Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats
Abstract
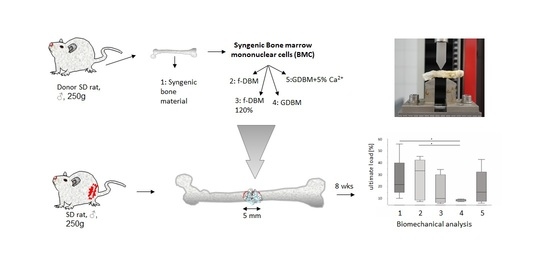
:1. Introduction
2. Materials and Methods
2.1. Ethics and Animal Care
2.2. Preparation of Fibrous Scaffold
2.3. Scaffolds
2.4. BMC Isolation and Seeding
2.5. Surgical Procedure
2.6. Biomechanical Characterisation
2.7. Histological Assessment
2.8. µCT-Analysis
2.9. Statistics
3. Results
3.1. Animal Care/Complications
3.2. Similar Biomechanical Properties of Bone Defects Treated with f-DBM and Syngenic Cancellous Bone
3.3. Similar Bone and Cartilage Formation in All Groups but Significantly Bigger Remnant Defect Size in GDBM
3.4. High Bone Healing Scores in f-DBM and Syngenic Cancellous Bone Groups
3.5. Bone Mineral Density Significantly Higher in SCB Group Than Fiber Groups
3.6. Significantly Better Vascularization in GDBM Group Than GDBM with Calcium Group
4. Discussion
4.1. DBM in Bone Defect Treatment
4.2. Tighter Packing and Higher Concentration of Residual Calcium Phosphate Does Not Improve Bone Healing
4.3. Use of BMCs with f-DBM Leads to Gold-Standard-Like Bone Healing Results
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dawson, J.I.; Oreffo, R.O. Bridging the regeneration gap: Stem cells, biomaterials and clinical translation in bone tissue engineering. Arch. Biochem. Biophys. 2008, 473, 124–131. [Google Scholar] [CrossRef]
- Kneser, U.; Schaefer, D.J.; Polykandriotis, E.; Horch, R.E. Tissue engineering of bone: The reconstructive surgeon’s point of view. J. Cell. Mol. Med. 2006, 10, 7–19. [Google Scholar] [CrossRef] [Green Version]
- Mobini, S.; Hoyer, B.; Solati-Hashjin, M.; Lode, A.; Nosoudi, N.; Samadikuchaksaraei, A.; Gelinsky, M. Fabrication and characterization of regenerated silk scaffolds reinforced with natural silk fibers for bone tissue engineering. J. Biomed. Mater. Res. Part. A 2013, 101, 2392–2404. [Google Scholar] [CrossRef] [PubMed]
- Jordana, F.; Le Visage, C.; Weiss, P. Bone substitutes. Med. Sci. 2017, 33, 60–65. (In French) [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Seebach, C.; Henrich, D.; Kähling, C.; Wilhelm, K.; Tami, A.E.; Alini, M.; Marzi, I. Endothelial progenitor cells and mesenchymal stem cells seeded onto β-TCP granules enhance early vascularization and bone healing in a critical-sized bone defect in rats. Tissue Eng. Part. A 2010, 16, 1961–1970. [Google Scholar] [CrossRef]
- Seebach, C.; Henrich, D.; Wilhelm, K.; Barker, J.H.; Marzi, I. Endothelial progenitor cells improve directly and indirectly early vascularization of mesenchymal stem cell-driven bone regeneration in a critical bone defect in Rats. Cell Transplant. 2012, 21, 1667–1677. [Google Scholar] [CrossRef] [Green Version]
- Henrich, D.; Seebach, C.; Kaehling, C.; Scherzed, A.; Wilhelm, K.; Tewksbury, R.; Powerski, M.; Marzi, I. Simultaneous cultivation of human endothelial–like differentiated precursor cells and human marrow stromal cells on β-tricalcium phosphate. Tissue Eng. Part. C Methods 2009, 15, 551–560. [Google Scholar] [CrossRef]
- Eldesoqi, K.; Henrich, D.; El-Kady, A.M.; Sweify, K.M.; Relja, B.; Abd El-Hady, B. Improved bone formation by differentiated mesenchymal stem cells and endothelial progenitor cells seeded on high concentrated bioglass-polylactic acid composite in calvarial rat bone defect. J. Stem Cells Res. Dev. Ther. 2015, 2, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Han, Z.-B.; Song, Y.-P.; Han, Z.C. Safety of mesenchymal stem cells for clinical application. Stem Cells Int. 2012, 2012, 652034. [Google Scholar] [CrossRef] [Green Version]
- Henrich, D.; Verboket, R.; Schaible, A.; Kontradowitz, K.; Oppermann, E.; Brune, J.C.; Nau, C.; Meier, S.; Bonig, H.; Marzi, I.; et al. Characterization of bone marrow mononuclear cells on biomaterials for bone tissue engineering in vitro. BioMed Res. Int. 2015, 2015, 762407. [Google Scholar] [CrossRef] [Green Version]
- Seebach, C.; Henrich, D.; Meier, S.; Nau, C.; Bonig, H.; Marzi, I. Safety and feasibility of cell-based therapy of autologous bone marrow-derived mononuclear cells in plate-stabilized proximal humeral fractures in humans. J. Transl. Med. 2016, 14, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Verboket, R.; Leiblein, M.; Seebach, C.; Nau, C.; Janko, M.; Bellen, M.; Bönig, H.; Henrich, D.; Marzi, I. Autologous cell-based therapy for treatment of large bone defects: From bench to bedside. Eur. J. Trauma Emerg. Surg. 2018, 44, 649–665. [Google Scholar] [CrossRef] [Green Version]
- Assmus, B.; Rolf, A.; Erbs, S.; Elsasser, A.; Haberbosch, W.; Hambrecht, R.; Tillmanns, H.; Yu, J.; Corti, R.; Mathey, D.G.; et al. clinical outcome 2 years after intracoronary administration of bone marrow-derived progenitor cells in acute myocardial infarction. Circ. Heart Fail. 2010, 3, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Henrich, D.; Seebach, C.; Sterlepper, E.; Tauchmann, C.; Marzi, I.; Frank, J. RIA reamings and hip aspirate: A comparative evaluation of osteoprogenitor and endothelial progenitor cells. Injury 2010, 41, S62–S68. [Google Scholar] [CrossRef]
- Kuci, Z.; Kuçi, S.; Zircher, S.; Koller, S.; Schubert, R.; Bönig, H.; Henschler, R.; Lieberz, R.; Klingebiel, T.; Bader, P. Mesenchymal stromal cells derived from CD271+ bone marrow mononuclear cells exert potent allosuppressive properties. Cytotherapy 2011, 13, 1193–1204. [Google Scholar] [CrossRef]
- Jager, M.; Jelinek, E.; Wess, K.; Scharfstadt, A.; Jacobson, M.; Kevy, S.; Krauspe, R. Bone marrow concentrate: A novel strategy for bone defect treatment. Curr. Stem Cell Res. Ther. 2009, 4, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seebach, C.; Henrich, D.; Schaible, A.; Relja, B.; Jugold, M.; Bönig, H.; Marzi, I. Cell-based therapy by implanted human bone marrow-derived mononuclear cells improved bone healing of large bone defects in rats. Tissue Eng. Part. A 2015, 21, 1565–1578. [Google Scholar] [CrossRef]
- Janko, M.; Sahm, J.; Schaible, A.; Brune, J.C.; Bellen, M.; Schroder, K.; Seebach, C.; Marzi, I.; Henrich, D. Comparison of three different types of scaffolds preseeded with human bone marrow mononuclear cells on the bone healing in a femoral critical size defect model of the athymic rat. J. Tissue Eng. Regen. Med. 2018, 12, 653–666. [Google Scholar] [CrossRef] [PubMed]
- Janko, M.; Pöllinger, S.; Schaible, A.; Bellen, M.; Schröder, K.; Heilani, M.; Fremdling, C.; Marzi, I.; Nau, C.; Henrich, D.; et al. Determination of the effective dose of bone marrow mononuclear cell therapy for bone healing in vivo. Eur. J. Trauma Emerg. Surg. 2020, 46, 265–276. [Google Scholar] [CrossRef] [Green Version]
- Söhling, N.; Leiblein, M.; Schaible, A.; Janko, M.; Schwäble, J.; Seidl, C.; Brune, J.; Nau, C.; Marzi, I.; Henrich, D.; et al. First human leucocyte antigen (HLA) response and safety evaluation of fibrous demineralized bone matrix in a critical size femoral defect model of the Sprague-Dawley rat. Materials 2020, 13, 3120. [Google Scholar] [CrossRef] [PubMed]
- Udeabor, S.E.; Herrera-Vizcaíno, C.; Sader, R.; Kirkpatrick, C.J.; Al-Maawi, S.; Ghanaati, S. Characterization of the cellular reaction to a collagen-based matrix: An in vivo histological and histomorphometrical analysis. Materials 2020, 13, 2730. [Google Scholar] [CrossRef] [PubMed]
- Leiblein, M.; Winkenbach, A.; Koch, E.; Schaible, A.; Büchner, H.; Marzi, I.; Henrich, D.; Nau, C. Impact of scaffold granule size use in Masquelet technique on periosteal reaction: A study in rat femur critical size bone defect model. Eur. J. Trauma Emerg. Surg. 2020, 1–9. [Google Scholar] [CrossRef]
- Hogrebe, N.; Reinhardt, J.W.; Gooch, K.J. Biomaterial microarchitecture: A potent regulator of individual cell behavior and multicellular organization. J. Biomed. Mater. Res. Part. A 2017, 105, 640–661. [Google Scholar] [CrossRef]
- Barradas, A.M.; Fernandes, H.A.; Groena, N.; ChinChaibc, Y.; Schrootencd, J.; van de Peppel, J.; van Leeuwen, J.P.; van Blitterswijk, C.A.; de Boer, J. A calcium-induced signaling cascade leading to osteogenic differentiation of human bone marrow-derived mesenchymal stromal cells. Biomaterials 2012, 33, 3205–3215. [Google Scholar] [CrossRef]
- Chai, Y.; Carlier, A.; Bolander, J.; Roberts, S.; Geris, L.; Schrooten, J.; Van Oosterwyck, H.; Luyten, F. Current views on calcium phosphate osteogenicity and the translation into effective bone regeneration strategies. Acta Biomater. 2012, 8, 3876–3887. [Google Scholar] [CrossRef] [PubMed]
- Nakade, O.; Takahashi, K.; Takuma, T.; Aoki, T.; Kaku, T. Effect of extracellular calcium on the gene expression of bone morphogenetic protein-2 and -4 of normal human bone cells. J. Bone Miner. Metab. 2001, 19, 13–19. [Google Scholar] [CrossRef]
- Eldesoqi, K.; Seebach, C.; Ngoc, C.N.; Meier, S.; Nau, C.; Schaible, A.; Marzi, I.; Henrich, D. High calcium bioglass enhances differentiation and survival of endothelial progenitor cells, inducing early vascularization in critical size bone defects. PLoS ONE 2013, 8, e79058. [Google Scholar] [CrossRef] [PubMed]
- Eldesoqi, K.; Henrich, D.; El-Kady, A.M.; Arbid, M.S.; El-Hady, B.M.A.; Marzi, I.; Seebach, C. Safety evaluation of a bioglass-polylactic acid composite scaffold seeded with progenitor cells in a rat skull critical-size bone defect. PLoS ONE 2014, 9, e87642. [Google Scholar] [CrossRef]
- Wildemann, B.; Kadow-Romacker, A.; Haas, N.P.; Schmidmaier, G. Quantification of various growth factors in different demineralized bone matrix preparations. J. Biomed. Mater. Res. Part. A 2007, 81, 437–442. [Google Scholar] [CrossRef]
- Pruss, A.; Göbel, U.B.; Pauli, G.; Kao, M.; Seibold, M.; Mönig, H.-J.; Hansen, A.; Von Versen, R. Peracetic acid-ethanol treatment of allogeneic avital bone tissue transplants—A reliable sterilization method. Ann. Transplant. 2003, 8, 34–42. [Google Scholar]
- Drosse, I.; Volkmer, E.; Seitz, S.; Seitz, H.; Penzkofer, R.; Zahn, K.; Matis, U.; Mutschler, W.; Augat, P.; Schieker, M. Validation of a femoral critical size defect model for orthotopic evaluation of bone healing: A biomechanical, veterinary and trauma surgical perspective. Tissue Eng. Part. C Methods 2008, 14, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Garvey, W.; Fathi, A.; Bigelow, F.; Carpenter, B.; Jimenez, C. Improved Movat pentachrome stain. Stain. Technol. 1986, 61, 60–62. [Google Scholar] [CrossRef]
- Limandjaja, G.; Belien, J.; Scheper, R.; Niessen, F.; Gibbs, S. Hypertrophic and keloid scars fail to progress from the CD 34−/α-smooth muscle actin (α-SMA) + immature scar phenotype and show gradient differences in α- SMA and p16 expression. Br. J. Dermatol. 2019, 182, 974–986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Z.; Bhavsar, M.; Leppik, L.; Oliveira, K.M.; Barker, J.H. Histological scoring method to assess bone healing in critical size bone defect models. Tissue Eng. Part. C Methods 2018, 24, 272–279. [Google Scholar] [CrossRef] [PubMed]
- Decambron, A.; Manassero, M.; Bensidhoum, M.; Lecuelle, B.; Logeart-Avramoglou, D.; Petite, H.; Viateau, V. A comparative study of tissue-engineered constructs from Acropora and Porites coral in a large animal bone defect model. Bone Jt. Res. 2017, 6, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Van der Stok, J.; Hartholt, K.A.; Schoenmakers, D.A.L.; Arts, J.J.C. The available evidence on demineralised bone matrix in trauma and orthopaedic surgery: A systematic review. Bone Jt. Res. 2017, 6, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Powers, R.M., Jr.; Wolfinbarger, L., Jr. Effect(s) of the demineralization process on the osteoinductivity of demineralized bone matrix. J. Periodontol. 1997, 68, 1085–1092. [Google Scholar] [CrossRef]
- Huber, E.; Pobloth, A.-M.; Bormann, N.; Kolarczik, N.; Schmidt-Bleek, K.; Schell, H.; Schwabe, P.; Duda, G.N.; Wildemann, B. Demineralized bone matrix as a carrier for bone morphogenetic protein-2: Burst release combined with long-term binding and osteoinductive activity evaluated in vitro and in vivo. Tissue Eng. Part. A 2017, 23, 1321–1330. [Google Scholar] [CrossRef]
- Loi, F.; Córdova, L.A.; Pajarinen, J.; Lin, T.-H.; Yao, Z.; Goodman, S.B. Inflammation, fracture and bone repair. Bone 2016, 86, 119–130. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Li, J.; Zhao, Q.; Zhou, Z.; Yuan, X.; Yang, D.; Chen, X. Dynamic compression promotes proliferation and neovascular networks of endothelial progenitor cells in demineralized bone matrix scaffold seed. J. Appl. Physiol. 2012, 113, 619–626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stumbras, A.; Kuliesius, P.; Januzis, G.; Juodzbalys, G. Alveolar ridge preservation after tooth extraction using different bone graft materials and autologous platelet concentrates: A systematic review. J. Oral Maxillofac. Res. 2019, 10, e2. [Google Scholar] [CrossRef] [PubMed]
- Baumann, F.; Krutsch, W.; Pfeifer, C.; Neumann, C.; Nerlich, M.; Loibl, M. Posterolateral fusion in acute traumatic thoracolumbar fractures: A comparison of demineralized bone matrix and autologous bone graft. Acta Chir. Orthop. Traumatol. Cechoslov. 2015, 82, 119–125. [Google Scholar]
- Frenkel, S.R.; Moskovich, R.; Spivak, J.; Zhang, Z.-H.; Prewett, A.B. Demineralized bone matrix. Enhancement of spinal fusion. Spine 1993, 18, 1634–1639. [Google Scholar] [CrossRef] [PubMed]
- Urist, M.R.; Dawson, E. Intertransverse process fusion with the aid of chemosterilized autolyzed antigen-extracted allogeneic (AAA) bone. Clin. Orthop. Relat. Res. 1981, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Aghdasi, B.; Montgomery, S.; Daubs, M.; Wang, J. A review of demineralized bone matrices for spinal fusion: The evidence for efficacy. Surgeon 2013, 11, 39–48. [Google Scholar] [CrossRef]
- Stancoven, B.W.; Lee, J.; Dixon, D.R.; McPherson, J.; Bisch, F.C.; Wikesjö, U.M.E.; Susin, C. Effect of bone morphogenetic protein-2, demineralized bone matrix and systemic parathyroid hormone (1-34) on local bone formation in a rat calvaria critical-size defect model. J. Periodontal Res. 2013, 48, 243–251. [Google Scholar] [CrossRef]
- Verboket, R.D.; Leiblein, M.; Janko, M.; Schaible, A.; Brune, J.C.; Schröder, K.; Heilani, M.; Fremdling, C.; Busche, Y.; Irrle, T.; et al. From two stages to one: Acceleration of the induced membrane (Masquelet) technique using human acellular dermis for the treatment of non-infectious large bone defects. Eur. J. Trauma Emerg. Surg. 2020, 46, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Carlier, A.; Geris, L.; van Gastel, N.; Carmeliet, G.; Van Oosterwyck, H. Oxygen as a critical determinant of bone fracture healing—A multiscale model. J. Theor. Biol. 2015, 365, 247–264. [Google Scholar] [CrossRef] [Green Version]
- Probe, R. Bone healing of tibial lengthening is enhanced by hyperbaric oxygen therapy: A study of bone mineral density and torsional strength on rabbits—Comment. J. Trauma 1998, 44, 681. [Google Scholar] [CrossRef]
- Malhotra, A.; Habibovic, P. Calcium phosphates and angiogenesis: Implications and advances for bone regeneration. Trends Biotechnol. 2016, 34, 983–992. [Google Scholar] [CrossRef] [Green Version]
- Henrich, D.; Seebach, C.; Verboket, R.; Schaible, A.; Marz, I.; Bonig, H. The osteo-inductive activity of bone-marrow-derived mononuclear cells resides within the CD14+ population and is independent of the CD34+ population. Eur. Cells Mater. 2018, 35, 165–177. [Google Scholar] [CrossRef]
- Asahara, T.; Murohara, T.; Sullivan, A.; Silver, M.; Van Der Zee, R.; Li, T.; Witzenbichler, B.; Schatteman, G.; Isner, J.M. Isolation of putative progenitor endothelial cells for angiogenesis. Science 1997, 275, 964–966. [Google Scholar] [CrossRef]







| Material | Histology | Radiology/Biomechanical Testing |
|---|---|---|
| syngenic cancellous bone (SCB) | n = 5 | n = 8 |
| fibrous demineralized bone matrix (f-DBM) | n = 5 | n = 8 |
| fibrous demineralized bone matrix densely packed (f-DBM 120%) | n = 5 | n = 8 |
| DBM granules (GDBM) | n = 5 | n = 8 |
| DBM granules 5% calcium phosphate (GDBM5%Ca2+) | n = 5 | n = 8 |
| Material. | Bone Healing Score |
|---|---|
| syngenic cancellous bone (SCB) | 25 |
| fibrous demineralized bone matrix (f-DBM) | 25 |
| fibrous demineralized bone matrix densely packed (f-DBM 120%) | 22 |
| DBM granules (GDBM) | 23 |
| DBM granules 5% calcium phosphate (GDBM5%Ca2+) | 20 |
| Material | Bone Volume/Total Volume (BV/TV) |
|---|---|
| syngenic cancellous bone (SCB) | 0.8530 (±5.3%) |
| fibrous demineralized bone matrix (f-DBM) | 0.8303 (±4.8%) |
| fibrous demineralized bone matrix densely packed (f-DBM 120%) | 0.8803 (±5.5%) |
| DBM granules (GDBM) | 0.7514 (±5.9%) |
| DBM granules 5% calcium phosphate (GDBM5%Ca2+) | 0.7988 (±4.4%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Verboket, R.D.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.C.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. https://doi.org/10.3390/cells10051249
Verboket RD, Irrle T, Busche Y, Schaible A, Schröder K, Brune JC, Marzi I, Nau C, Henrich D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells. 2021; 10(5):1249. https://doi.org/10.3390/cells10051249
Chicago/Turabian StyleVerboket, René D., Tanja Irrle, Yannic Busche, Alexander Schaible, Katrin Schröder, Jan C. Brune, Ingo Marzi, Christoph Nau, and Dirk Henrich. 2021. "Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats" Cells 10, no. 5: 1249. https://doi.org/10.3390/cells10051249
APA StyleVerboket, R. D., Irrle, T., Busche, Y., Schaible, A., Schröder, K., Brune, J. C., Marzi, I., Nau, C., & Henrich, D. (2021). Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells, 10(5), 1249. https://doi.org/10.3390/cells10051249