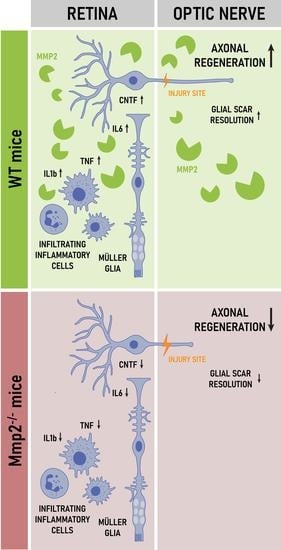
MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals
2.2. Surgical Procedures
2.2.1. Heterologous Bone Marrow Transplantations
2.2.2. Intraorbital Optic Nerve Crush Model
2.2.3. Intravitreal Injection
2.3. Immunohistochemistry on Retinal Whole Mounts
2.4. Immunohistochemistry on Retinal and Optic Nerve Cryosections
2.5. Visualisation of Axonal Tracing
2.6. Flow Cytometry and Fluorescence-Activated Cell Sorting (FACS) of Myeloid Cells
2.7. Quantitative Real-Time PCR (qRT-PCR)
2.8. Statistics
3. Results
3.1. MMP2 Has a Beneficial Effect on Inflammation-Induced Axonal Regeneration
3.2. The Pro-Regenerative Effect of MMP2 Is in Part Due to Resolution of the Glial Scar
3.3. MMP2 Expression in the Infiltrating Inflammatory Cells Is Important for Axonal Regeneration
3.4. MMP2 Does Not Alter the Proliferation or Influx of Inflammatory Cells after Optic Nerve Crush Combined with Inflammatory Stimulation
3.5. MMP2 Does Alter the Expression Profile after Optic Nerve Crush Combined with Inflammatory Stimulation
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andries, L.; van Hove, I.; Moons, L.; de Groef, L. Matrix Metalloproteinases During Axonal Regeneration, a Multifactorial Role from Start to Finish. Mol. Neurobiol. 2017, 54, 2114–2125. [Google Scholar] [CrossRef] [PubMed]
- Andries, L.; de Groef, L.; Moons, L. Neuroinflammation and Optic Nerve Regeneration: Where Do We Stand in Elucidating Underlying Cellular and Molecular Players? Curr. Eye Res. 2020, 45, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, E.; Salinas-Navarro, M.; Andries, L.; Noterdaeme, L.; Etienne, I.; van Wonterghem, E.; Vinckier, S.; Davis, B.M.; van Bergen, T.; van Hove, I.; et al. Tightening the retinal glia limitans attenuates neuroinflammation after optic nerve injury. Glia 2020, 68, 2643–2660. [Google Scholar] [CrossRef]
- Leon, S.; Yin, Y.; Nguyen, J.; Irwin, N.; Benowitz, L.I. Lens injury stimulates axon regeneration in the mature rat optic nerve. J. Neurosci. 2000, 20, 4615–4626. [Google Scholar] [CrossRef]
- De Lima, S.; Koriyama, Y.; Kurimoto, T.; Oliveira, J.T.; Yin, Y.; Li, Y.; Gilbert, H.Y.; Fagiolini, M.; Martinez, A.M.B.; Benowitz, L. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc. Natl. Acad. Sci. USA 2012, 109, 9149–9154. [Google Scholar] [CrossRef]
- Hauk, T.G.; Leibinger, M.; Müller, A.; Andreadaki, A.; Knippschild, U.; Fischer, D. Stimulation of Axon Regeneration in Mature Optic Nerve by Intravitreal Application of te Toll-like Receptor 2 Agonist Pam3Cys. Investig. Ophthalmol. Vis. Sci. 2010, 51, 459–464. [Google Scholar] [CrossRef]
- Hauk, T.G.; Müller, A.; Lee, J.; Schwendener, R.; Fischer, D. Neuroprotective and axon growth promoting effects of intraocular inflammation do not depend on oncomodulin or the presence of large numbers of activated macrophages. Exp. Neurol. 2008, 209, 469–482. [Google Scholar] [CrossRef]
- De Groef, L.; Gaublomme, D.; Janssens, E.; Dekeyster, E.; Moons, L. Retinal MMP expression is upregulated in an excitotoxic mouse model of glaucoma. Acta Ophthalmol. 2012, 11, 4657. [Google Scholar] [CrossRef]
- De Groef, L.; van Hove, I.; Dekeyster, E.; Stalmans, I.; Moons, L. MMPs in the neuroretina and optic nerve: Modulators of glaucoma pathogenesis and repair? Invest. Ophthalmol. Vis. Sci. 2014, 55, 1953–1964. [Google Scholar] [CrossRef] [PubMed]
- Gaublomme, D.; Buyens, T.; de Groef, L.; Stakenborg, M.; Janssens, E.; Ingvarsen, S.; Porse, A.; Behrendt, N.; Moons, L. Matrix metalloproteinase 2 and membrane type 1 matrix metalloproteinase co-regulate axonal outgrowth of mouse retinal ganglion cells. J. Neurochem. 2014, 129, 966–979. [Google Scholar] [CrossRef] [PubMed]
- Verslegers, M.; Lemmens, K.; van Hove, I.; Moons, L. Matrix metalloproteinase-2 and -9 as promising benefactors in development, plasticity and repair of the nervous system. Prog. Neurobiol. 2013, 105, 60–78. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.; Bollaerts, I.; Bhumika, S.; de Groef, L.; van Houcke, J.; Darras, V.M.; van Hove, I.; Moons, L. Matrix metalloproteinases as promising regulators of axonal regrowth in the injured adult zebrafish retinotectal system. J. Comp. Neurol. 2016, 524, 1472–1493. [Google Scholar] [CrossRef]
- Agapova, O.A.; Ricard, C.S.; Salvador-Silva, M.; Hernandez, M.R. Expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in human optic nerve head astrocytes. Glia 2001, 33, 205–216. [Google Scholar] [CrossRef]
- Agapova, O.A.; Kaufman, P.L.; Lucarelli, M.J.; Gabelt, B.T.; Hernandez, M.R. Differential expression of matrix metalloproteinases in monkey eyes with experimental glaucoma or optic nerve transection. Brain Res. 2003, 967, 132–143. [Google Scholar] [CrossRef]
- Limb, G.A.; Daniels, J.T.; Pleass, R.; Charteris, D.G.; Luthert, P.J.; Khaw, P.T. Differential Expression of Matrix Metalloproteinases 2 and 9 by Glial Müller Cells. Am. J. Pathol. 2002, 160, 1847–1855. [Google Scholar] [CrossRef]
- Janssens, E.; Gaublomme, D.; de Groef, L.; Darras, V.M.; Arckens, L.; Delorme, N.; Claes, F.; van Hove, I.; Moons, L. Matrix metalloproteinase 14 in the zebrafish: an eye on retinal and retinotectal development. PLoS One 2013, 8, e52915. [Google Scholar] [CrossRef]
- De Groef, L.; Andries, L.; Lemmens, K.; van Hove, I.; Moons, L. Matrix metalloproteinases in the mouse retina: A comparative study of expression patterns and MMP antibodies. BMC Ophthalmol. 2015, 15, 187. [Google Scholar] [CrossRef]
- Ahmed, Z.; Dent, R.G.; Leadbeater, W.E.; Smith, C.; Berry, M.; Logan, A. Matrix metalloproteases: Degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol. Cell. Neurosci. 2005, 28, 64–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Klassen, H.J.; Tucker, B.A.; Perez, M.-T.R.; Young, M.J. CNS progenitor cells promote a permissive environment for neurite outgrowth via a matrix metalloproteinase-2-dependent mechanism. J. Neurosci. 2007, 27, 4499–4506. [Google Scholar] [CrossRef]
- Duchossoy, Y.; Horvat, J.C.; Stettler, O. MMP-related gelatinase activity is strongly induced in scar tissue of injured adult spinal cord and forms pathways for ingrowing neurites. Mol. Cell. Neurosci. 2001, 17, 945–956. [Google Scholar] [CrossRef] [PubMed]
- Pizzi, M.A.; Crowe, M.J. Matrix metalloproteinases and proteoglycans in axonal regeneration. Exp. Neurol. 2007, 204, 496–511. [Google Scholar] [CrossRef]
- Charalambous, P.; Hurst, L.A.; Thanos, S. Engrafted chicken neural tube-derived stem cells support the innate propensity for axonal regeneration within the rat optic nerve. Invest. Ophthalmol. Vis. Sci. 2008, 49, 3513–3524. [Google Scholar] [CrossRef]
- Pastrana, E.; Moreno-Flores, M.T.; Gurzov, E.N.; Avila, J.; Wandosell, F.; Diaz-Nido, J. Genes associated with adult axon regeneration promoted by olfactory ensheathing cells: A new role for matrix metalloproteinase 2. J. Neurosci. 2006, 26, 5347–5359. [Google Scholar] [CrossRef] [PubMed]
- Filous, A.R.; Miller, J.H.; Coulson-Thomas, Y.M.; Horn, K.P.; Alilain, W.J.; Silver, J. Immature astrocytes promote CNS axonal regeneration when combined with chondroitinase ABC. Dev. Neurobiol. 2010, 70, 826–841. [Google Scholar] [CrossRef]
- Webber, C.A.; Hocking, J.C.; Yong, V.W.; Stange, C.L.; McFarlane, S. Metalloproteases and guidance of retinal axons in the developing visual system. J. Neurosci. 2002, 22, 8091–8100. [Google Scholar] [CrossRef] [PubMed]
- Hehr, C.L.; Hocking, J.C.; McFarlane, S. Matrix metalloproteinases are required for retinal ganglion cell axon guidance at select decision points. Development 2005, 132, 3371–3379. [Google Scholar] [CrossRef] [PubMed]
- Lemmens, K.; van Hove, I.; Moons, L. Complementary research in mammals and fish indicates MMP-2 as a pleiotropic contributor to optic nerve regeneration. Neural Regen. Res. 2016, 11, 740–742. [Google Scholar]
- Itoh, T.; Ikeda, T.; Gomi, H.; Nakao, S.; Suzuki, T.; Itohara, S. Unaltered Secretion of -Amyloid Precursor Protein in Gelatinase A (Matrix Metalloproteinase 2)-deficient Mice. J. Biol. Chem. 1997, 272, 22389–22392. [Google Scholar] [CrossRef]
- Shen, F.W.; Saga, Y.; Litman, G.; Freeman, G.; Tung, J.S.; Cantor, H.; Boyse, E.A. Cloning of Ly-5 cDNA. Proc. Natl. Acad. Sci. USA 1985, 82, 7360–7363. [Google Scholar] [CrossRef]
- Madaan, A.; Verma, R.; Singh, A.T.; Jain, S.K.; Jaggi, M. A stepwise procedure for isolation of murine bone marrow and generation of dendritic cells. J. Biol. Methods 2014, 1, 1. [Google Scholar] [CrossRef]
- Dekeyster, E.; Geeraerts, E.; Buyens, T.; van den Haute, C.; Baekelandt, V.; de Groef, L.; Salinas-Navarro, M.; Moons, L. Tackling Glaucoma from within the Brain: An Unfortunate Interplay of BDNF and TrkB. PLoS One 2015, 10, e0142067. [Google Scholar] [CrossRef] [PubMed]
- Geeraerts, E.; Dekeyster, E.; Gaublomme, D.; Salinas-Navarro, M.; de Groef, L.; Moons, L. A freely available semi-automated method for quantifying retinal ganglion cells in entire retinal flatmounts. Exp. Eye Res. 2016, 147, 105–113. [Google Scholar] [CrossRef]
- De Groef, L.; Dekeyster, E.; Geeraerts, E.; Lefevere, E.; Stalmans, I.; Salinas-navarro, M.; Moons, L. Differential visual system organization and susceptibility to experimental models of optic neuropathies in three commonly used mouse strains. Exp. Eye Res. 2016, 145, 235–247. [Google Scholar] [CrossRef] [PubMed]
- Pernet, V.; Schwab, M.E. The role of Nogo-A in axonal plasticity, regrowth and repair. Cell Tissue Res. 2012, 349, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Devoldere, J.; Peynshaert, K.; Dewitte, H.; Vanhove, C.; de Groef, L.; Moons, L.; Özcan, S.Y.; Dalkara, D.; de Smedt, S.C.; Remaut, K. Non-viral delivery of chemically modified mRNA to the retina: Subretinal versus intravitreal administration. J. Control. Release 2019, 307, 315–330. [Google Scholar] [CrossRef] [PubMed]
- Pearson, C.S.; Mencio, C.P.; Barber, A.C.; Martin, K.R.; Geller, H.M. Identification of a critical sulfation in chondroitin that inhibits axonal regeneration. Elife 2018, 7. [Google Scholar] [CrossRef]
- Van Hove, H.; Martens, L.; Scheyltjens, I.; de Vlaminck, K.; Pombo Antunes, A.R.; de Prijck, S.; Vandamme, N.; de Schepper, S.; van Isterdael, G.; Scott, C.L.; et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nat. Neurosci. 2019, 22, 1021–1035. [Google Scholar] [CrossRef]
- Vandesompele, J.; de Preter, K.; Pattyn, F.; Poppe, B.; van Roy, N.; de Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3. [Google Scholar] [CrossRef]
- Chintala, S.K.; Zhang, X.; Austin, J.S.; Fini, M.E. Deficiency in matrix metalloproteinase gelatinase B (MMP-9) protects against retinal ganglion cell death after optic nerve ligation. J. Biol. Chem. 2002, 277, 47461–47468. [Google Scholar] [CrossRef]
- Leibinger, M.; Andreadaki, A.; Gobrecht, P.; Levin, E.; Diekmann, H.; Fischer, D. Boosting Central Nervous System Axon Regeneration by Circumventing Limitations of Natural Cytokine Signaling. Mol. Ther. 2016, 24, 1712–1725. [Google Scholar] [CrossRef]
- Fischer, D. Hyper-IL-6: A potent and efficacious stimulator of RGC regeneration. Eye 2016. [Google Scholar] [CrossRef]
- Zhang, X. Kainic Acid-Mediated Upregulation of Matrix Metalloproteinase-9 Promotes Retinal Degeneration. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2374–2383. [Google Scholar] [CrossRef] [PubMed]
- Becker, T.; Becker, C.G. Axonal regeneration in zebrafish. Curr. Opin. Neurobiol. 2014, 27, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Shetty, A.K.; Mishra, V.; Kodali, M.; Hattiangady, B. Blood brain barrier dysfunction and delayed neurological deficits in mild traumatic brain injury induced by blast shock waves. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef]
- Abdul-Muneer, P.M.; Schuetz, H.; Wang, F.; Skotak, M.; Jones, J.; Gorantla, S.; Zimmerman, M.C.; Chandra, N.; Haorah, J. Induction of oxidative and nitrosative damage leads to cerebrovascular inflammation in an animal model of mild traumatic brain injury induced by primary blast. Free Radic. Biol. Med. 2013, 60, 282–291. [Google Scholar] [CrossRef]
- Van Hove, I.; Lefevere, E.; de Groef, L.; Sergeys, J.; Salinas-Navarro, M.; Libert, C.; Vandenbroucke, R.; Moons, L. MMP-3 deficiency alleviates endotoxin-induced acute inflammation in the posterior eye segment. Int. J. Mol. Sci. 2016, 17, 1825. [Google Scholar] [CrossRef]
- Boato, F.; Rosenberger, K.; Nelissen, S.; Geboes, L.; Peters, E.M.; Nitsch, R.; Hendrix, S. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J. Neuroinflamm. 2013, 10, 792. [Google Scholar] [CrossRef]
- Sato, A.; Ohtaki, H.; Tsumuraya, T.; Song, D.; Ohara, K.; Asano, M.; Iwakura, Y.; Atsumi, T.; Shioda, S. Interleukin-1 participates in the classical and alternative activation of microglia/macrophages after spinal cord injury. J. Neuroinflamm. 2012, 9, 553. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, M.; Solomon, A.; Lavie, V.; Ben-Bassat, S.; Belkin, M.; Cohen, A. Tumor necrosis factor facilitates regeneration of injured central nervous system axons. Brain Res. 1991, 545, 334–338. [Google Scholar] [CrossRef]
- Saleh, A.; Smith, D.R.; Balakrishnan, S.; Dunn, L.; Martens, C.; Tweed, C.W.; Fernyhough, P. Tumor necrosis factor-α elevates neurite outgrowth through an NF-κB-dependent pathway in cultured adult sensory neurons: Diminished expression in diabetes may contribute to sensory neuropathy. Brain Res. 2011, 1423, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Kato, K.; Liu, H.; Kikuchi, S.; Myers, R.R.; Shubayev, V.I. Immediate anti-tumor necrosis factor-α (etanercept) therapy enhances axonal regeneration after sciatic nerve crush. J. Neurosci. Res. 2010, 88, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic control of proinflammatory cytokines Il-1β and Tnf-α by macrophages in zebrafish spinal cord regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef]
- Van Dyck, A.; Bollaerts, I.; Beckers, A.; Vanhunsel, S.; Glorian, N.; houcke, J.; Ham, T.J.; De Groef, L.; Andries, L.; Moons, L. Müller glia–myeloid cell crosstalk accelerates optic nerve regeneration in the adult zebrafish. Glia 2021, 69, 1444–1463. [Google Scholar] [CrossRef]
- Leibinger, M.; Müller, A.; Gobrecht, P.; Diekmann, H.; Andreadaki, A.; Fischer, D. Interleukin-6 contributes to CNS axon regeneration upon inflammatory stimulation. Cell Death Dis. 2013, 4, e609. [Google Scholar] [CrossRef] [PubMed]
- Leibinger, M.; Müller, A.; Andreadaki, A.; Hauk, T.G.; Kirsch, M.; Fischer, D. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J. Neurosci. 2009, 29, 14334–14341. [Google Scholar] [CrossRef] [PubMed]








| Condition | Explanation | Müller Glial | Infiltrating Inflammatory Cells |
|---|---|---|---|
| WT + WT BM | CD45.1+ bone marrow in CD45.2+ mice | WT | WT |
| Mmp2-/- + Mmp2-/- BM | CD45.2+ Mmp2-/- bone marrow in CD45.2+ Mmp2-/- mice | Mmp2-/- | Mmp2-/- |
| WT + Mmp2-/- BM | CD45.2+ Mmp2-/- bone marrow in CD45.1+ mice | WT | Mmp2-/- |
| Mmp2-/- + WT BM | CD45.1+ bone marrow in CD45.2+ Mmp2-/- mice | Mmp2-/- | WT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andries, L.; Masin, L.; Salinas-Navarro, M.; Zaunz, S.; Claes, M.; Bergmans, S.; Brouwers, V.; Lefevere, E.; Verfaillie, C.; Movahedi, K.; et al. MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System. Cells 2021, 10, 1672. https://doi.org/10.3390/cells10071672
Andries L, Masin L, Salinas-Navarro M, Zaunz S, Claes M, Bergmans S, Brouwers V, Lefevere E, Verfaillie C, Movahedi K, et al. MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System. Cells. 2021; 10(7):1672. https://doi.org/10.3390/cells10071672
Chicago/Turabian StyleAndries, Lien, Luca Masin, Manuel Salinas-Navarro, Samantha Zaunz, Marie Claes, Steven Bergmans, Véronique Brouwers, Evy Lefevere, Catherine Verfaillie, Kiavash Movahedi, and et al. 2021. "MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System" Cells 10, no. 7: 1672. https://doi.org/10.3390/cells10071672
APA StyleAndries, L., Masin, L., Salinas-Navarro, M., Zaunz, S., Claes, M., Bergmans, S., Brouwers, V., Lefevere, E., Verfaillie, C., Movahedi, K., De Groef, L., & Moons, L. (2021). MMP2 Modulates Inflammatory Response during Axonal Regeneration in the Murine Visual System. Cells, 10(7), 1672. https://doi.org/10.3390/cells10071672