Deleterious Role of Th9 Cells in Pulmonary Fibrosis
Abstract
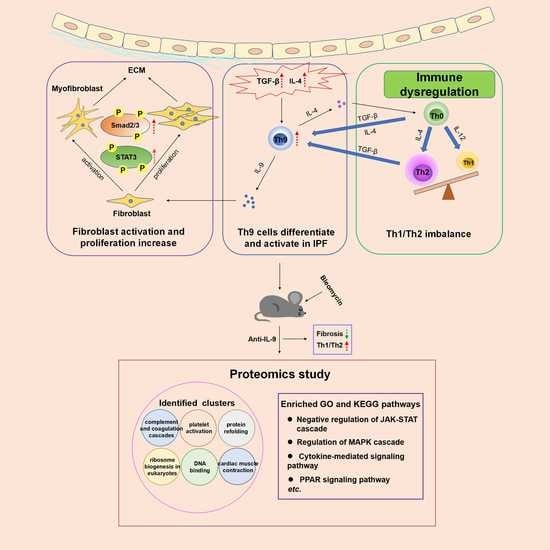
:1. Introduction
2. Results
2.1. Th9 Differentiation and Activation Increase in the PBMC and Lung Tissue of Patients with IPF
2.2. Th9 Differentiation and Activation Increase in Lung Tissue of BLM-Induced Lung Fibrosis Mice
2.3. IL-9 Promotes Fibroblast Proliferation and Activation In Vitro
2.4. Th9 Cells Promote Th0 Cells to Differentiate into Th2 Cells and Induce Lung Fibroblasts to Secrete More Collagen
2.5. Preventive Treatment of Neutralizing IL-9 Reduces Pulmonary Fibrosis and Collagen Secretion
2.6. Effects of IL-9 Neutralizing Antibody on the Ratio of Th9 Cells, Th2 Cells, and Th1/Th2 in Lung Lymphocytes of BLM Mice in Preventive Treatment
2.7. Proteomics Study Identify Additional Signal Pathways Involved in the Effect of IL-9 Neutralizing Antibody on Pulmonary Fibrosis
3. Discussion
4. Materials and Methods
4.1. Reagents and Antibodies
4.2. Human Subjects
4.3. Bleomycin-Induced Pulmonary Fibrosis Mice Model and Intervention Study
4.4. CCK Assay
4.5. Histology and Fibrosis Assessment
4.6. Hydroxyproline Assay
4.7. RNA Extraction and Quantitative Real-Time PCR (qRT-PCR)
4.8. Flow Cytometry
4.8.1. Human
4.8.2. Mouse
4.9. Isolation and Culture of Primary Fibroblasts
4.10. Western Blot Analysis
4.11. Co-Culture
4.12. Immunofluorescence and Image Analysis
4.13. Mass Spectrometry and Data Analysis
4.14. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Raghu, G.; Remy-Jardin, M.; Myers, J.L.; Richeldi, L.; Ryerson, C.J.; Lederer, D.J.; Behr, J.; Cottin, V.; Danoff, S.K.; Morell, F.; et al. Diagnosis of Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2018, 198, e44–e68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.N.; Tang, X.X. New Perspectives on the Aberrant Alveolar Repair of Idiopathic Pulmonary Fibrosis. Front. Cell Dev. Biol. 2020, 8, 580026. [Google Scholar] [CrossRef] [PubMed]
- Ley, B.; Collard, H.R.; King, T.E. Clinical Course and Prediction of Survival in Idiopathic Pulmonary Fibrosis. Am. J. Respir. Crit. Care Med. 2011, 183, 431–440. [Google Scholar] [CrossRef]
- Mora, A.L.; Rojas, M.; Pardo, A.; Selman, M. Emerging therapies for idiopathic pulmonary fibrosis, a progressive age-related disease. Nat. Rev. Drug Discov. 2017, 16, 755–772. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.J.; Collard, H.R.; Pardo, A.; Raghu, G.; Richeldi, L.; Selman, M.; Swigris, J.J.; Taniguchi, H.; Wells, A.U. Idiopathic pulmonary fibrosis. Nat. Rev. Dis. Primers 2017, 3, 17074. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Jakubzick, C.; Osterburg, A.R.; Nelson, R.L.; Gupta, N.; McCormack, F.X.; Borchers, M.T. Dendritic Cell Trafficking and Function in Rare Lung Diseases. Am. J. Respir. Cell Mol. Biol. 2017, 57, 393–402. [Google Scholar] [CrossRef]
- Smolen, J.S.; Kay, J.; Doyle, M.K.; Landewé, R.; Matteson, E.L.; Wollenhaupt, J.; Gaylis, N.; Murphy, F.T.; Neal, J.S.; Zhou, Y.; et al. Golimumab in patients with active rheumatoid arthritis after treatment with tumour necrosis factor α inhibitors (GO-AFTER study): A multicentre, randomised, double-blind, placebo-controlled, phase III trial. Lancet 2009, 374, 210–221. [Google Scholar] [CrossRef]
- Keane, M.P.; Belperio, J.A.; Burdick, M.D.; Strieter, R.M. IL-12 attenuates bleomycin-induced pulmonary fibrosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2001, 281, L92–L97. [Google Scholar] [CrossRef] [PubMed]
- Saito, A.; Okazaki, H.; Sugawara, I.; Yamamoto, K.; Takizawa, H. Potential action of IL-4 and IL-13 as fibrogenic factors on lung fibroblasts in vitro. Int. Arch. Allergy Immunol. 2003, 132, 168–176. [Google Scholar] [CrossRef]
- Hashimoto, S.; Gon, Y.; Takeshita, I.; Maruoka, S.; Horie, T. IL-4 and IL-13 induce myofibroblastic phenotype of human lung fibroblasts through c-Jun NH2-terminal kinase-dependent pathway. J. Allergy Clin. Immunol. 2001, 107, 1001–1008. [Google Scholar] [CrossRef]
- Kraft, M.; Lewis, C.; Pham, D.; Chu, H.W. IL-4, IL-13, and dexamethasone augment fibroblast proliferation in asthma. J. Allergy Clin. Immunol. 2001, 107, 602–606. [Google Scholar] [CrossRef]
- Park, S.W.; Ahn, M.H.; Jang, H.K.; Jang, A.S.; Kim, D.J.; Koh, E.S.; Park, J.S.; Uh, S.T.; Kim, Y.H.; Park, J.S.; et al. Interleukin-13 and its receptors in idiopathic interstitial pneumonia: Clinical implications for lung function. J. Korean Med. Sci. 2009, 24, 614–620. [Google Scholar] [CrossRef] [Green Version]
- Alhamad, E.H.; Cal, J.G.; Shakoor, Z.; Almogren, A.; AlBoukai, A.A. Cytokine gene polymorphisms and serum cytokine levels in patients with idiopathic pulmonary fibrosis. BMC Med. Genet. 2013, 14, 66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papiris, S.A.; Tomos, I.P.; Karakatsani, A.; Spathis, A.; Korbila, I.; Analitis, A.; Kolilekas, L.; Kagouridis, K.; Loukides, S.; Karakitsos, P.; et al. High levels of IL-6 and IL-8 characterize early-on idiopathic pulmonary fibrosis acute exacerbations. Cytokine 2018, 102, 168–172. [Google Scholar] [CrossRef]
- Cai, F.; Hornauer, H.; Peng, K.; Schofield, C.A.; Scheerens, H.; Morimoto, A.M. Bioanalytical challenges and improved detection of circulating levels of IL-13. Bioanalysis 2016, 8, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Heukels, P.; Moor, C.C.; von der Thüsen, J.H.; Wijsenbeek, M.S.; Kool, M. Inflammation and immunity in IPF pathogenesis and treatment. Respir. Med. 2019, 147, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Tatler, A.L.; Jenkins, G. TGF-beta activation and lung fibrosis. Proc. Am. Thorac. Soc. 2012, 9, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H. Th9 cells: Differentiation and disease. Immunol. Rev. 2013, 252, 104–115. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, Z.; Chang, C.; Lu, L.; Lau, C.S.; Lu, Q. Th9 cells and IL-9 in autoimmune disorders: Pathogenesis and therapeutic potentials. Hum. Immunol. 2017, 78, 120–128. [Google Scholar] [CrossRef]
- Zhao, P.; Xiao, X.; Ghobrial, R.M.; Li, X.C. IL-9 and Th9 cells: Progress and challenges. Int. Immunol. 2013, 25, 547–551. [Google Scholar] [CrossRef] [Green Version]
- Overed-Sayer, C.; Rapley, L.; Mustelin, T.; Clarke, D.L. Are mast cells instrumental for fibrotic diseases? Front. Pharmacol. 2013, 4, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noelle, R.J.; Nowak, E.C. Cellular sources and immune functions of interleukin-9. Nat. Rev. Immunol. 2010, 10, 683–687. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. A brief history of IL-9. J. Immunol. 2011, 186, 3283–3288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugimoto, N.; Suzukawa, M.; Nagase, H.; Koizumi, Y.; Ro, S.; Kobayashi, K.; Yoshihara, H.; Kojima, Y.; Kamiyama-Hara, A.; Hebisawa, A.; et al. IL-9 Blockade Suppresses Silica-induced Lung Inflammation and Fibrosis in Mice. Am. J. Respir. Cell Mol. Biol. 2019, 60, 232–243. [Google Scholar] [CrossRef]
- Arras, M.; Huaux, F.; Vink, A.; Delos, M.; Coutelier, J.P.; Many, M.C.; Barbarin, V.; Renauld, J.C.; Lison, D. Interleukin-9 reduces lung fibrosis and type 2 immune polarization induced by silica particles in a murine model. Am. J. Respir. Cell Mol. Biol. 2001, 24, 368–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weng, D.; Chen, X.Q.; Qiu, H.; Zhang, Y.; Li, Q.H.; Zhao, M.M.; Wu, Q.; Chen, T.; Hu, Y.; Wang, L.S.; et al. The Role of Infection in Acute Exacerbation of Idiopathic Pulmonary Fibrosis. Mediat. Inflamm. 2019, 2019, 5160694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, Z.; Ouyang, H.; Liu, Z.; Li, L.; Shi, Y. Aberrant expression of cytokine interleukin 9 along with interleukin 4 and interferon gamma in connective tissue disease-associated interstitial lung disease: Association with severity of pulmonary fibrosis. Arch. Med. Sci. 2016, 12, 101–106. [Google Scholar] [CrossRef] [Green Version]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Sato, S. Serum interleukin 9 levels are increased in patients with systemic sclerosis: Association with lower frequency and severity of pulmonary fibrosis. J. Rheumatol. 2011, 38, 2193–2197. [Google Scholar] [CrossRef]
- Saeki, M.; Kaminuma, O.; Nishimura, T.; Kitamura, N.; Mori, A.; Hiroi, T. Th9 cells elicit eosinophil-independent bronchial hyperresponsiveness in mice. Allergol. Int. 2016, 65, S24–S29. [Google Scholar] [CrossRef] [Green Version]
- Jakubzick, C.; Choi, E.S.; Joshi, B.H.; Keane, M.P.; Kunkel, S.L.; Puri, R.K.; Hogaboam, C.M. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J. Immunol. 2003, 171, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Chong, M.M.; Littman, D.R. Plasticity of CD4+ T cell lineage differentiation. Immunity 2009, 30, 646–655. [Google Scholar] [CrossRef] [Green Version]
- Tan, C.; Aziz, M.K.; Lovaas, J.D.; Vistica, B.P.; Shi, G.; Wawrousek, E.F.; Gery, I. Antigen-specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J. Immunol. 2010, 185, 6795–6801. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Qiu-Lan, H.; Lei, R.E.; Shi, C.; Jiang, H.X.; Qin, S.Y. Interleukin-9 Promotes Pancreatic Cancer Cells Proliferation and Migration via the miR-200a/Beta-Catenin Axis. Biomed. Res. Int. 2017, 2017, 2831056. [Google Scholar] [CrossRef] [PubMed]
- Lei, R.-E.; Shi, C.; Zhang, P.-L.; Hu, B.-L.; Jiang, H.-X.; Qin, S.-Y. IL-9 promotes proliferation and metastasis of hepatocellular cancer cells by activating JAK2/STAT3 pathway. Int J. Clin. Exp. Pathol. 2017, 10, 7940–7946. [Google Scholar]
- Prele, C.M.; Yao, E.; O’Donoghue, R.J.; Mutsaers, S.E.; Knight, D.A. STAT3: A central mediator of pulmonary fibrosis? Proc. Am. Thorac. Soc. 2012, 9, 177–182. [Google Scholar] [CrossRef]
- Walton, K.L.; Johnson, K.E.; Harrison, C.A. Targeting TGF-beta Mediated SMAD Signaling for the Prevention of Fibrosis. Front. Pharmacol. 2017, 8, 461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moeller, A.; Ask, K.; Warburton, D.; Gauldie, J.; Kolb, M. The bleomycin animal model: A useful tool to investigate treatment options for idiopathic pulmonary fibrosis? Int. J. Biochem. Cell Biol. 2008, 40, 362–382. [Google Scholar] [CrossRef] [Green Version]
- Hoppenot, D.; Malakauskas, K.; Lavinskienė, S.; Bajoriūnienė, I.; Kalinauskaitė, V.; Sakalauskas, R. Peripheral blood Th9 cells and eosinophil apoptosis in asthma patients. Medicina 2015, 51, 10–17. [Google Scholar] [CrossRef]
- Moretti, S.; Renga, G.; Oikonomou, V.; Galosi, C.; Pariano, M.; Iannitti, R.G.; Borghi, M.; Puccetti, M.; De Zuani, M.; Pucillo, C.E.; et al. A mast cell-ILC2-Th9 pathway promotes lung inflammation in cystic fibrosis. Nat. Commun. 2017, 8, 14017. [Google Scholar] [CrossRef]
- Arras, M.; Louahed, J.; Simoen, V.; Barbarin, V.; Misson, P.; van den Brûle, S.; Delos, M.; Knoops, L.; Renauld, J.-C.; Lison, D.; et al. B Lymphocytes Are Critical for Lung Fibrosis Control and Prostaglandin E2 Regulation in IL-9 Transgenic Mice. Am. J. Respir. Cell Mol. Biol. 2006, 34, 573–580. [Google Scholar] [CrossRef]
- Arras, M.; Louahed, J.; Heilier, J.F.; Delos, M.; Brombacher, F.; Renauld, J.C.; Lison, D.; Huaux, F. IL-9 protects against bleomycin-induced lung injury: Involvement of prostaglandins. Am. J. Pathol. 2005, 166, 107–115. [Google Scholar] [CrossRef]
- Frankenstein, Z.; Alon, U.; Cohen, I.R. The immune-body cytokine network defines a social architecture of cell interactions. Biol. Direct. 2006, 1, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoyle, G.W.; Brody, A.R. IL-9 and lung fibrosis: A Th2 good guy? Am. J. Respir. Cell Mol. Biol. 2001, 24, 365–367. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, E.; Germann, T.; Goedert, S.; Hoehn, P.; Huels, C.; Koelsch, S.; Kühn, R.; Müller, W.; Palm, N.; Rüde, E. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-beta and IL-4, and is inhibited by IFN-gamma. J. Immunol. 1994, 153, 3989–3996. [Google Scholar] [PubMed]
- Veldhoen, M.; Uyttenhove, C.; van Snick, J.; Helmby, H.; Westendorf, A.; Buer, J.; Martin, B.; Wilhelm, C.; Stockinger, B. Transforming growth factor-beta ’reprograms’ the differentiation of T helper 2 cells and promotes an interleukin 9-producing subset. Nat. Immunol. 2008, 9, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [PubMed]
- Machino, T.; Hashimoto, S.; Gon, Y.; Kujime, K.; Maruoka, S.; Horie, T. Interleukin-4 and interleukin-13 induce fibronectin production by human lung fibroblasts. Allergol. Int. 2001, 50, 197–202. [Google Scholar] [CrossRef] [Green Version]
- Arendse, B.; Van Snick, J.; Brombacher, F. IL-9 is a susceptibility factor in Leishmania major infection by promoting detrimental Th2/type 2 responses. J. Immunol. 2005, 174, 2205–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munitz, A.; Foster, P.S. T(H)9 cells: In front and beyond T(H). J. Allergy Clin. Immunol. 2012, 129, 1011–1013. [Google Scholar] [CrossRef]
- Cai, L.; Zhang, Y.; Zhang, Y.; Chen, H.; Hu, J. Effect of Th9/IL-9 on the growth of gastric cancer in nude mice. Onco Targets Ther. 2019, 12, 2225–2234. [Google Scholar] [CrossRef] [Green Version]
- Chakraborty, D.; Šumová, B.; Mallano, T.; Chen, C.W.; Distler, A.; Bergmann, C.; Ludolph, I.; Horch, R.E.; Gelse, K.; Ramming, A.; et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat. Commun. 2017, 8, 1130. [Google Scholar] [CrossRef] [Green Version]
- Pedroza, M.; Le, T.T.; Lewis, K.; Karmouty-Quintana, H.; To, S.; George, A.T.; Blackburn, M.R.; Tweardy, D.J.; Agarwal, S.K. STAT-3 contributes to pulmonary fibrosis through epithelial injury and fibroblast-myofibroblast differentiation. FASEB J. 2016, 30, 129–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derynck, R.; Zhang, Y.; Feng, X.H. Smads: Transcriptional activators of TGF-beta responses. Cell 1998, 95, 737–740. [Google Scholar] [CrossRef] [Green Version]
- Motzer, R.J.; Tannir, N.M.; McDermott, D.F.; Arén Frontera, O.; Melichar, B.; Choueiri, T.K.; Plimack, E.R.; Barthélémy, P.; Porta, C.; George, S.; et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N. Engl. J. Med. 2018, 378, 1277–1290. [Google Scholar] [CrossRef]
- Esfahani, K.; Buhlaiga, N.; Thébault, P.; Lapointe, R.; Johnson, N.A.; Miller, W.H., Jr. Alemtuzumab for Immune-Related Myocarditis Due to PD-1 Therapy. N. Engl. J. Med. 2019, 380, 2375–2376. [Google Scholar] [CrossRef] [PubMed]
- University of Alabama at Birmingham; National Institutes of Health (NIH). Autoantibody Reduction Therapy in Patients with Idiopathic Pulmonary Fibrosis (ART-IPF); National Library of Medicine (US): Bethesda, MD, USA, 2018. Available online: https://clinicaltrials.gov/ct2/show/NCT01969409 (accessed on 5 November 2021).
- Khalil, N.; Manganas, H.; Ryerson, C.J.; Shapera, S.; Cantin, A.M.; Hernandez, P.; Turcotte, E.E.; Parker, J.M.; Moran, J.E.; Albert, G.R.; et al. Phase 2 clinical trial of PBI-4050 in patients with idiopathic pulmonary fibrosis. Eur. Respir. J. 2018, 53, 1800663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MediciNova. A Randomized, Placebo-Controlled, Double-Blind Six Month Study Followed by an Open-Label Extension Phase to Evaluate the Efficacy, Safety and Tolerability of MN-001 in Subjects with Idiopathic Pulmonary Fibrosis (IPF); National Library of Medicine (US): Bethesda, MD, USA, 2020. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02503657 (accessed on 5 November 2021).
- Ng, B.; Dong, J.; D’Agostino, G.; Viswanathan, S.; Widjaja, A.A.; Lim, W.-W.; Ko, N.S.J.; Tan, J.; Chothani, S.P.; Huang, B.; et al. Interleukin-11 is a therapeutic target in idiopathic pulmonary fibrosis. Sci. Transl. Med. 2019, 11, eaaw1237. [Google Scholar] [CrossRef] [PubMed]
- Oh, K.; Park, H.-B.; Byoun, O.-J.; Shin, D.-M.; Jeong, E.M.; Kim, Y.W.; Kim, Y.S.; Melino, G.; Kim, I.-G.; Lee, D.-S. Epithelial transglutaminase 2 is needed for T cell interleukin-17 production and subsequent pulmonary inflammation and fibrosis in bleomycin-treated mice. J. Exp. Med. 2011, 208, 1707–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hubner, R.H.; Gitter, W.; El Mokhtari, N.E.; Mathiak, M.; Both, M.; Bolte, H.; Freitag-Wolf, S.; Bewig, B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 2008, 44, 507–511,514–517. [Google Scholar] [CrossRef] [PubMed]
- Szapiel, S.V.; Elson, N.A.; Fulmer, J.D.; Hunninghake, G.W.; Crystal, R.G. Bleomycin-induced interstitial pulmonary disease in the nude, athymic mouse. Am. Rev. Respir. Dis. 1979, 120, 893–899. [Google Scholar] [PubMed]
- Seluanov, A.; Vaidya, A.; Gorbunova, V. Establishing Primary Adult Fibroblast Cultures From Rodents. J. Vis. Exp. 2010, 44, 2033. [Google Scholar] [CrossRef] [PubMed] [Green Version]

















| Characteristic | Patients with IPF (n = 16) | Healthy Controls (n = 19) | p Value |
|---|---|---|---|
| Age (years) | 64.13 ± 6.62 | 65.45 ± 7.39 | 0.60 |
| Male, n (%) | 16 (100%) | 19 (100%) | - |
| Characteristic | Patients with IPF (n = 14) | Controls (n = 4) | p Value |
|---|---|---|---|
| Age (years) | 58.86 ± 8.75 | 60.5 ± 3 | 0.72 |
| Male, n (%) | 12 (85.71%) | 4 (100%) | - |
| FEV1 (%pre) | 88.43 ± 8.75 | 94.25 ± 5.06 | 0.15 |
| FVC (%pre) | 73.21 ± 5.96 | 88.5 ± 3 | <0.001 *** |
| DLCO (%pre) | 62.43 ± 6.38 | 87.25 ± 3.77 | <0.001 *** |
| Reagent | Source | Catalog# |
|---|---|---|
| LEAFTM Purified anti-mouse IL-9 antibody | Biolegend | 504802 |
| Ultra-LEAFTM Purified Armenian Hamster IgG Isotype Control | Biolegend | 400940 |
| Recombinant Mouse IL-9 | R&D Systems | 409-ML |
| Recombinant Mouse TGF-beta 1 | R&D Systems | 7666-MB |
| Recombinant Mouse IL-4 | R&D Systems | 404-ML |
| Mouse cross linked C-telopeptide of type I collagen (CTX-I) ELISA Kit | CUSABIO | CSB-E12782m |
| TB Green® Premix Ex TaqTM II (Tli RNaseH Plus) | Takara | RR820A |
| PrimeScriptTM RT reagent Kit with gDNA Eraser (Perfect Real Time) | Takara | RR047A |
| Bleomycin (BLM) | Hanhui Pharmaceuticals |
| Primary Antibody | Application/Dilution | Species | Source (Catalog#) |
|---|---|---|---|
| PU.1 | ICH (1:200) | Human | Abcam (ab76543) |
| IL-9 | ICH (1:200) | Human | Abcam (181397) |
| α-SMA | WB (1:1000), IF (1:200) | Rabbit | Abcam (ab5694) |
| COL1A1 | WB (1:500) | Rabbit | Abclonal (A1352) |
| STAT3 | WB (1:1000) | Rabbit | Cell Signaling Technology (4904) |
| p-STAT3 | WB (1:1000) | Rabbit | Cell Signaling Technology (9145) |
| SMAD2/3 | WB (1:1000) | Rabbit | Cell Signaling Technology (8685) |
| p-SMAD2/3 | WB (1:1000) | Rabbit | Cell Signaling Technology (8828) |
| β-actin | WB (1:5000) | Mouse | EASYBIO (BE0021-100) |
| Gene | Mouse Primer Sequence(F/R) |
|---|---|
| Col1a1 | CCCGTTGGCAAAGATGGTAG |
| ACCTTGGCTACCCTGAGAAC | |
| α-SMA | GCTGGTGATGATGCTCCCA |
| GCCCATTCCAACCATTACTCC | |
| PU.1 | GTTCTCGTCCAAGCACAAGG |
| TTCTTCACCTCGCCTGTCTT | |
| Irf4 | AGACCAGACTTGCAAGCTCT |
| CACCAAAGCACAGAGTCACC | |
| Gapdh | AACGACCCCTTCATTGACCT |
| CATTCTCGGCCTTGACTGTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, K.M.; Yang, X.S.; Luo, Q.; She, Y.X.; Yu, Q.Y.; Tang, X.X. Deleterious Role of Th9 Cells in Pulmonary Fibrosis. Cells 2021, 10, 3209. https://doi.org/10.3390/cells10113209
Deng KM, Yang XS, Luo Q, She YX, Yu QY, Tang XX. Deleterious Role of Th9 Cells in Pulmonary Fibrosis. Cells. 2021; 10(11):3209. https://doi.org/10.3390/cells10113209
Chicago/Turabian StyleDeng, Kui Miao, Xiang Sheng Yang, Qun Luo, Yi Xin She, Qing Yang Yu, and Xiao Xiao Tang. 2021. "Deleterious Role of Th9 Cells in Pulmonary Fibrosis" Cells 10, no. 11: 3209. https://doi.org/10.3390/cells10113209
APA StyleDeng, K. M., Yang, X. S., Luo, Q., She, Y. X., Yu, Q. Y., & Tang, X. X. (2021). Deleterious Role of Th9 Cells in Pulmonary Fibrosis. Cells, 10(11), 3209. https://doi.org/10.3390/cells10113209