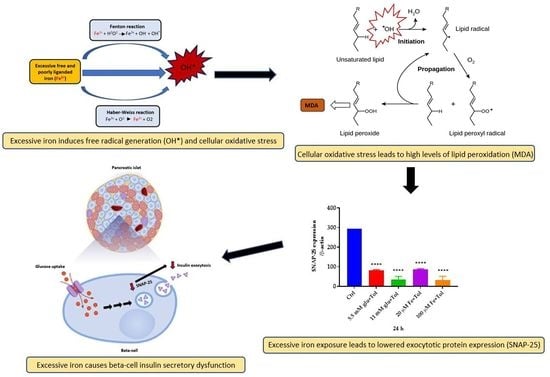
Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. MIN6 Cell Culture
2.3. MIN6 Cell Exposure to Glucose and Iron
2.4. Intracellular Total Iron Quantification
2.5. Intracellular Ferritin Quantification
2.6. Lipid Peroxidation Assessment
2.7. Measurement of Cellular Cytotoxicity
2.8. Estimation of Insulin Secretion and Content
2.9. SNAP-25 Protein Expression
2.10. Cell Culture with Transferrin Treatments
2.11. Statistical Analysis
3. Results
3.1. Assessment of Total Cellular Iron Content upon Exposure to Transferrin
3.2. Total Iron Content in the Absence of Transferrin
3.3. Cellular Ferritin Content
3.4. Estimation of Lipid Peroxidation
3.5. Assessment of Cellular Viability
3.6. Assessment of Insulin Content
3.7. Assessment of Insulin Secretion
3.8. Expression of SNAP-25–Mediated Insulin Exocytosis
4. Discussion
4.1. Exposure of MIN6 β-Cells to High Iron Levels Results in Significantly Elevated Cellular Iron Accumulation
4.2. Excess Iron in MIN6 β-Cells Results in ROS Generation, Lipid Peroxidation and Cytotoxic Effects
4.3. Excess Iron in MIN6 β-Cells Results in Dysfunctions in Insulin Secretory Machinery
4.4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Guariguata, L.; Whiting, D.R.; Hambleton, I.; Beagley, J.; Linnenkamp, U.; Shaw, J.E. Global Estimates of Diabetes Prevalence for 2013 and Projections for 2035. Diabetes Res. Clin. Pract. 2014, 103, 137–149. [Google Scholar] [CrossRef]
- Wild, S.; Roglic, G.; Green, A.; Sicree, R.; King, H. Global Prevalence of Diabetes: Estimates for the Year 2000 and Projections for 2030. Diabetes Care 2004, 27, 1047–1053. [Google Scholar] [CrossRef] [Green Version]
- Tripathy, D.; Chavez, A.O. Defects in Insulin Secretion and Action in the Pathogenesis of Type 2 Diabetes Mellitus. Curr. Diab. Rep. 2010, 10, 184–191. [Google Scholar] [CrossRef]
- Jewell, J.L.; Oh, E.; Thurmond, D.C. Exocytosis Mechanisms Underlying Insulin Release and Glucose Uptake: Conserved Roles for Munc18c and Syntaxin 4. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R517–R531. [Google Scholar] [CrossRef] [Green Version]
- Gerber, P.A.; Rutter, G.A. The Role of Oxidative Stress and Hypoxia in Pancreatic Beta-Cell Dysfunction in Diabetes Mellitus. Antioxid. Redox Signal. 2017, 26, 501–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lenzen, S.; Drinkgern, J.; Tiedge, M. Low Antioxidant Enzyme Gene Expression in Pancreatic Islets Compared with Various Other Mouse Tissues. Free Radic. Biol. Med. 1996, 20, 463–466. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed]
- McCord, J.M. The Evolution of Free Radicals and Oxidative Stress. Am. J. Med. 2000, 108, 652–659. [Google Scholar] [CrossRef]
- Ayala, A.; Muñoz, M.F.; Argüelles, S. Lipid Peroxidation: Production, Metabolism, and Signaling Mechanisms of Malondialdehyde and 4-Hydroxy-2-Nonenal. Oxid. Med. Cell. Longev. 2014, 2014, 360438. [Google Scholar] [CrossRef] [PubMed]
- Jezek, P.; Hlavatá, L. Mitochondria in Homeostasis of Reactive Oxygen Species in Cell, Tissues, and Organism. Int. J. Biochem. Cell Biol. 2005, 37, 2478–2503. [Google Scholar] [CrossRef]
- Ma, Z.A.; Zhao, Z.; Turk, J. Mitochondrial Dysfunction and β-Cell Failure in Type 2 Diabetes Mellitus. Exp. Diabetes Res. 2012, 2012, 703538. [Google Scholar] [CrossRef] [Green Version]
- Simcox, J.A.; McClain, D.A. Iron and Diabetes Risk. Cell Metab. 2013, 17, 329–341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jellinger, K.; Paulus, W.; Grundke-Iqbal, I.; Riederer, P.; Youdim, M.B. Brain Iron and Ferritin in Parkinson’s and Alzheimer’s Diseases. J. Neural Transm. Park. Dis. Dement. Sect. 1990, 2, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Andrews, N.C. Disorders of Iron Metabolism. N. Engl. J. Med. 1999, 341, 1986–1995. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J. Iron Nutrition in the UK: Getting the Balance Right. Proc. Nutr. Soc. 2004, 63, 519–528. [Google Scholar] [CrossRef] [Green Version]
- Hansen, J.B.; Moen, I.W.; Mandrup-Poulsen, T. Iron: The Hard Player in Diabetes Pathophysiology. Acta Physiol. 2014, 210, 717–732. [Google Scholar] [CrossRef] [PubMed]
- Lenzen, S. Oxidative Stress: The Vulnerable Beta-Cell. Biochem. Soc. Trans. 2008, 36, 343–347. [Google Scholar] [CrossRef]
- Jiang, R.; Manson, J.E.; Meigs, J.B.; Ma, J.; Rifai, N.; Hu, F.B. Body Iron Stores in Relation to Risk of Type 2 Diabetes in Apparently Healthy Women. JAMA 2004, 291, 711–717. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.; Shepherd, R.M.; Gill, D.; Gorman, T.; Smith, D.M.; Dunne, M.J. Glucose-Dependent Modulation of Insulin Secretion and Intracellular Calcium Ions by GKA50, a Glucokinase Activator. Diabetes 2007, 56, 1694–1702. [Google Scholar] [CrossRef]
- Riemer, J.; Hoepken, H.H.; Czerwinska, H.; Robinson, S.R.; Dringen, R. Colorimetric Ferrozine-Based Assay for the Quantitation of Iron in Cultured Cells. Anal. Biochem. 2004, 331, 370–375. [Google Scholar] [CrossRef]
- Kautzky-Willer, A.; Harreiter, J.; Pacini, G. Sex and Gender Differences in Risk, Pathophysiology and Complications of Type 2 Diabetes Mellitus. Endocr. Rev. 2016, 37, 278–316. [Google Scholar] [CrossRef] [Green Version]
- Paul, B.T.; Manz, D.H.; Torti, F.M.; Torti, S.V. Mitochondria and Iron: Current Questions. Expert Rev. Hematol. 2017, 10, 65–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evan, G.I.; Vousden, K.H. Proliferation, Cell Cycle and Apoptosis in Cancer. Nature 2001, 411, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Ford, E.S.; Cogswell, M.E. Diabetes and Serum Ferritin Concentration among U.S. Adults. Diabetes Care 1999, 22, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Aregbesola, A.; Voutilainen, S.; Virtanen, J.K.; Mursu, J.; Tuomainen, T.-P. Body Iron Stores and the Risk of Type 2 Diabetes in Middle-Aged Men. Eur. J. Endocrinol. 2013, 169, 247–253. [Google Scholar] [CrossRef] [Green Version]
- Juckett, M.B.; Balla, J.; Balla, G.; Jessurun, J.; Jacob, H.S.; Vercellotti, G.M. Ferritin Protects Endothelial Cells from Oxidized Low Density Lipoprotein in Vitro. Am. J. Pathol. 1995, 147, 782–789. [Google Scholar] [PubMed]
- Halliwell, B. The Role of Oxygen Radicals in Human Disease, with Particular Reference to the Vascular System. Haemostasis 1993, 23 (Suppl. 1), 118–126. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.-L.; Cheung, T.T.; Lam, K.S.L.; Cheung, B.M.Y. High Ferritin and Low Transferrin Saturation Are Associated with Pre-Diabetes among a National Representative Sample of U.S. Adults. Clin. Nutr. 2013, 32, 1055–1060. [Google Scholar] [CrossRef]
- Esterbauer, H.; Schaur, R.J.; Zollner, H. Chemistry and Biochemistry of 4-Hydroxynonenal, Malonaldehyde and Related Aldehydes. Free Radic. Biol. Med. 1991, 11, 81–128. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Burdon, R.H. Free Radical Damage and Its Control; Elsevier: Amsterdam, The Netherlands, 1994; ISBN 9780080860886. [Google Scholar]
- Newsholme, P.; Krause, M. Nutritional Regulation of Insulin Secretion: Implications for Diabetes. Clin. Biochem. Rev. 2012, 33, 35–47. [Google Scholar]
- Cooksey, R.C.; Jouihan, H.A.; Ajioka, R.S.; Hazel, M.W.; Jones, D.L.; Kushner, J.P.; McClain, D.A. Oxidative Stress, Beta-Cell Apoptosis, and Decreased Insulin Secretory Capacity in Mouse Models of Hemochromatosis. Endocrinology 2004, 145, 5305–5312. [Google Scholar] [CrossRef]
- Kasuga, M. Insulin Resistance and Pancreatic Beta Cell Failure. J. Clin. Investig. 2006, 116, 1756–1760. [Google Scholar] [CrossRef]
- Shaaban, M.A.; Dawod, A.E.A.; Nasr, M.A. Role of Iron in Diabetes Mellitus and Its Complications. Menoufia Med J. 2016, 29, 11. [Google Scholar]
- Ježek, P.; Jabůrek, M.; Plecitá-Hlavatá, L. Contribution of Oxidative Stress and Impaired Biogenesis of Pancreatic β-Cells to Type 2 Diabetes. Antioxid. Redox Signal. 2019, 31, 722–751. [Google Scholar] [CrossRef] [Green Version]
- Bao, Y.-M.; Sun, S.-J.; Li, M.; Li, L.; Cao, W.-L.; Luo, J.; Tang, H.-J.; Huang, J.; Wang, Z.-F.; Wang, J.-F.; et al. Overexpression of the Qc-SNARE Gene OsSYP71 Enhances Tolerance to Oxidative Stress and Resistance to Rice Blast in Rice (Oryza Sativa L.). Gene 2012, 504, 238–244. [Google Scholar] [CrossRef] [PubMed]
- Munhoz, A.C.; Riva, P.; Simões, D.; Curi, R.; Carpinelli, A.R. Control of Insulin Secretion by Production of Reactive Oxygen Species: Study Performed in Pancreatic Islets from Fed and 48-Hour Fasted Wistar Rats. PLoS ONE 2016, 11, e0158166. [Google Scholar] [CrossRef]
- Bock, L.V.; Hutchings, B.; Grubmüller, H.; Woodbury, D.J. Chemomechanical Regulation of SNARE Proteins Studied with Molecular Dynamics Simulations. Biophys. J. 2010, 99, 1221–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daraio, T.; Bombek, L.K.; Gosak, M.; Valladolid-Acebes, I.; Klemen, M.S.; Refai, E.; Berggren, P.-O.; Brismar, K.; Rupnik, M.S.; Bark, C. SNAP-25b-Deficiency Increases Insulin Secretion and Changes Spatiotemporal Profile of Ca2 Oscillations in β Cell Networks. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslamy, A.; Thurmond, D.C. Exocytosis Proteins as Novel Targets for Diabetes Prevention And/or Remediation? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2017, 312, R739–R752. [Google Scholar] [CrossRef] [Green Version]
- Hou, J.C.; Min, L.; Pessin, J.E. Insulin Granule Biogenesis, Trafficking and Exocytosis. Vitam. Horm. 2009, 80, 473–506. [Google Scholar] [PubMed] [Green Version]











| Name of Condition | Treatment | Glucose Concentrations (mM) | Iron Concentrations (µM) |
|---|---|---|---|
| Ctrl | Basal Glu | 1.1 | 0 |
| C1 | Glu | 5.5 | 0 |
| C2 | Glu | 11 | 0 |
| C3 | Fe | 0 | 20 |
| C4 | Fe | 0 | 100 |
| C5 | Glu + Fe | 5.5 | 20 |
| C6 | Glu + Fe | 5.5 | 100 |
| C7 | Glu + Fe | 11 | 20 |
| C8 | Glu + Fe | 11 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blesia, V.; Patel, V.B.; Al-Obaidi, H.; Renshaw, D.; Zariwala, M.G. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells 2021, 10, 1141. https://doi.org/10.3390/cells10051141
Blesia V, Patel VB, Al-Obaidi H, Renshaw D, Zariwala MG. Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells. 2021; 10(5):1141. https://doi.org/10.3390/cells10051141
Chicago/Turabian StyleBlesia, Voni, Vinood B. Patel, Hisham Al-Obaidi, Derek Renshaw, and Mohammed Gulrez Zariwala. 2021. "Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells" Cells 10, no. 5: 1141. https://doi.org/10.3390/cells10051141
APA StyleBlesia, V., Patel, V. B., Al-Obaidi, H., Renshaw, D., & Zariwala, M. G. (2021). Excessive Iron Induces Oxidative Stress Promoting Cellular Perturbations and Insulin Secretory Dysfunction in MIN6 Beta Cells. Cells, 10(5), 1141. https://doi.org/10.3390/cells10051141