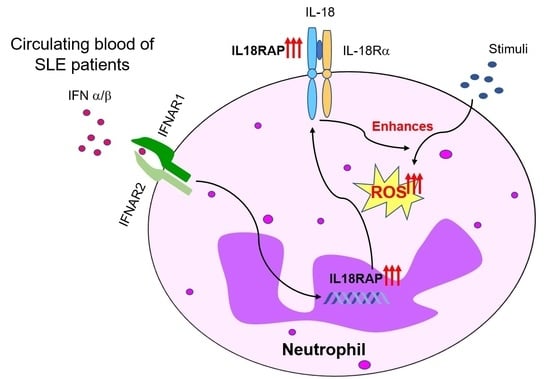
Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Isolation and Stimulation of Human Neutrophils
2.3. Real-Time Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
2.4. Western Blotting
2.5. Measurement of Reactive Oxygen Species (ROS)
2.6. Statistical Analyses
3. Results
3.1. Neutrophils of SLE Patients Show Elevated IL18RAP Expression, Which Correlates Positively with Disease Activity and Renal Involvement
3.2. IL18RAP Expression in Neutrophils Is Regulated by Type I Interferon
3.3. IL-18 Enhances fMLP-Mediated ROS Generation in SLE Neutrophils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ANCA | anti-neutrophil cytoplasm autoantibody |
| C3 | complement 3 |
| C4 | complement 4 |
| DHR123 | dihydrorhodamine 123 |
| eQTL | expression quantitative trait loci |
| fMLP | N-formyl-methionyl-leucyl-phenylalanine |
| FS | fluorescence signal |
| GAPDH | glyceraldehyde-3-phosphate dehydrogenase |
| GWAS | genome-wide association studies |
| HC | healthy control |
| IFIT1 | interferon induced protein with tetratricopeptide repeats 1 |
| IFN | interferon |
| IFN-a | interferon-alpha |
| IFN-g | interferon-gamma |
| IL-18 | interleukin-18 |
| IL-18R | interleukin-18 receptor |
| IL-18R1/IL-18Ra | interleukin-18 receptor alpha |
| IL18RAP/IL-18Rb | interleukin-18 receptor accessory protein/interleukin-18 receptor beta |
| IL-1R | interleukin-1 receptor |
| IQR | interquartile range |
| ISGs | interferon-stimulated genes |
| LDGs | low density-gradient granulocytes |
| LN | lupus nephritis |
| MAPK pathway | mitogen-activated protein kinase pathway |
| mtROS | mitochondrial reactive oxygen species |
| MyD88 | myeloid differentiation primary response protein 88 |
| NA | not available/not applicable |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NETs | neutrophil extracellular traps |
| NF-kB | nuclear factor kappa-light-chain-enhancer of activated B cells |
| NKcells | natural killer cells |
| NLN | non-nephritic lupus |
| PBMCs | peripheral blood mononuclear cells |
| PMA | phorbol-myristate acetate |
| qRT-PCR | real-time quantitative reverse transcription polymerase chain reaction |
| rhIL-18 | recombinant human interleukin-18 |
| RNP-IC | ribonucleoprotein immune complex |
| ROS | reactive oxygen species |
| RQ | relative quantity |
| SJIA | systemic-onset juvenile idiopathic arthritis |
| SLE | systemic lupus erythematosus |
| SLEDAI-2K | systemic lupus erythematosus disease activity index 2000 |
| SLICC | Systemic Lupus International Collaborating Clinics |
| SNP | single nucleotide polymorphism |
| Th-1 cells | type 1 T helper cells |
| TIR | toll-interleukin-1 receptor |
| TLR | toll-like receptor |
References
- Mak, A.; Isenberg, D.A.; Lau, C.S. Global trends, potential mechanisms and early detection of organ damage in SLE. Nat. Rev. Rheumatol. 2013, 9, 301–310. [Google Scholar] [CrossRef]
- Fava, A.; Petri, M. Systemic lupus erythematosus: Diagnosis and clinical management. J. Autoimmun. 2019, 96, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tsokos, G.C. Systemic Lupus Erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, D.Y.; Chan, T.M. Lupus Nephritis in Asia: Clinical Features and Management. Kidney Dis. 2015, 1, 100–109. [Google Scholar] [CrossRef]
- Tsokos, G.C.; Lo, M.S.; Reis, P.C.; Sullivan, K.E. New insights into the immunopathogenesis of systemic lupus erythematosus. Nat. Rev. Rheumatol. 2016, 12, 716–730. [Google Scholar] [CrossRef] [PubMed]
- Jacob, N.; Stohl, W. Cytokine disturbances in systemic lupus erythematosus. Arthritis Res. Ther. 2011, 13, 228. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, N.; Kimura, T.; Arita, K.; Ariyoshi, M.; Ohnishi, H.; Yamamoto, T.; Zuo, X.; Maenaka, K.; Park, E.Y.; Kondo, N.; et al. The structural basis for receptor recognition of human interleukin-18. Nat. Commun. 2014, 5, 5340. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Sakorafas, P.; Miller, R.; McCarthy, D.; Scesney, S.; Dixon, R.; Ghayur, T. IL-18 Receptor β-Induced Changes in the Presentation of IL-18 Binding Sites Affect Ligand Binding and Signal Transduction. J. Immunol. 2003, 170, 5571–5577. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A.; Novick, D.; Kim, S.; Kaplanski, G. Interleukin-18 and IL-18 Binding Protein. Front. Immunol. 2013, 4, 289. [Google Scholar] [CrossRef] [Green Version]
- Rex, D.A.B.; Agarwal, N.; Prasad, T.S.K.; Kandasamy, R.K.; Subbannayya, Y.; Pinto, S.M. A comprehensive pathway map of IL-18-mediated signalling. J. Cell Commun. Signal. 2020, 14, 257–266. [Google Scholar] [CrossRef]
- Wong, C.K.; Li, E.K.; Ho, C.Y.; Lam, C.W.K. Elevation of plasma interleukin-18 concentration is correlated with disease activity in systemic lupus erythematosus. Rheumatology 2000, 39, 1078–1081. [Google Scholar] [CrossRef] [Green Version]
- Novick, D.; Elbirt, D.; Miller, G.; Dinarello, C.A.; Rubinstein, M.; Sthoeger, Z.M. High circulating levels of free interleukin-18 in patients with active SLE in the presence of elevated levels of interleukin-18 binding protein. J. Autoimmun. 2010, 34, 121–126. [Google Scholar] [CrossRef]
- Wu, C.Y.; Yang, H.Y.; Yao, T.C.; Liu, S.H.; Huang, J.L. Serum IL-18 as biomarker in predicting long-term renal outcome among pediatric-onset systemic lupus erythematosus patients. Medicine 2016, 95, e5037. [Google Scholar] [CrossRef]
- Calvani, N.; Richards, H.B.; Tucci, M.; Pannarale, G.; Silvestris, F. Up-regulation of IL-18 and predominance of a Th1 immune response is a hallmark of lupus nephritis. Clin. Exp. Immunol. 2004, 138, 171–178. [Google Scholar] [CrossRef]
- Tucci, M.; Quatraro, C.; Lombardi, L.; Pellegrino, C.; Dammacco, F.; Silvestris, F. Glomerular accumulation of plasmacytoid dendritic cells in active lupus nephritis: Role of interleukin-18. Arthritis Rheum. 2008, 58, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Mende, R.; Vincent, F.B.; Kandane-Rathnayake, R.; Koelmeyer, R.; Lin, E.; Chang, J.; Hoi, A.Y.; Morand, E.F.; Harris, J.; Lang, T. Analysis of Serum Interleukin (IL)-1beta and IL-18 in Systemic Lupus Erythematosus. Front. Immunol. 2018, 9, 1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, J.; Menke, J.; Kriegsmann, J.; Kelley, V.R.; Mayet, W.J.; Galle, P.R.; Schwarting, A. Correlation of renal tubular epithelial cell-derived interleukin-18 up-regulation with disease activity in MRL-Faslpr mice with autoimmune lupus nephritis. Arthritis Rheum. 2002, 46, 3083–3095. [Google Scholar] [CrossRef] [PubMed]
- Esfandiari, E.; McInnes, I.B.; Lindop, G.; Huang, F.P.; Field, M.; Komai-Koma, M.; Wei, X.; Liew, F.Y. A Proinflammatory Role of IL-18 in the Development of Spontaneous Autoimmune Disease. J. Immunol. 2001, 167, 5338–5347. [Google Scholar] [CrossRef]
- Bossu, P.; Neumann, D.; Del Giudice, E.; Ciaramella, A.; Gloaguen, I.; Fantuzzi, G.; Dinarello, C.A.; Di Carlo, E.; Musiani, P.; Meroni, P.L.; et al. IL-18 cDNA vaccination protects mice from spontaneous lupus-like autoimmune disease. Proc. Natl. Acad. Sci. USA 2003, 100, 14181–14186. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, K.; Yamagata, T.; Nozaki, Y.; Sugiyama, M.; Ikoma, S.; Funauchi, M.; Kanamaru, A. Blockade of IL-18 Receptor Signaling Delays the Onset of Autoimmune Disease in MRL-Faslpr Mice. J. Immunol. 2004, 173, 5312–5318. [Google Scholar] [CrossRef] [Green Version]
- Neumann, D.; Del Giudice, E.; Ciaramella, A.; Boraschi, D.; Bossu, P. Lymphocytes from Autoimmune MRL lpr/lpr Mice Are Hyperresponsive to IL-18 and Overexpress the IL-18 Receptor Accessory Chain. J. Immunol. 2001, 166, 3757–3762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvani, N.; Tucci, M.; Richards, H.B.; Tartaglia, P.; Silvestris, F. Th1 cytokines in the pathogenesis of lupus nephritis: The role of IL-18. Autoimmun. Rev. 2005, 4, 542–548. [Google Scholar] [CrossRef]
- Favilli, F.; Anzilotti, C.; Martinelli, L.; Quattroni, P.; De Martino, S.; Pratesi, F.; Neumann, D.; Beermann, S.; Novick, D.; Dinarello, C.A.; et al. IL-18 Activity in Systemic Lupus Erythematosus. Ann. N. Y. Acad. Sci. 2009, 1173, 301–309. [Google Scholar] [CrossRef] [PubMed]
- Sareneva, T.; Julkunen, I.; Matikainen, S. IFN-α and IL-12 Induce IL-18 Receptor Gene Expression in Human NK and T Cells. J. Immunol. 2000, 165, 1933–1938. [Google Scholar] [CrossRef]
- Leung, B.P.; Culshaw, S.; Gracie, J.A.; Hunter, D.; Canetti, C.A.; Campbell, C.; Cunha, F.; Liew, F.Y.; McInnes, I.B. A Role for IL-18 in Neutrophil Activation. J. Immunol. 2001, 167, 2879–2886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baechler, E.C.; Batliwalla, F.M.; Karypis, G.; Gaffney, P.M.; Ortmann, W.A.; Espe, K.J.; Shark, K.B.; Grande, W.J.; Hughes, K.M.; Kapur, V.; et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. USA 2003, 100, 2610–2615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winterbourn, C.C.; Kettle, A.J.; Hampton, M.B. Reactive Oxygen Species and Neutrophil Function. Annu. Rev. Biochem. 2016, 85, 765–792. [Google Scholar] [CrossRef]
- Bashir, S.; Stanworth, S.; Massey, E.; Goddard, F.; Cardigan, R. Neutrophil function is preserved in a pooled granulocyte component prepared from whole blood donations. Br. J. Haematol. 2008, 140, 701–711. [Google Scholar] [CrossRef]
- van de Geer, A.; Gazendam, R.P.; Tool, A.T.; van Hamme, J.L.; de Korte, D.; van den Berg, T.K.; Zeerleder, S.S.; Kuijpers, T.W. Characterization of buffy coat-derived granulocytes for clinical use: A comparison with granulocyte colony-stimulating factor/dexamethasone-pretreated donor-derived products. Vox Sang. 2017, 112, 173–182. [Google Scholar] [CrossRef]
- Cheung, H.; Chen, N.J.; Cao, Z.; Ono, N.; Ohashi, P.S.; Yeh, W.C. Accessory Protein-Like is Essential for IL-18-Mediated Signaling. J. Immunol. 2005, 174, 5351–5357. [Google Scholar] [CrossRef]
- Smyth, D.J.; Plagnol, V.; Walker, N.M.; Cooper, J.D.; Downes, K.; Yang, J.H.; Howson, J.M.; Stevens, H.; McManus, R.; Wijmenga, C.; et al. Shared and Distinct Genetic Variants in Type 1 Diabetes and Celiac Disease. N. Engl. J. Med. 2008, 359, 2767–2777. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Myhr, C.B.; Hulme, M.A.; Wasserfall, C.H.; Hong, P.J.; Lakshmi, P.S.; Schatz, D.A.; Haller, M.J.; Brusko, T.M.; Atkinson, M.A. The autoimmune disease-associated SNP rs917997 of IL18RAP controls IFNgamma production by PBMC. J. Autoimmun. 2013, 44, 8–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andiappan, A.K.; Melchiotti, R.; Poh, T.Y.; Nah, M.; Puan, K.J.; Vigano, E.; Haase, D.; Yusof, N.; Luis, B.S.; Lum, J.; et al. Genome-wide analysis of the genetic regulation of gene expression in human neutrophils. Nat. Commun. 2015, 6, 7971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badot, V.; Galant, C.; Toukap, A.N.; Theate, I.; Maudoux, A.L.; Van den Eynde, B.J.; Durez, P.; Houssiau, F.A.; Lauwerys, B.R. Gene expression profiling in the synovium identifies a predictive signature of absence of response to adalimumab therapy in rheumatoid arthritis. Arthritis Res. Ther. 2009, 11, R57. [Google Scholar] [CrossRef] [Green Version]
- Brown, R.A.; Henderlight, M.; Do, T.; Yasin, S.; Grom, A.A.; DeLay, M.; Thornton, S.; Schulert, G.S. Neutrophils from Children with Systemic Juvenile Idiopathic Arthritis Exhibit Persistent Proinflammatory Activation Despite Long-Standing Clinically Inactive Disease. Front. Immunol. 2018, 9. [Google Scholar] [CrossRef]
- de Jager, W.; Vastert, S.J.; Beekman, J.M.; Wulffraat, N.M.; Kuis, W.; Coffer, P.J.; Prakken, B.J. Defective phosphorylation of interleukin-18 receptor beta causes impaired natural killer cell function in systemic-onset juvenile idiopathic arthritis. Arthritis Rheum. 2009, 60, 2782–2793. [Google Scholar] [CrossRef]
- Crow, M.K. Type I Interferon in the Pathogenesis of Lupus. J. Immunol. 2014, 192, 5459–5468. [Google Scholar] [CrossRef]
- Feng, X.; Wu, H.; Grossman, J.M.; Hanvivadhanakul, P.; FitzGerald, J.D.; Park, G.S.; Dong, X.; Chen, W.; Kim, M.H.; Weng, H.H.; et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006, 54, 2951–2962. [Google Scholar] [CrossRef]
- Bennett, L.; Palucka, A.K.; Arce, E.; Cantrell, V.; Borvak, J.; Banchereau, J.; Pascual, V. Interferon and Granulopoiesis signature in Systemic Lupus Erythematosus Blood. J. Exp. Med. 2003, 176, 711–723. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting Neutrophils Are Major Inducers of Type I IFN Production in Pediatric Systemic Lupus Erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, M.; Miyazaki, H.; Ono, S.; Inatsu, A.; Nakashima, H.; Tsujimoto, H.; Shinomiya, N.; Saitoh, D.; Seki, S. Enhancement of Neutrophil Function by Interleukin-18 Therapy Protects Burn-Injured Mice from Methicillin-Resistant Staphylococcus aureus. Infect. Immun. 2011, 79, 2670–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hewins, P.; Morgan, M.D.; Holden, N.; Neil, D.; Williams, J.M.; Savage, C.O.; Harper, L. IL-18 is upregulated in the kidney and primes neutrophil responsiveness in ANCA-associated vasculitis. Kidney Int. 2006, 69, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Tsai, C.Y.; Li, K.J.; Hsieh, S.C.; Liao, H.T.; Yu, C.L. What’s wrong with neutrophils in lupus? Clin. Exp. Rheumatol. 2019, 37, 684–693. [Google Scholar]
- Perazzio, S.F.; Salomao, R.; Silva, N.P.; Andrade, L.E. Increased neutrophil oxidative burst metabolism in systemic lupus erythematosus. Lupus 2012, 21, 1543–1551. [Google Scholar] [CrossRef]
- Bengtsson, A.A.; Pettersson, A.; Wichert, S.; Gullstrand, B.; Hansson, M.; Hellmark, T.; Johansson, A.C.M. Low production of reactive oxygen species in granulocytes is associated with organ damage in systemic lupus erythematosus. Arthritis Res. Ther. 2014, 16, R120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef] [Green Version]
- Stoiber, W.; Obermayer, A.; Steinbacher, P.; Krautgartner, W.D. The Role of Reactive Oxygen Species (ROS) in the Formation of Extracellular Traps (ETs) in Humans. Biomolecules 2015, 5, 702–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Migliorini, P.; Anzilotti, C.; Pratesi, F.; Quattroni, P.; Bargagna, M.; Dinarello, C.A.; Boraschi, D. Serum and urinary levels of IL-18 and its inhibitor IL-18BP in systemic lupus erythematosus. Eur. Cytokine Netw. 2010, 21, 264–271. [Google Scholar] [CrossRef]
- Liu, X.; Bao, C.; Hu, D. Elevated interleukin-18 and skewed Th1:Th2 immune response in lupus nephritis. Rheumatol. Int. 2012, 32, 223–229. [Google Scholar] [CrossRef]
- Gwinner, W.; Grone, H.J. Role of reactive oxygen species in glomerulonephritis. Nephrol. Dial. Transplant. 2000, 15, 1127–1132. [Google Scholar] [CrossRef] [Green Version]




| Study Group | HC (n = 30) | NLN (n = 40) | LN (n = 55) |
|---|---|---|---|
| Demographics | |||
| Male: Female, n (%) | 1:29 (3:97) | 2:38 (5:95) | 4:51 (7:93) |
| Age, median (IQR) | 48 (41–53) | 53 (47–61) | 49 (42–59) |
| Serological parameters, median (IQR) | |||
| Anti-dsDNA antibodies [IU/mL] | NA | 28 (9–81) | 47 (19–106) |
| C3 [mg/dL] | NA | 83 (63–100) | 79 (63–93) |
| C4 [mg/dL] | NA | 14 (11–18) | 16 (12–22) |
| Urea [mmol/L] | NA | 5 (4–6) | 5 (4–7) |
| Creatinine [mmol/L] | NA | 64 (55–71) | 69 (57–83) |
| Albumin [g/L] | NA | 43 (40–45) | 41 (37–44) |
| Globulin [g/L] | NA | 35 (32–38) | 30 (28–34) |
| SLEDAI-2K score | NA | 2 (0–4) | 4 (0–5) |
| Organ involvement a, n (%) | |||
| Central nervous system | NA | 0 (0) | 0 (0) |
| Vascular | NA | 0 (0) | 0 (0) |
| Musculoskeletal | NA | 0 (0) | 0 (0) |
| Renal | NA | 0 (0) | 12 (22) |
| Dermal | NA | 6 (15) | 8 (15) |
| Serosal | NA | 0 (0) | 0 (0) |
| Immunological | NA | 25 (63) | 39 (71) |
| Haematological | NA | 7 (18) | 3 (6) |
| Constitutional | NA | 0 (0) | 0 (0) |
| Medication, n (%) | |||
| Prednisolone | NA | 26 (65) | 50 (91) |
| Azathioprine | NA | 8 (20) | 8 (15) |
| Hydroxychloroquine | NA | 26 (65) | 32 (58) |
| Mycophenolate mofetil | NA | 6 (15) | 36 (66) |
| Methotrexate | NA | 1 (3) | 0 (0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Lam, I.K.Y.; Lau, C.-S.; Chan, V.S.F. Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients. Cells 2021, 10, 964. https://doi.org/10.3390/cells10050964
Ma J, Lam IKY, Lau C-S, Chan VSF. Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients. Cells. 2021; 10(5):964. https://doi.org/10.3390/cells10050964
Chicago/Turabian StyleMa, Jie, Ian Kar Yin Lam, Chak-Sing Lau, and Vera Sau Fong Chan. 2021. "Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients" Cells 10, no. 5: 964. https://doi.org/10.3390/cells10050964
APA StyleMa, J., Lam, I. K. Y., Lau, C. -S., & Chan, V. S. F. (2021). Elevated Interleukin-18 Receptor Accessory Protein Mediates Enhancement in Reactive Oxygen Species Production in Neutrophils of Systemic Lupus Erythematosus Patients. Cells, 10(5), 964. https://doi.org/10.3390/cells10050964