Leptin Is an Important Endocrine Player That Directly Activates Gonadotropic Cells in Teleost Fish, Chub Mackerel
Abstract
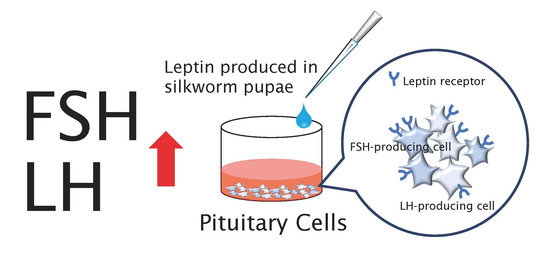
:1. Introduction
2. Materials and Methods
2.1. Production and Purification of Recombinant Leptin
2.2. Luciferase Reporter Gene Assay
2.3. In Vitro Leptin Bioassay
2.4. RNA Preparation and Quantitative Real-Time PCR
2.5. Dual-Label In Situ Hybridization
2.6. Statistical Analysis
3. Results
3.1. Production of Recombinant Leptin-a
3.2. Effect of Recombinant Leptin on GTH Secretion
3.3. Effect of Leptin on GTH Gene Expression
3.4. Expression of lepr in GTH-Producing Cells
3.5. Localization Analysis of lepr Using Frozen Sections
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Tartaglia, L.A. The leptin receptor. J. Biol. Chem. 1997, 272, 6093–6096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Mayor, R.V.; Andrade, M.A.; Rios, M.; Lage, M.; Diéguez, C.; Casanueva, F.F. Serum Leptin Levels in Normal Children: Relationship to Age, Gender, Body Mass Index, Pituitary-Gonadal Hormones, and Pubertal Stage. J. Clin. Endocrinol. Metab. 1997, 82, 2849–2855. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef]
- Chehab, F.F.; Mounzih, K.; Lu, R.; Lim, M.E. Early Onset of Reproductive Function in Normal Female Mice Treated with Leptin. Science 1997, 275, 88–90. [Google Scholar] [CrossRef]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef]
- Finn, P.D.; Cunningham, M.J.; Pau, K.-Y.F.; Spies, H.G.; Clifton, D.K.; Steiner, R.A. The Stimulatory Effect of Leptin on the Neuroendocrine Reproductive Axis of the Monkey. Endocrinology 1998, 139, 4652–4662. [Google Scholar] [CrossRef]
- Quennell, J.H.; Mulligan, A.C.; Tups, A.; Liu, X.; Phipps, S.J.; Kemp, C.J.; Herbison, A.; Grattan, D.; Anderson, G.M. Leptin Indirectly Regulates Gonadotropin-Releasing Hormone Neuronal Function. Endocrinology 2009, 150, 2805–2812. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Burguera, B.G.; Couce, M.E.; Scheithauer, B.W.; Lamsan, J.; Eberhardt, N.L.; Kulig, E.; Lloyd, R.V. Leptin and Leptin Receptor Expression in Normal and Neoplastic Human Pituitary: Evidence of a Regulatory Role for Leptin on Pituitary Cell Proliferation. J. Clin. Endocrinol. Metab. 1999, 84, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Pompolo, S.; Considine, R.V.; Clarke, I.J. Localization of Leptin Receptor-Like Immunoreactivity in the Corticotropes, Somatotropes, and Gonadotropes in the Ovine Anterior Pituitary. Endocrinology 2000, 141, 1515–1520. [Google Scholar] [CrossRef] [PubMed]
- Akhter, N.; CarlLee, T.; Syed, M.M.; Odle, A.K.; Cozart, M.A.; Haney, A.C.; Allensworth-James, M.L.; Beneš, H.; Childs, G.V. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology 2014, 155, 4027–4042. [Google Scholar] [CrossRef]
- Yu, W.H.; Walczewska, A.; Karanth, S.; McCann, S.M. Nitric oxide mediates leptin-induced luteinizing hormone-releasing hormone (LHRH) and LHRH and leptin-induced LH release from the pituitary gland. Endocrinology 1997, 138, 5055–5058. [Google Scholar] [CrossRef]
- Yu, W.H.; Kimura, M.; Walczewska, A.; Karanth, S.; McCann, S.M. Role of leptin in hypothalamic-pituitary function. Proc. Natl. Acad. Sci. USA 1997, 94, 1023–1028. [Google Scholar] [CrossRef] [Green Version]
- Ogura, K.; Irahara, M.; Kiyokawa, M.; Tezuka, M.; Matsuzaki, T.; Yasui, T.; Kamada, M.; Aono, T. Effects of leptin on secretion of LH and FSH from primary cultured female rat pituitary cells. Eur. J. Endocrinol. 2001, 144, 653–658. [Google Scholar] [CrossRef] [Green Version]
- De Biasi, S.N.; Apfelbaum, L.I.; Apfelbaum, M.E. In vitro effect of leptin on LH release by anterior pituitary glands from female rats at the time of spontaneous and steroid-induced LH surge. Eur. J. Endocrinol. 2001, 145, 659–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezuka, M.; Irahara, M.; Ogura, K.; Kiyokawa, M.; Tamura, T.; Matsuzaki, T.; Yasui, T.; Aono, T. Effects of leptin on gonadotropin secretion in juvenile female rat pituitary cells. Eur. J. Endocrinol. 2002, 146, 261–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peyon, P.; Zanuy, S.; Carrillo, M. Action of Leptin on In Vitro Luteinizing Hormone Release in the European Sea Bass (Dicentrarchus labrax). Biol. Reprod. 2001, 65, 1573–1578. [Google Scholar] [CrossRef] [Green Version]
- Weil, C.; Le Bail, P.; Sabin, N.; Le Gac, F. In vitro action of leptin on FSH and LH production in rainbow trout (Onchorynchus mykiss) at different stages of the sexual cycle. Gen. Comp. Endocrinol. 2003, 130, 2–12. [Google Scholar] [CrossRef]
- Kurokawa, T.; Uji, S.; Suzuki, T. Identification of cDNA coding for a homologue to mammalian leptin from pufferfish, Takifugu rubripes. Peptides 2005, 26, 745–750. [Google Scholar] [CrossRef]
- Shpilman, M.; Hollander-Cohen, L.; Ventura, T.; Gertler, A.; Levavi-Sivan, B. Production, gene structure and characterization of two orthologs of leptin and a leptin receptor in tilapia. Gen. Comp. Endocrinol. 2014, 207, 74–85. [Google Scholar] [CrossRef]
- Ohga, H.; Ito, K.; Matsumori, K.; Kimura, R.; Ohta, K.; Matsuyama, M. Leptin stimulates gonadotropin release and ovarian development in marine teleost chub mackerel. Gen. Comp. Endocrinol. 2020, 292, 113442. [Google Scholar] [CrossRef] [PubMed]
- Engler, C.; Kandzia, R.; Marillonnet, S. A One Pot, One Step, Precision Cloning Method with High Throughput Capability. PLoS ONE 2008, 3, e3647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fujita, R.; Hino, M.; Ebihara, T.; Nagasato, T.; Masuda, A.; Lee, J.M.; Fujii, T.; Mon, H.; Kakino, K.; Nagai, R.; et al. Efficient production of recombinant SARS-CoV-2 spike protein using the baculovirus-silkworm system. Biochem. Biophys. Res. Commun. 2020, 529, 257–262. [Google Scholar] [CrossRef]
- Ono, C.; Nakatsukasa, T.; Nishijima, Y. Construction of the BmNPV T3 bacmid system and its application to the functional analysis of BmNPV he65. J. Insect Biotechnol. Sericol. 2007, 76, 161–163. [Google Scholar]
- Yano, T.; Lee, J.M.; Mon, H.; Xu, J.; Morifuji, Y.; Masuda, A.; Hino, M.; Morokuma, D.; Fujita, R.; Takahashi, M.; et al. Expression of the thermostable Moloney murine leukemia virus reverse transcriptase by silkworm-baculovirus expression system. J. Asia-Pac. Entomol. 2019, 22, 453–457. [Google Scholar] [CrossRef]
- Lumayno, S.D.P.; Ohga, H.; Selvaraj, S.; Nyuji, M.; Yamaguchi, A.; Matsuyama, M. Molecular characterization and functional analysis of pituitary GnRH receptor in a commercial scombroid fish, chub mackerel (Scomber japonicus). Gen. Comp. Endocrinol. 2017, 247, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Ohga, H.; Matsuyama, M. In vitro action of leptin on gonadotropin secretion in pre-pubertal male chub mackerel. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2021, 253, 110856. [Google Scholar] [CrossRef]
- Prat, F.; Sumpter, J.P.; Tyler, C.R.; Graham, C.H.; Watson, J.D.; Blumenfeld, A.J.; Pang, S.C. Validation of Radioimmunoassays for Two Salmon Gonadotropins (GTH I and GTH II) and Their Plasma Concentrations Throughout the Reproductive Cycle in Male and Female Rainbow Trout (Oncorhynchus Mykiss). Biol. Reprod. 1996, 54, 1375–1382. [Google Scholar] [CrossRef] [Green Version]
- Gomez, J.M.; Weil, C.; Ollitrault, M.; Le Bail, P.-Y.; Breton, B.; Le Gac, F. Growth Hormone (GH) and Gonadotropin Subunit Gene Expression and Pituitary and Plasma Changes during Spermatogenesis and Oogenesis in Rainbow Trout (Oncorhynchus mykiss). Gen. Comp. Endocrinol. 1999, 113, 413–428. [Google Scholar] [CrossRef]
- Campbell, B.; Dickey, J.; Swanson, P. Endocrine Changes during Onset of Puberty in Male Spring Chinook Salmon, Oncorhynchus tshawytscha. Biol. Reprod. 2003, 69, 2109–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyuji, M.; Kodama, R.; Kato, K.; Yamamoto, S.; Yamaguchi, A.; Matsuyama, M. Gonadal Development and Gonadotropin Gene Expression during Puberty in Cultured Chub Mackerel (Scomber japonicus). Zool. Sci. 2014, 31, 398–406. [Google Scholar] [CrossRef]
- Fernald, R.D.; White, R.B. Gonadotropin-Releasing Hormone Genes: Phylogeny, Structure, and Functions. Front. Neuroendocrinol. 1999, 20, 224–240. [Google Scholar] [CrossRef] [Green Version]
- Selvaraj, S.; Kitano, H.; Fujinaga, Y.; Amano, M.; Takahashi, A.; Shimizu, A.; Yoneda, M.; Yamaguchi, A.; Matsuyama, M. Immunological Characterization and Distribution of Three GnRH Forms in the Brain and Pituitary Gland of Chub Mackerel (Scomber japonicus). Zool. Sci. 2009, 26, 828–839. [Google Scholar] [CrossRef]
- Ohga, H.; Adachi, H.; Kitano, H.; Yamaguchi, A.; Matsuyama, M. Kiss1 hexadecapeptide directly regulates gonadotropin-releasing hormone 1 in the scombroid fish, chub mackerel. Biol. Reprod. 2017, 96, 376–388. [Google Scholar] [CrossRef]
- Okuzawa, K.; Kazeto, Y.; Uji, S.; Yamaguchi, T.; Tanaka, H.; Nyuji, M.; Gen, K. Development of a homologous radioimmunoassay for red seabream follicle stimulating hormone and regulation of gonadotropins by GnRH in red seabream, Pagrus major. Gen. Comp. Endocrinol. 2016, 239, 4–12. [Google Scholar] [CrossRef]
- Takahashi, A.; Kanda, S.; Abe, T.; Oka, Y. Evolution of the Hypothalamic-Pituitary-Gonadal Axis Regulation in Vertebrates Revealed by Knockout Medaka. Endocrinology 2016, 157, 3994–4002. [Google Scholar] [CrossRef] [PubMed]
- Ohga, H.; Kaneko, K.; Shimizu, A.; Kitano, H.; Selvaraj, S.; Nyuji, M.; Adachi, H.; Yamaguchi, A.; Matsuyama, M. Steroidogenic and maturation-inducing potency of native gonadotropic hormones in female chub mackerel, Scomber japonicus. Reprod. Biol. Endocrinol. 2012, 10, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nyuji, M.; Kitano, H.; Shimizu, A.; Lee, J.M.; Kusakabe, T.; Yamaguchi, A.; Matsuyama, M. Characterization, Localization, and Stage-Dependent Gene Expression of Gonadotropin Receptors in Chub Mackerel (Scomber japonicus) Ovarian Follicles. Biol. Reprod. 2013, 88, 148. [Google Scholar] [CrossRef]
- Chen, J.; Cao, M.; Zhang, A.; Shi, M.; Tao, B.; Li, Y.; Wang, Y.; Zhu, Z.; Trudeau, V.L.; Hu, W. Growth Hormone Overexpression Disrupts Reproductive Status through Actions on Leptin. Front. Endocrinol. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohga, H.; Matsuyama, M. Effects of LPXRFamide peptides on chub mackerel gonadotropin secretion. Biol. Reprod. 2021, 105, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Kitahashi, T.; Alok, D.; Ando, H.; Kaeriyama, M.; Zohar, Y.; Ueda, H.; Urano, A. GnRH Analog Stimulates Gonadotropin II Gene Expression in Maturing Sockeye Salmon. Zool. Sci. 1998, 15, 761–765. [Google Scholar] [CrossRef] [Green Version]
- Mateos, J.; Mañanos, E.; Carrillo, M.; Zanuy, S. Regulation of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) gene expression by gonadotropin-releasing hormone (GnRH) and sexual steroids in the Mediterranean Sea bass. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2002, 132, 75–86. [Google Scholar] [CrossRef]
- Nyuji, M.; Shiraishi, T.; Selvaraj, S.; Van In, V.; Kitano, H.; Yamaguchi, A.; Okamoto, K.; Onoue, S.; Shimizu, A.; Matsuyama, M. Immunoreactive changes in pituitary FSH and LH cells during seasonal reproductive and spawning cycles of female chub mackerel Scomber japonicus. Fish. Sci. 2011, 77, 731–739. [Google Scholar] [CrossRef]
- Escobar, S.; Rocha, A.; Felip, A.; Carrillo, M.; Zanuy, S.; Kah, O.; Servili, A. Leptin receptor gene in the European sea bass (Dicentrarchus labrax): Cloning, phylogeny, tissue distribution and neuroanatomical organization. Gen. Comp. Endocrinol. 2016, 229, 100–111. [Google Scholar] [CrossRef]
- Ohga, H.; Matsumori, K.; Kodama, R.; Kitano, H.; Nagano, N.; Yamaguchi, A.; Matsuyama, M. Two leptin genes and a leptin receptor gene of female chub mackerel (Scomber japonicus): Molecular cloning, tissue distribution and expression in different obesity indices and pubertal stages. Gen. Comp. Endocrinol. 2015, 222, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Ohga, H.; Hirata, D.; Matsumori, K.; Kitano, H.; Nagano, N.; Yamaguchi, A.; Matsuyama, M. Possible role of the leptin system in controlling puberty in the male chub mackerel, Scomber japonicus. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 159–166. [Google Scholar] [CrossRef]
- Kurokawa, T.; Murashita, K.; Suzuki, T.; Uji, S. Genomic characterization and tissue distribution of leptin receptor and leptin receptor overlapping transcript genes in the pufferfish, Takifugu rubripes. Gen. Comp. Endocrinol. 2008, 158, 108–114. [Google Scholar] [CrossRef] [PubMed]







| cDNA | Primer Sequence (5′–3′) | |
|---|---|---|
| fshb | Fw | TGTGAAGGACAGTGTTACCACAGGG |
| Rv | TCATAGGTCCAGTCACCGC | |
| lhb | Fw | GAAACAACCATCTGCAGCG |
| Rv | AAAAGTCCCGATACGYGCAC | |
| rpl8 | Fw | CCGCGCTTCAGGAAACTAC |
| Rv | TCAATACGACCACCTCCAGC | |
| cDNA | Primer Sequence (5′–3′) | |
|---|---|---|
| fshb | Fw | CAAAGCAACAAATCTCTACAGGCG |
| Rv | GCAACAAGGAAGGACAATGGG | |
| lhb | Fw | GACACCGGCTGAGATTCACTA |
| Rv | GTGTGACAACCTTTATTTAGCACAAC | |
| lepr | Fw | TGCAGACTATTGAGGCAGAAC |
| Rv | CAGACGGGATGGCACCTC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohga, H.; Ito, K.; Kakino, K.; Mon, H.; Kusakabe, T.; Lee, J.M.; Matsuyama, M. Leptin Is an Important Endocrine Player That Directly Activates Gonadotropic Cells in Teleost Fish, Chub Mackerel. Cells 2021, 10, 3505. https://doi.org/10.3390/cells10123505
Ohga H, Ito K, Kakino K, Mon H, Kusakabe T, Lee JM, Matsuyama M. Leptin Is an Important Endocrine Player That Directly Activates Gonadotropic Cells in Teleost Fish, Chub Mackerel. Cells. 2021; 10(12):3505. https://doi.org/10.3390/cells10123505
Chicago/Turabian StyleOhga, Hirofumi, Kosuke Ito, Kohei Kakino, Hiroaki Mon, Takahiro Kusakabe, Jae Man Lee, and Michiya Matsuyama. 2021. "Leptin Is an Important Endocrine Player That Directly Activates Gonadotropic Cells in Teleost Fish, Chub Mackerel" Cells 10, no. 12: 3505. https://doi.org/10.3390/cells10123505
APA StyleOhga, H., Ito, K., Kakino, K., Mon, H., Kusakabe, T., Lee, J. M., & Matsuyama, M. (2021). Leptin Is an Important Endocrine Player That Directly Activates Gonadotropic Cells in Teleost Fish, Chub Mackerel. Cells, 10(12), 3505. https://doi.org/10.3390/cells10123505