric Antigen Receptor T Cells for Improved Anticancer Immunotherapy. Int. J. Mol. Sci. 2019, 20, 6223. [Google Scholar] [CrossRef] [Green Version]
- Wucherpfennig, K.W.; Gagnon, E.; Call, M.J.; Huseby, E.S.; Call, M.E. Structural Biology of the T-cell Receptor: Insights into Receptor Assembly, Ligand Recognition, and Initiation of Signaling. Cold Spring Harb. Perspect. Biol. 2010, 2, a005140. [Google Scholar] [CrossRef] [Green Version]
- Meuer, S.C.; Acuto, O.; Hussey, R.E.; Hodgdon, J.C.; Fitzgerald, K.A.; Schlossman, S.F.; Reinherz, E.L. Evidence for the T3-associated 90K heterodimer as the T-cell antigen receptor. Nature 1983, 303, 808–810. [Google Scholar] [CrossRef]
- Mayer, A.; Zhang, Y.; Perelson, A.S.; Wingreen, N.S. Regulation of T cell expansion by antigen presentation dynamics. Proc. Natl. Acad. Sci. USA 2019, 116, 5914–5919. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeg, J.; Axmann, M.; Matic, J.; Liapis, A.; Depoil, D.; Afrose, J.; Curado, S.; Dustin, M.L.; Spatz, J.P. T cell activation is determined by the number of presented antigens. Nano Lett. 2013, 13, 5619–5626. [Google Scholar] [CrossRef]
- Kaech, S.M.; Wherry, E.J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 2007, 27, 393–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stingl, L.A.; Sinska, A.; Landesmann, U.; Smolen, J.S. Induction of interleukin 2 receptiveness and proliferation in resting peripheral T cells by monoclonal anti-CD3 (T3) antibodies does not require the presence of macrophages. Clin. Exp. Immunol. 1987, 68, 146–155. [Google Scholar]
- Plouffe, B.D.; Murthy, S.K.; Lewis, L.H. Fundamentals and application of magnetic particles in cell isolation and enrichment: A review. Rep. Prog. Phys. 2015, 78, 16601. [Google Scholar] [CrossRef]
- Clark, J.R.; Scott, S.D.; Jack, A.L.; Lee, H.; Mason, J.; Carter, G.I.; Pearce, L.; Jackson, T.; Clouston, H.; Sproul, A.; et al. Monitoring of chimerism following allogeneic haematopoietic stem cell transplantation (HSCT): Technical recommendations for the use of short tandem repeat (STR) based techniques, on behalf of the United Kingdom National External Quality Assessment Service for Leucocyte Immunophenotyping Chimerism Working Group. Br. J. Haematol. 2015, 168, 26–37. [Google Scholar] [CrossRef]
- Tajti, G.; Szanto, T.G.; Csoti, A.; Racz, G.; Evaristo, C.; Hajdu, P.; Panyi, G. Immunomagnetic separation is a suitable method for electrophysiology and ion channel pharmacology studies on T cells. Channels 2021, 15, 53–66. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weiss, R.; Gerdes, W.; Berthold, R.; Sack, U.; Koehl, U.; Hauschildt, S.; Grahnert, A. Comparison of Three CD3-Specific Separation Methods Leading to Labeled and Label-Free T Cells. Cells 2021, 10, 2824. https://doi.org/10.3390/cells10112824
Weiss R, Gerdes W, Berthold R, Sack U, Koehl U, Hauschildt S, Grahnert A. Comparison of Three CD3-Specific Separation Methods Leading to Labeled and Label-Free T Cells. Cells. 2021; 10(11):2824. https://doi.org/10.3390/cells10112824
Chicago/Turabian StyleWeiss, Ronald, Wilhelm Gerdes, Rommy Berthold, Ulrich Sack, Ulrike Koehl, Sunna Hauschildt, and Anja Grahnert. 2021. "Comparison of Three CD3-Specific Separation Methods Leading to Labeled and Label-Free T Cells" Cells 10, no. 11: 2824. https://doi.org/10.3390/cells10112824
APA StyleWeiss, R., Gerdes, W., Berthold, R., Sack, U., Koehl, U., Hauschildt, S., & Grahnert, A. (2021). Comparison of Three CD3-Specific Separation Methods Leading to Labeled and Label-Free T Cells. Cells, 10(11), 2824. https://doi.org/10.3390/cells10112824