Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative
Abstract
:1. Introduction
2. Materials and Methods
2.1. Neuroimaging
2.1.1. Region of Interest (ROI) Masks
2.1.2. BrainPAD Measure Calculation
2.1.3. Voxel Based Morphometry (VBM) Analyses
2.1.4. Relationship between LC Volume and Attention
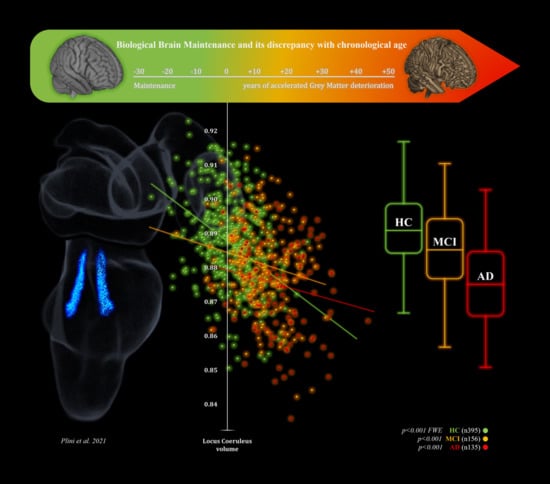
2.1.5. Relationship between LC Volume and Biological Brain Maintenance
2.2. Mediation Analyses
2.3. Bayes Factors Calculation
2.4. Rationale for the Neuromodulatory Subcortical System ROI Selection
3. Results
3.1. Sociodemographic Characteristics and Indices of Reserve and Attention
3.2. 1st Branch of VBM Analyses: Multiple Regressions—TMT-A (Attention—Visuo-Motor Speed Processing)
3.3. 2nd Branch of VBM Analyses: Multiple Regressions—BrainPAD (Reserve—Brain Maintenance)
3.4. Mediation Analyses

| Brain Regions | Side | MNI Coordinates | Peak T Value a | Peak Z-Score b | Peak Cluster Ke c | p-Value Uncorr d | FEW e | FDR f | Total Number of Voxels p < 0.001 Threshold g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | |||||||||
| HC (n. 395) | |||||||||||
| Locus Coeruleus | / | / | / | / | / | / | / | / | / | / | / |
| Dorsal Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Median Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Ventral Tegmental Area | / | / | / | / | / | / | / | / | / | / | / |
| Nucleus Basalis of Meynert | right | 14 | −4 | −12 | 3.35 | 3.32 | 3 | 0.000 | 1.000 | 0.571 | 3 |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
| MCI (n. 156) | |||||||||||
| Locus Coeruleus | left | −4 | −40 | −28 | 3.57 | 3.49 | 7 | 0.000 | 1.000 | 0.639 | 21 |
| Dorsal Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Median Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Ventral Tegmental Area | / | / | / | / | / | / | / | / | / | / | / |
| Nucleus Basalis of Meynert | / | / | / | / | / | / | / | / | / | / | / |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
| AD (n. 135) | |||||||||||
| Locus Coeruleus | right | 6 | −36 | −20 | 4.41 | 4.25 | 8 | 0.000 | 0.992 | 0.463 | 9 |
| Dorsal Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Median Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Ventral Tegmental Area | left | −4 | −22 | −16 | 3.78 | 3.68 | 13 | 0.000 | 1.000 | 0.618 | 14 |
| Nucleus Basalis of Meynert | right | 14 | −4 | −12 | 3.83 | 3.72 | 19 | 0.000 | 1.000 | 0.583 | 19 |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
3.5. Bayes Factors: Parameters of Evidence Strength for the Six ROIs Involved in Attention and Brain Maintenance across the Three Groups
| Brain Regions | Side | MNI Coordinates | Peak T Value a | Peak Zscore b | Peak Cluster Ke c | p-Value Uncorr d | FEW e | FDR f | Total Number of Voxels p < 0.001 FWE Threshold g | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| x | y | Z | |||||||||
| HC (n. 395) | |||||||||||
| Locus Coeruleus | left | −2 | −38 | −22 | 9.10 | inf | 106 | 0.000 | 0.000 | 0.000 | 153 |
| Dorsal Raphe | right | 2 | −32 | −12 | 8.14 | 7.81 | 71 | 0.000 | 0.000 | 0.000 | 71 |
| Median Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Ventral Tegmental Area | / | / | / | / | / | / | / | / | / | / | / |
| Nucleus Basalis of Meynert | / | / | / | / | / | / | / | / | / | / | / |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
| MCI (n. 156) | total number of voxels for p < 0.001 threshold g | ||||||||||
| Locus Coeruleus | left | −2 | −34 | −14 | 4.16 | 4.04 | 16 | 0.000 | 1.000 | 0.125 | 59 |
| Dorsal Raphe | right | 2 | −30 | −8 | 5.78 | 5.48 | 109 | 0.000 | 0.021 | 0.563 | 109 |
| Median Raphe | / | / | / | / | / | / | / | / | / | / | / |
| Ventral Tegmental Area | left | −2 | −16 | −14 | 3.22 | 3.16 | 1 | 0.001 | 1.000 | 0.369 | 2 |
| Nucleus Basalis of Meynert | right | 14 | −8 | −10 | 4.15 | 4.03 | 12 | 0.000 | 0.999 | 0.093 | 12 |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
| AD (n. 135) | |||||||||||
| Locus Coeruleus | left | −4 | −36 | −16 | 5.56 | 5.25 | 94 | 0.000 | 0.065 | 0.005 | 192 |
| Dorsal Raphe | left | −2 | −32 | −12 | 4.74 | 4.54 | 90 | 0.000 | 0.786 | 0.037 | 90 |
| Median Raphe | / | 0 | −34 | −22 | 4.19 | 4.05 | 20 | 0.000 | 1.000 | 0.135 | 20 |
| Ventral Tegmental Area | left | −4 | −22 | −16 | 4.49 | 4.32 | 9 | 0.000 | 0.976 | 0.067 | 9 |
| Nucleus Basalis of Meynert | right | 14 | −6 | −12 | 3.41 | 3.33 | 5 | 0.000 | 1.000 | 0.655 | 5 |
| Control Pontine ROI | / | / | / | / | / | / | / | / | / | / | / |
3.6. Brief Summary of the 3rd, 4th, 5th and 6th VBM Analyses
4. Discussion
5. Limitations
6. Conclusions and Clinical Implications
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Robertson, I.H. A noradrenergic theory of cognitive reserve: Implications for Alzheimer’s disease. Neurobiol. Aging 2013, 34, 298–308. [Google Scholar] [CrossRef]
- Robertson, I.H. A right hemisphere role in cognitive reserve. Neurobiol. Aging 2014, 35, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Tucker, A.M. Cognitive Reserve in Aging. Curr. Alzheimer Res. 2011, 8, 354–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenzuela, M.J.; Sachdev, P. Brain reserve and dementia: A systematic review. Psychol. Med. 2005, 36, 441–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stern, Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012, 11, 1006–1012. [Google Scholar] [CrossRef] [Green Version]
- Stern, Y. Cognitive reserve. Neuropsychologia 2009, 47, 2015–2028. [Google Scholar] [CrossRef]
- Stern, Y.; Urquiljo, E.M.A.; Bartrés-Faz, D.; Belleville, S.; Cantillon, M.; Chetelat, G.; Ewers, M.; Franzmeier, N.; Kempermann, G.; Kremen, W.S.; et al. Whitepaper: Defining and investigating cognitive reserve, brain reserve, and brain maintenance. Alzheimer’s Dement. 2020, 16, 1305–1311. [Google Scholar] [CrossRef]
- Cabeza, R.; Albert, M.; Belleville, S.; Craik, F.I.M.; Duarte, A.; Grady, C.L.; Lindenberger, U.; Nyberg, L.; Park, D.C.; Reuter-Lorenz, P.A.; et al. Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nat. Rev. Neurosci. 2018, 19, 772. [Google Scholar] [CrossRef]
- Barulli, D.; Stern, Y. Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends Cogn. Sci. 2013, 17, 502–509. [Google Scholar] [CrossRef] [Green Version]
- Gaser, C.; Franke, K.; Klöppel, S.; Koutsouleris, N.; Sauer, H.; Initiative, A.D.N. BrainAGE in Mild Cognitive Impaired Patients: Predicting the Conversion to Alzheimer’s Disease. PLoS ONE 2013, 8, e67346. [Google Scholar] [CrossRef]
- Habeck, C.; Razlighi, Q.; Gazes, Y.; Barulli, D.; Steffener, J.; Stern, Y. Cognitive Reserve and Brain Maintenance: Orthogonal Concepts in Theory and Practice. Cereb. Cortex 2016, 27, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Boyle, R.; Jollans, L.; Rueda-Delgado, L.M.; Rizzo, R.; Yener, G.G.; McMorrow, J.P.; Knight, S.P.; Carey, D.; Robertson, I.H.; Emek-Savaş, D.D.; et al. Brain-predicted age difference score is related to specific cognitive functions: A multi-site replication analysis. Brain Imaging Behav. 2021, 15, 327–345. [Google Scholar] [CrossRef] [PubMed]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Mai, J.K.; Paxinos, G. The Human Nervous System, 3rd ed.; Academic Press: Cambridge, MA, USA, 2012; ISBN 9780123742360. [Google Scholar] [CrossRef]
- Chandler, D.J.; Jensen, P.; McCall, J.G.; Pickering, A.E.; Schwarz, L.A.; Totah, N.K. Redefining Noradrenergic Neuromodulation of Behavior: Impacts of a Modular Locus Coeruleus Architecture. J. Neurosci. 2019, 39, 8239–8249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borodovitsyna, O.; Flamini, M.; Chandler, D. Noradrenergic Modulation of Cognition in Health and Disease. Neural Plast. 2017, 2017, 6031478. [Google Scholar] [CrossRef] [Green Version]
- Sara, S.J. The locus coeruleus and noradrenergic modulation of cognition. Nat. Rev. Neurosci. 2009, 10, 211–223. [Google Scholar] [CrossRef]
- Brunnström, H.; Friberg, N.; Lindberg, E.; Englund, E. Differential degeneration of the locus coeruleus in dementia subtypes. Clin. Neuropathol. 2011, 30, 104–110. [Google Scholar] [CrossRef]
- Benarroch, E.E. The locus ceruleus norepinephrine system: Functional organization and potential clinical significance. Neurologhy 2009, 73, 1699–1704. [Google Scholar] [CrossRef]
- Kelly, S.; He, B.; Perez, S.E.; Ginsberg, S.D.; Mufson, E.J.; Counts, S.E. Locus coeruleus cellular and molecular pathology during the progression of Alzheimer’s disease. Acta Neuropathol. Commun. 2017, 5, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Mather, M.; Harley, C.W. The Locus Coeruleus: Essential for Maintaining Cognitive Function and the Aging Brain. Trends Cogn. Sci. 2016, 20, 214–226. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, P.; Lagarde, J.; Lehericy, S.; Valabrègue, R.; Michel, A.; Macé, P.; Caillé, F.; Gervais, P.; Bottlaender, M.; Sarazin, M. Early alteration of the locus coeruleus in phenotypic variants of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 1345–1351. [Google Scholar] [CrossRef] [Green Version]
- Mravec, B.; Lejavova, K.; Cubinkova, V. Locus (coeruleus) minoris resistentiae in pathogenesis of Alzheimer’s disease. Curr. Alzheimer Res. 2014, 11, 992–1001. [Google Scholar] [CrossRef]
- Grudzien, A.; Shaw, P.; Weintraub, S.; Bigio, E.; Mash, D.C.; Mesulam, M.M. Locus coeruleus neurofibrillary degeneration in aging, mild cognitive impairment and early Alzheimer’s disease. Neurobiol. Aging 2007, 28, 327–335. [Google Scholar] [CrossRef]
- Ehrenberg, A.J.; Nguy, A.K.; Theofilas, P.; Dunlop, S.; Suemoto, C.K.; Alho, A.T.D.L.; Leite, R.P.; Rodriguez, R.D.; Mejia, M.B.; Rüb, U.; et al. Quantifying the accretion of hyperphosphorylated tau in the locus coeruleus and dorsal raphe nucleus: The pathological building blocks of early Alzheimer’s disease. Neuropathol. Appl. Neurobiol. 2017, 43, 393–408. [Google Scholar] [CrossRef]
- Braak, H.; Thal, D.R.; Ghebremedhin, E.; Del Tredici, K. Stages of the pathologic process in Alzheimer disease: Age categories from 1 to 100 years. J. Neuropathol. Exp. Neurol. 2011, 70, 960–969. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, F.S.; Ryskalin, L.; Ruffoli, R.; Biagioni, F.; Limanaqi, F.; Ferrucci, M.; Busceti, C.L.; Bonuccelli, U.; Fornai, F. The Neuroanatomy of the Reticular Nucleus Locus Coeruleus in Alzheimer’s Disease. Front. Neuroanat. 2017, 11, 80. [Google Scholar] [CrossRef] [Green Version]
- Satoh, A.; Iijima, K.M. Roles of tau pathology in the locus coeruleus (LC) in age-associated pathophysiology and Alzheimer’s disease pathogenesis: Potential strategies to protect the LC against aging. Brain Res. 2019, 1702, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Pamphlett, R.; Jew, S.K. Different Populations of Human Locus Ceruleus Neurons Contain Heavy Metals or Hyperphosphorylated Tau: Implications for Amyloid-β and Tau Pathology in Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 45, 437–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Betts, M.J.; Kirilina, E.; Otaduy, M.C.G.; Ivanov, D.; Acosta-Cabronero, J.; Callaghan, M.F.; Lambert, C.; Cardenas-Blanco, A.; Pine, K.; Passamonti, L.; et al. Locus coeruleus imaging as a biomarker for noradrenergic dysfunction in neurodegenerative diseases. Brain 2019, 142, 2558–2571. [Google Scholar] [CrossRef]
- Wilson, R.S.; Nag, S.; Boyle, P.A.; Hizel, L.; Yu, L.; Buchman, A.S.; Schneider, J.A.; Bennett, D.A. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology 2013, 80, 1202–1208. [Google Scholar] [CrossRef] [Green Version]
- Bondareff, W.; Mountjoy, C.Q.; Roth, M. Loss of neurons of origin of the adrenergic projection to cerebral cortex (nucleus locus ceruleus) in senile dementia. Neurology 1982, 32, 164. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Düzel, S.; Bodammer, N.C.; Lindenberger, U.; Kühn, S.; Werkle-Bergner, M. Rostral locus coeruleus integrity is associated with better memory performance in older adults. Nat. Hum. Behav. 2019, 3, 1203–1214. [Google Scholar] [CrossRef]
- Liu, K.Y.; Marijatta, F.; Hämmerer, D.; Acosta-Cabronero, J.; Düzel, E.; Howard, R.J. Magnetic resonance imaging of the human locus coeruleus: A systematic review. Neurosci. Biobehav. Rev. 2017, 83, 325–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Nadrigny, F.; Regen, T.; Martinez-Hernandez, A.; Dumitrescu-Ozimek, L.; Terwel, D.; Jardanhazi-Kurutz, D.; Walter, J.; Kirchhoff, F.; Hanisch, U.-K.; et al. Locus ceruleus controls Alzheimer’s disease pathology by modulating microglial functions through norepinephrine. Proc. Natl. Acad. Sci. USA 2010, 107, 6058–6063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chalermpalanupap, T.; Schroeder, J.P.; Rorabaugh, J.M.; Liles, L.C.; Lah, J.J.; Levey, A.I.; Weinshenker, D. Locus Coeruleus Ablation Exacerbates Cognitive Deficits, Neuropathology, and Lethality in P301S Tau Transgenic Mice. J. Neurosci. 2018, 38, 74–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duffy, K.B.; Ray, B.; Lahiri, D.K.; Tilmont, E.M.; Tinkler, G.P.; Herbert, R.L.; Greig, N.H.; Ingram, D.K.; Ottinger, M.A.; Mattison, J.A. Effects of Reducing Norepinephrine Levels via DSP4 Treatment on Amyloid-β Pathology in Female Rhesus Macaques (Macaca Mulatta). J. Alzheimer’s Dis. 2019, 68, 115–126. [Google Scholar] [CrossRef]
- Heneka, M.T.; Ramanathan, M.; Jacobs, A.H.; Dumitrescu-Ozimek, L.; Bilkei-Gorzo, A.; Debeir, T.; Sastre, M.; Galldiks, N.; Zimmer, A.; Hoehn, M.; et al. Locus Ceruleus Degeneration Promotes Alzheimer Pathogenesis in Amyloid Precursor Protein 23 Transgenic Mice. J. Neurosci. 2006, 26, 1343–1354. [Google Scholar] [CrossRef] [Green Version]
- Kjær, M.; Secher, N.; Galbo, H. Physical stress and catecholamine release. Baillieres Clin. Endocrinol. Metab. 1987, 1, 279–298. [Google Scholar] [CrossRef]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Troadec, J.D.; Marien, M.; Darios, F.; Hartmann, A.; Ruberg, M.; Colpaert, F.; Michel, P.P. Noradrenaline provides long-term protec-tion to dopaminergic neurons by reducing oxidative stress. J. Neurochem. 2001, 79, 200–210. [Google Scholar] [CrossRef]
- Feinstein, D.L.; Heneka, M.T.; Gavrilyuk, V.; Russo, C.D.; Weinberg, G.; Galea, E. Noradrenergic regulation of inflammatory gene expression in brain. Neurochem. Int. 2002, 41, 357–365. [Google Scholar] [CrossRef]
- Giorgi, F.S.; Saccaro, L.F.; Galgani, A.; Busceti, C.L.; Biagioni, F.; Frati, A.; Fornai, F. The role of Locus Coeruleus in neuroinflammation occurring in Alzheimer’s disease. Brain Res. Bull. 2019, 153, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Traver, S.; Salthun-Lassalle, B.; Marien, M.; Hirsch, E.; Colpaert, F.; Michel, P.P. The Neurotransmitter Noradrenaline Rescues Septal Cholinergic Neurons in Culture from Degeneration Caused by Low-Level Oxidative Stress. Mol. Pharmacol. 2005, 67, 1882–1891. [Google Scholar] [CrossRef] [PubMed]
- Counts, S.E.; Mufson, E.J. Noradrenaline activation of neurotrophic pathways protects against neuronal amyloid toxicity. J. Neurochem. 2010, 113, 649–660. [Google Scholar] [CrossRef] [Green Version]
- Mannari, C.; Origlia, N.; Scatena, A.; Del Debbio, A.; Catena, M.; Dell’Agnello, G.; Barraco, A.; Giovannini, L.; Dell’Osso, L.; Domenici, L.; et al. BDNF Level in the Rat Prefrontal Cortex Increases Following Chronic but Not Acute Treatment with Duloxetine, a Dual Acting Inhibitor of Noradrenaline and Serotonin Re-uptake. Cell. Mol. Neurobiol. 2008, 28, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Aghajanov, M.; Chavushyan, V.; Matinyan, S.; Danielyan, M.; Yenkoyan, K. Alzheimer’s disease-like pathology-triggered oxidative stress, alterations in monoamines levels, and structural damage of locus coeruleus neurons are partially recovered by a mix of proteoglycans of embryonic genesis. Neurochem. Int. 2019, 131, 104531. [Google Scholar] [CrossRef]
- Hassani, O.K.; Rymar, V.V.; Nguyen, K.Q.; Huo, L.; Cloutier, J.-F.; Miller, F.D.; Sadikot, A.F. The noradrenergic system is necessary for survival of vulnerable midbrain dopaminergic neurons: Implications for development and Parkinson’s disease. Neurobiol. Aging 2020, 85, 22–37. [Google Scholar] [CrossRef]
- Clewett, D.V.; Lee, T.-H.; Greening, S.; Ponzio, A.; Margalit, E.; Mather, M. Neuromelanin marks the spot: Identifying a locus coeruleus biomarker of cognitive reserve in healthy aging. Neurobiol. Aging 2016, 37, 117–126. [Google Scholar] [CrossRef] [Green Version]
- Del Tredici, K.; Braak, H. To stage, or not to stage. Curr. Opin. Neurobiol. 2020, 61, 10–22. [Google Scholar] [CrossRef]
- Andrés-Benito, P.; Fernández-Dueñas, V.; Carmona, M.; Escobar, L.A.; Torrejón-Escribano, B.; Aso, E.; Ciruela, F.; Ferrer, I. Locus coeruleus at asymptomatic early and middle Braak stages of neurofibrillary tangle pathology. Neuropathol. Appl. Neurobiol. 2017, 43, 373–392. [Google Scholar] [CrossRef] [Green Version]
- Simic, G.; Stanic, G.; Mladinov, M.; Jovanov-Milosevic, N.; Kostovic, I.; Hof, P.R. Does Alzheimer’s disease begin in the brainstem? Neuropathol. Appl. Neurobiol. 2009, 35, 532–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simić, G.; Babić Leko, M.; Wray, S.; Harrington, C.R.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; De Silva, R.; Di Giovanni, G.; et al. Monoaminergic neuropathology in Alzheimer’s disease. Prog. Neurobiol. 2017, 151, 101–138. [Google Scholar] [CrossRef] [Green Version]
- Szot, P.; White, S.; Greenup, J.; Leverenz, J.; Peskind, E.; Raskind, M. Changes in adrenoreceptors in the prefrontal cortex of subjects with dementia: Evidence of compensatory changes. Neuroscience 2007, 146, 471–480. [Google Scholar] [CrossRef] [Green Version]
- Szot, P.; White, S.S.; Greenup, J.L.; Leverenz, J.; Peskind, E.R.; Raskind, M.A. Compensatory Changes in the Noradrenergic Nervous System in the Locus Ceruleus and Hippocampus of Postmortem Subjects with Alzheimer’s Disease and Dementia with Lewy Bodies. J. Neurosci. 2006, 26, 467–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theofilas, P.; Ehrenberg, A.; Dunlop, S.; Alho, A.T.D.L.; Nguy, A.; Leite, R.E.P.; Rodriguez, R.D.; Mejia, M.B.; Suemoto, C.K.; Ferretti-Rebustini, R.E.D.L.; et al. Locus coeruleus volume and cell population changes during Alzheimer’s disease progression: A stereological study in human postmortem brains with potential implication for early-stage biomarker discovery. Alzheimer’s Dement. 2017, 13, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Minzenberg, M.J.; Watrous, A.J.; Yoon, J.H.; Ursu, S.; Carter, C.S. Modafinil Shifts Human Locus Coeruleus to Low-Tonic, High-Phasic Activity During Functional MRI. Science 2008, 322, 1700–1702. [Google Scholar] [CrossRef] [Green Version]
- Mather, M.; Huang, R.; Clewett, D.; Nielsen, S.E.; Velasco, R.; Tu, K.P.; Han, S.; Kennedy, B. Isometric exercise facilitates attention to salient events in women via the noradrenergic system. NeuroImage 2020, 210, 116560. [Google Scholar] [CrossRef]
- Holland, N.; Robbins, T.W.; Rowe, J.B. The role of noradrenaline in cognition and cognitive disorders. Brain 2021. [Google Scholar] [CrossRef]
- Klimek, V.; Stockmeier, C.; Overholser, J.; Meltzer, H.Y.; Kalka, S.; Dilley, G.; Ordway, G.A. Reduced Levels of Norepinephrine Transporters in the Locus Coeruleus in Major Depression. J. Neurosci. 1997, 17, 8451–8458. [Google Scholar] [CrossRef] [Green Version]
- Hoogendijk, W.J.; Feenstra, M.G.; Botterblom, M.H.; Gilhuis, J.; Sommer, I.E.; Kamphorst, W.; Eikelenboom, P.; Swaab, D.F. Increased activity of surviving locus ceruleus neurons in Alzheimer’s disease. Ann. Neurol. 1999, 45, 82–91. [Google Scholar] [CrossRef]
- Mueller, S.G.; Weiner, M.W.; Thal, L.J.; Petersen, R.C.; Jack, C.; Jagust, W.; Trojanowski, J.Q.; Toga, A.W.; Beckett, L. The Alzheimer’s disease neuroimaging initiative. Neuroimaging Clin. N. Am. 2005, 15, 869–877. [Google Scholar] [CrossRef] [Green Version]
- Mueller, S.G.; Weiner, M.W.; Thal, L.J.; Petersen, R.C.; Jack, C.R.; Jagust, W.; Trojanowski, J.Q.; Toga, A.W.; Beckett, L. Ways toward an early diagnosis in Alzheimer’s disease: The Alzheimer’s Disease Neuroimaging Initiative (ADNI). Alzheimer’s Dement. 2005, 1, 55–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.; Wang, H.; Zhu, M.; He, Y.; Zhang, H.; Chen, X.; Gao, W.; Fu, Y.; Alzheimer’s Disease Neuroimaging Initiative. Brainstem atrophy in the early stage of Alzheimer’s disease: A voxel-based morphometry study. Brain Imaging Behav. 2021, 15, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Ryan, J.; Andreescu, C.; Aizenstein, H.; Lim, H.K. Brainstem morphological changes in Alzheimer’s disease. Neuroreport 2015, 26, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.; Chen, X.; Liu, M.; Liu, S.; Ma, L.; Yu, S. Volume gain of periaqueductal gray in medication-overuse headache. J. Headache Pain 2017, 18, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Protopopescu, X.; Pan, H.; Tuescher, O.; Cloitre, M.; Goldstein, M.; Engelien, A.; Yang, Y.; Gorman, J.; LeDoux, J.; Stern, E.; et al. Increased brainstem volume in panic disorder: A voxel-based morphometric study. Neuroreport 2006, 17, 361–363. [Google Scholar] [CrossRef]
- Dutt, S.; Li, Y.; Mather, M.; Nation, D.A. Brainstem substructures and cognition in prodromal Alzheimer’s disease. Brain Imaging Behav. 2021, 1–11. [Google Scholar] [CrossRef]
- Dutt, S.; Li, Y.; Mather, M.; Nation, D.A.; Alzheimer’s Disease Neuroimaging Initiative. Brainstem Volumetric Integrity in Preclin-ical and Prodromal Alzheimer’s Disease. J. Alzheimers Dis. 2020, 77, 1579–1594. [Google Scholar] [CrossRef]
- Dahl, M.J.; Mather, M.; Werkle-Bergner, M.; Kennedy, B.L.; Guzman, S.; Hurth, K.; Miller, C.A.; Qiao, Y.; Shi, Y.; Chui, H.C.; et al. Locus coeruleus integrity is related to tau burden and memory loss in autosomal-dominant Alzheimer’s disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Takahashi, J.; Shibata, T.; Sasaki, M.; Kudo, M.; Yanezawa, H.; Obara, S.; Kudo, K.; Ito, K.; Yamashita, F.; Terayama, Y. Detection of changes in the locus coeruleus in patients with mild cognitive impairment and A lzheimer’s disease: High-resolution fast spin-echo T 1-weighted imaging. Geriatr. Gerontol. Int. 2015, 15, 334–340. [Google Scholar] [CrossRef]
- Ayzenberg, I.; Nastos, I.; Strassburger-Krogias, K.; Obermann, M.; Gold, R.; Krogias, C. Hypoechogenicity of brainstem raphe nuclei is associated with increased attack frequency in episodic migraine. Cephalalgia 2016, 36, 800–806. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, J.; Lan, L.; Cheng, S.; Sun, R.; Gong, Q.; Wintermark, M.; Zeng, F.; Liang, F. Concurrent brain structural and functional alterations in patients with migraine without aura: An fMRI study. J. Headache Pain 2020, 21, 1–9. [Google Scholar] [CrossRef]
- Supprian, T.; Reiche, W.; Schmitz, B.; Grunwald, I.; Backens, M.; Hofmann, E.; Georg, T.; Falkai, P.; Reith, W. MRI of the brainstem in patients with major depression, bipolar affective disorder and normal controls. Psychiatry Res. 2004, 131, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-Y.; Tae, W.S.; Yoon, H.-K.; Lee, B.-T.; Paik, J.-W.; Son, K.-R.; Oh, Y.-W.; Lee, M.-S.; Ham, B.-J. Demonstration of decreased gray matter concentration in the midbrain encompassing the dorsal raphe nucleus and the limbic subcortical regions in major depressive disorder: An optimized voxel-based morphometry study. J. Affect. Disord. 2011, 133, 128–136. [Google Scholar] [CrossRef] [PubMed]
- De Marco, M.; Venneri, A. Volume and Connectivity of the Ventral Tegmental Area are Linked to Neurocognitive Signatures of Alzheimer’s Disease in Humans. J. Alzheimer’s Dis. 2018, 63, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Khan, A.R.; Hiebert, N.M.; Vo, A.; Wang, B.T.; Owen, A.M.; Seergobin, K.N.; Macdonald, P.A. Biomarkers of Parkinson’s disease: Striatal sub-regional structural morphometry and diffusion MRI. Neuroimage Clin. 2019, 21, 101597. [Google Scholar] [CrossRef]
- Liu, J.; Chen, L.; Chen, X.; Hu, K.; Tu, Y.; Lin, M.; Huang, J.; Liu, W.; Wu, J.; Qiu, Z.; et al. Modulatory effects of different exercise modalities on the functional connectivity of the periaqueductal grey and ventral tegmental area in patients with knee osteoarthritis: A randomised multimodal magnetic resonance imaging study. Br. J. Anaesth. 2019, 123, 506–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Z.-Y.; Chen, X.-Y.; Liu, M.-Q.; Ma, L.; Yu, S.-Y. Volume Gain of Brainstem on Medication-Overuse Headache Using Voxel-Based Morphometry. Chin. Med. J. 2018, 131, 2158–2163. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.W.; Initiative, T.A.D.N.; Spreng, R.N. Basal forebrain degeneration precedes and predicts the cortical spread of Alzheimer’s pathology. Nat. Commun. 2016, 7, 13249. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Mufson, E.; Leurgans, S.; Shah, R.; Ferrari, C.; Detoledo-Morrell, L. MRI-based volumetric measurement of the substantia innominata in amnestic MCI and mild AD. Neurobiol. Aging 2011, 32, 1756–1764. [Google Scholar] [CrossRef] [Green Version]
- Schulz, J.; Pagano, G.; Bonfante, J.A.F.; Wilson, H.; Politis, M. Nucleus basalis of Meynert degeneration precedes and predicts cognitive impairment in Parkinson’s disease. Brain 2018, 141, 1501–1516. [Google Scholar] [CrossRef] [Green Version]
- Jethwa, K.; Dhillon, P.; Meng, D.; Auer, D.; Alzheimer’s Disease Neuroimaging Initiative. Are Linear Measurements of the Nucleus Basalis of Meynert Suitable as a Diagnostic Biomarker in Mild Cognitive Impairment and Alzheimer Disease? Am. J. Neuroradiol. 2019, 40, 2039–2044. [Google Scholar] [CrossRef] [PubMed]
- Colloby, S.J.; Elder, G.J.; Rabee, R.; O’Brien, J.T.; Taylor, J. Structural grey matter changes in the substantia innominata in Alzheimer’s disease and dementia with Lewy bodies: A DARTEL-VBM study. Int. J. Geriatr. Psychiatry 2016, 32, 615–623. [Google Scholar] [CrossRef]
- Ray, N.J.; Bradburn, S.; Murgatroyd, C.; Toseeb, U.; Mir, P.; Kountouriotis, G.K.; Teipel, S.J.; Grothe, M.J. In vivo cholinergic basal forebrain atrophy predicts cognitive decline in de novo Parkinson’s disease. Brain 2018, 141, 165–176. [Google Scholar] [CrossRef] [Green Version]
- Cantero, J.L.; Zaborszky, L.; Atienza, M. Volume Loss of the Nucleus Basalis of Meynert is Associated with Atrophy of Innervated Regions in Mild Cognitive Impairment. Cereb. Cortex 2016, 27, 3881–3889. [Google Scholar] [CrossRef]
- Muth, K.; Schönmeyer, R.; Matura, S.; Haenschel, C.; Schröder, J.; Pantel, J. Mild Cognitive Impairment in the Elderly is Associated with Volume Loss of the Cholinergic Basal Forebrain Region. Biol. Psychiatry 2010, 67, 588–591. [Google Scholar] [CrossRef] [PubMed]
- Van Hooren, R.W.; Verhey, F.R.; Ramakers, I.H.; Jansen, W.J.; Jacobs, H.I. Elevated norepinephrine metabolism is linked to cortical thickness in the context of Alzheimer’s disease pathology. Neurobiol. Aging 2021, 102, 17–22. [Google Scholar] [CrossRef]
- Tombaugh, T.N. Trail Making Test A and B: Normative data stratified by age and education. Arch. Clin. Neuropsychol. 2004, 19, 203–214. [Google Scholar] [CrossRef]
- Gaudino, E.A.; Geisler, M.W.; Squires, N.K. Construct validity in the trail making test: What makes part B harder? J. Clin. Exp. Neuropsychol. 1995, 17, 529–535. [Google Scholar] [CrossRef]
- Aston-Jones, G.; Waterhouse, B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 2016, 1645, 75–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palomero-Gallagher, N.; Amunts, K.; Zilles, K. Transmitter Receptor Distribution in the Human Brain. Brain Mapp. 2015, 261–275. [Google Scholar]
- Talwar, N.; Churchill, N.W.; Hird, M.A.; Tam, F.; Graham, S.J.; Schweizer, T.A. Functional magnetic resonance imaging of the trail-making test in older adults. PLoS ONE 2020, 15, e0232469. [Google Scholar] [CrossRef] [PubMed]
- Varjacic, A.; Mantini, D.; Demeyere, N.; Gillebert, C.R. Neural signatures of Trail Making Test performance: Evidence from lesion-mapping and neuroimaging studies. Neuropsychologia 2018, 115, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Shindo, A.; Terada, S.; Sato, S.; Ikeda, C.; Nagao, S.; Oshima, E.; Yokota, O.; Uchitomi, Y. Trail Making Test Part A and Brain Perfusion Imaging in Mild Alzheimer’s Disease. Dement. Geriatr. Cogn. Disord. Extra 2013, 3, 202–211. [Google Scholar] [CrossRef] [PubMed]
- Zakzanis, K.K.; Mraz, R.; Graham, S.J. An fMRI study of the Trail Making Test. Neuropsychologia 2005, 43, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Lezak, M.D.; Howieson, D.B.; Loring, D.W.; Hannay, H.J.; Fischer, J.S. Neuropsychological Assessment, 4th ed.; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Bowie, C.R.; Harvey, P.D. Administration and interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef] [PubMed]
- Gannon, M.; Wang, Q. Complex noradrenergic dysfunction in Alzheimer’s disease: Low norepinephrine input is not always to blame. Brain Res. 2019, 1702, 12–16. [Google Scholar] [CrossRef]
- Kang, S.S.; Liu, X.; Ahn, E.H.; Xiang, J.; Manfredsson, F.P.; Yang, X.; Luo, H.R.; Liles, L.C.; Weinshenker, D.; Ye, K. Norepinephrine metabolite DOPEGAL activates AEP and pathological Tau aggregation in locus coeruleus. J. Clin. Investig. 2019, 130, 422–437. [Google Scholar] [CrossRef] [Green Version]
- Petersen, R.C.; Aisen, P.S.; Beckett, L.A.; Donohue, M.; Gamst, A.C.; Harvey, D.J.; Jack, C.R.; Jagust, W.J.; Shaw, L.M.; Toga, A.W.; et al. Alzheimer’s Disease Neuroimaging Initiative (ADNI): Clinical characterization. Neurology 2009, 74, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Keren, N.I.; Lozar, C.T.; Harris, K.; Morgan, P.; Eckert, M.A. In vivo mapping of the human locus coeruleus. NeuroImage 2009, 47, 1261–1267. [Google Scholar] [CrossRef] [Green Version]
- Szot, P.; Leverenz, J.B.; Peskind, E.R.; Kiyasu, E.; Rohde, K.; Miller, M.A.; Raskind, M.A. Tyrosine hydroxylase and norepinephrine transporter mRNA expression in the locus coeruleus in Alzheimer’s disease. Mol. Brain Res. 2000, 84, 135–140. [Google Scholar] [CrossRef]
- Matchett, B.J.; Grinberg, L.T.; Theofilas, P.; Murray, M.E. The mechanistic link between selective vulnerability of the locus coeruleus and neurodegeneration in Alzheimer’s disease. Acta Neuropathol. 2021, 141, 631–650. [Google Scholar] [CrossRef]
- Ishimatsu, M.; Williams, J.T. Synchronous Activity in Locus Coeruleus Results from Dendritic Interactions in Pericoerulear Regions. J. Neurosci. 1996, 16, 5196–5204. [Google Scholar] [CrossRef]
- Janitzky, K. Impaired Phasic Discharge of Locus Coeruleus Neurons Based on Persistent High Tonic Discharge—A New Hypothesis with Potential Implications for Neurodegenerative Diseases. Front. Neurol. 2020, 11. [Google Scholar] [CrossRef]
- Keren, N.I.; Taheri, S.; Vazey, E.M.; Morgan, P.; Granholm, A.-C.E.; Aston-Jones, G.S.; Eckert, M.A. Histologic validation of locus coeruleus MRI contrast in post-mortem tissue. NeuroImage 2015, 113, 235–245. [Google Scholar] [CrossRef] [Green Version]
- Tona, K.-D.; Keuken, M.; De Rover, M.; Lakke, E.; Forstmann, B.U.; Nieuwenhuis, S.; Van Osch, M.J.P. In vivo visualization of the locus coeruleus in humans: Quantifying the test–retest reliability. Brain Struct. Funct. 2017, 222, 4203–4217. [Google Scholar] [CrossRef]
- Betts, M.J.; Cardenas-Blanco, A.; Kanowski, M.; Jessen, F.; Düzel, E. In vivo MRI assessment of the human locus coeruleus along its rostrocaudal extent in young and older adults. NeuroImage 2017, 163, 150–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.Y.; Acosta-Cabronero, J.; Cardenas-Blanco, A.; Loane, C.; Berry, A.; Betts, M.; Kievit, R.A.; Henson, R.; Düzel, E.; Howard, R.; et al. In vivo visualization of age-related differences in the locus coeruleus. Neurobiol. Aging 2019, 74, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Rong, Y.; Rua, C.; O’Callaghan, C.; Jones, P.S.; Hezemans, F.; Kaalund, S.S.; Tsvetanov, K.A.; Rodgers, C.T.; Williams, G.; Passamonti, L.; et al. An in vivo Probabilistic Atlas of the Human Locus Coeruleus at Ultra-high Field. bioRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Beliveau, V.; Svarer, C.; Frokjaer, V.; Knudsen, G.M.; Greve, D.N.; Fisher, P.M. Functional connectivity of the dorsal and median raphe nuclei at rest. NeuroImage 2015, 116, 187–195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baker, K.G.; Törk, I.; Hornung, J.-P.; Halasz, P. The human locus coeruleus complex: An immunohistochemical and three di-mensional reconstruction study. Exp. Brain Res. 1989, 77, 257–270. [Google Scholar] [CrossRef]
- German, D.C.; Walker, B.S.; Manaye, K.; Smith, W.K.; Woodward, D.J.; North, A.J. The human locus coeruleus: Computer re-construction of cellular distribution. J. Neurosci. 1988, 8, 1776–1788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohm, T.; Busch, C.; Bohl, J. Unbiased Estimation of Neuronal Numbers in the Human Nucleus Coeruleus during Aging. Neurobiol. Aging 1997, 18, 393–399. [Google Scholar] [CrossRef]
- Chan-Palay, E. Asan. Quantitation of catecholamine neurons in the locus coeruleus in human brains of normal young and older adults and in depression. J. Comp. Neurol. 1989, 287, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, P.; Regala, J.; Correia, F.; Gonçalves-Ferreira, A.J. The human locus coeruleus 3-D stereotactic anatomy. Surg. Radiol. Anat. 2012, 34, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Pauli, W.M.; Nili, A.N.; Tyszka, J.M. A high-resolution probabilistic in vivo atlas of human subcortical brain nuclei. Sci. Data 2018, 5, 180063. [Google Scholar] [CrossRef] [Green Version]
- Zaborszky, L.; Hoemke, L.; Mohlberg, H.; Schleicher, A.; Amunts, K.; Zilles, K. Stereotaxic probabilistic maps of the magnocellular cell groups in human basal forebrain. Neuroimage 2008, 42, 1127–1141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forstmann, B.U.; Keuken, M.; Jahfari, S.; Bazin, P.-L.; Neumann, J.; Schäfer, A.; Anwander, A.; Turner, R. Cortico-subthalamic white matter tract strength predicts interindividual efficacy in stopping a motor response. Neuroimage 2012, 60, 370–375. [Google Scholar] [CrossRef]
- Collins, D.L.; Holmes, C.J.; Peters, T.M.; Evans, A.C. Automatic 3-D model-based neuroanatomical segmentation. Hum. Brain Mapp. 1995, 3, 190–208. [Google Scholar] [CrossRef]
- Mazziotta, J.; Toga, A.; Evans, A.; Fox, P.; Lancaster, J.; Zilles, K.; Woods, R.; Paus, T.; Simpson, G.; Pike, B. A probabilistic atlas and reference system for the human brain: International Consortium for Brain Mapping (ICBM). Philos. Trans. R. Soc. London Ser. B Biol. Sci. 2001, 356, 1293–1322. [Google Scholar] [CrossRef]
- Makris, N.; Goldstein, J.M.; Kennedy, D.; Hodge, S.M.; Caviness, V.S.; Faraone, S.V.; Tsuang, M.T.; Seidman, L.J. Decreased volume of left and total anterior insular lobule in schizophrenia. Schizophr. Res. 2006, 83, 155–171. [Google Scholar] [CrossRef]
- Frazier, J.A.; Chiu, S.; Breeze, J.L.; Makris, N.; Lange, N.; Kennedy, D.N.; Herbert, M.R.; Bent, E.K.; Koneru, V.K.; Dieterich, M.E.; et al. Structural Brain Magnetic Resonance Imaging of Limbic and Thalamic Volumes in Pediatric Bipolar Disorder. Am. J. Psychiatry 2005, 162, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Desikan, R.S.; Ségonne, F.; Fischl, B.; Quinn, B.T.; Dickerson, B.C.; Blacker, D.; Buckner, R.L.; Dale, A.M.; Maguire, R.P.; Hyman, B.T.; et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based re-gions of interest. Neuroimage 2006, 31, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.M.; Seidman, L.J.; Makris, N.; Ahern, T.; O’Brien, L.M.; Caviness, V.S., Jr.; Kennedy, D.N.; Faraone, S.V.; Tsuang, M.T. Hypo-thalamic abnormalities in schizophrenia: Sex effects and genetic vulnerability. Biol. Psychiatry 2007, 61, 935–945. [Google Scholar] [CrossRef]
- Liu, A.K.L.; Chang, R.C.-C.; Pearce, R.K.B.; Gentleman, S.M. Nucleus basalis of Meynert revisited: Anatomy, history and differential involvement in Alzheimer’s and Parkinson’s disease. Acta Neuropathol. 2015, 129, 527–540. [Google Scholar] [CrossRef] [PubMed]
- Kilimann, I.; Grothe, M.; Heinsen, H.; Alho, E.J.L.; Grinberg, L.; Jr, E.A.; Dos Santos, G.A.B.; Da Silva, R.E.; Mitchell, A.J.; Frisoni, G.B.; et al. Subregional Basal Forebrain Atrophy in Alzheimer’s Disease: A Multicenter Study. J. Alzheimer’s Dis. 2014, 40, 687–700. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koulousakis, P.; Andrade, P.; Visser-Vandewalle, V.; Sesia, T. The Nucleus Basalis of Meynert and Its Role in Deep Brain Stimulation for Cognitive Disorders: A Historical Perspective. J. Alzheimer’s Dis. 2019, 69, 905–919. [Google Scholar] [CrossRef]
- Le, T.; Kuplicki, R.T.; McKinney, B.A.; Yeh, H.-W.; Thompson, W.K.; Paulus, M.; Aupperle, R.L.; Bodurka, J.; Cha, Y.-H.; Tulsa 1000 Investigators; et al. A Nonlinear Simulation Framework Supports Adjusting for Age When Analyzing BrainAGE. Front. Aging Neurosci. 2018, 10, 317. [Google Scholar] [CrossRef] [Green Version]
- Aletrino, M.; Vogels, O.; Van Domburg, P.; Donkelaar, H.T. Cell loss in the nucleus raphes dorsalis in alzheimer’s disease. Neurobiol. Aging 1992, 13, 461–468. [Google Scholar] [CrossRef]
- Krashia, P.; Nobili, A.; D’Amelio, M. Unifying Hypothesis of Dopamine Neuron Loss in Neurodegenerative Diseases: Focusing on Alzheimer’s Disease. Front. Mol. Neurosci. 2019, 12, 123. [Google Scholar] [CrossRef]
- Bozzali, M.; D’Amelio, M.; Serra, L. Ventral tegmental area disruption in Alzheimer’s disease. Aging 2019, 11, 1325–1326. [Google Scholar] [CrossRef]
- Herrera, A.Y.; Wang, J.; Mather, M. The gist and details of sex differences in cognition and the brain: How parallels in sex differences across domains are shaped by the locus coeruleus and catecholamine systems. Prog. Neurobiol. 2019, 176, 120–133. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Braak, E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991, 82, 239–259. [Google Scholar] [CrossRef] [PubMed]
- Koychev, I.; Hofer, M.; Friedman, N.C. Correlation of Alzheimer Disease Neuropathologic Staging with Amyloid and Tau Scintigraphic Imaging Biomarkers. J. Nucl. Med. 2020, 61, 1413–1418. [Google Scholar] [CrossRef] [PubMed]
- Bachman, S.L.; Dahl, M.J.; Werkle-Bergner, M.; Düzel, S.; Forlim, C.G.; Lindenberger, U.; Kühn, S.; Mather, M. Locus coeruleus MRI contrast is associated with cortical thickness in older adults. Neurobiol. Aging 2021, 100, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Sims, N.R.; Bowen, D.M.; Davison, A.N.; Esiri, M.M.; Neary, D.; Snowden, J.S.; Wilcock, G.K. Neurochemical Studies of Early-Onset Alzheimer’s Disease. N. Engl. J. Med. 1985, 313, 7–11. [Google Scholar] [CrossRef]
- Hoogendijk, W.J.G.; Sommer, I.E.C.; Pool, C.W.; Kamphorst, W.; Hofman, M.A.; Eikelenboom, P.; Swaab, D.F. Lack of association between depression and loss of neurons in the locus coeruleus in Alzheimer disease. Arch. Gen. Psychiatry 1999, 56, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Matthews, K.L.; Chen, C.P.-H.; Esiri, M.M.; Keene, J.; Minger, S.L.; Francis, P.T. Noradrenergic changes, aggressive behavior, and cognition in patients with dementia. Biol. Psychiatry 2002, 51, 407–416. [Google Scholar] [CrossRef]
- Gannon, M.; Che, P.; Chen, Y.; Jiao, K.; Roberson, E.; Wang, Q. Noradrenergic dysfunction in Alzheimer’s disease. Front. Neurosci. 2015, 9, 220. [Google Scholar] [CrossRef] [Green Version]
- Aston-Jones, G.; Rajkowski, J.; Cohen, J. Role of locus coeruleus in attention and behavioral flexibility. Biol. Psychiatry 1999, 46, 1309–1320. [Google Scholar] [CrossRef]
- Shine, J.M. Neuromodulatory Influences on Integration and Segregation in the Brain. Trends Cogn. Sci. 2019, 23, 572–583. [Google Scholar] [CrossRef]
- O’Callaghan, C.; Walpola, I.C.; Shine, J.M. Neuromodulation of the mind-wandering brain state: The interaction between neuromodulatory tone, sharp wave-ripples and spontaneous thought. Philos. Trans. R. Soc. B Biol. Sci. 2021, 376, 20190699. [Google Scholar] [CrossRef]
- Jacobs, H.I.L.; Riphagen, J.M.; Ramakers, I.H.G.B.; Verhey, F.R.J. Alzheimer’s disease pathology: Pathways between central norepinephrine activity, memory, and neuropsychiatric symptoms. Mol. Psychiatry 2021, 26, 897–906. [Google Scholar] [CrossRef]
- Löwe, L.C.; Gaser, C.; Franke, K.; Alzheimer’s Disease Neuroimaging Initiative. The Effect of the APOE Genotype on Individual BrainAGE in Normal Aging, Mild Cognitive Impairment, and Alzheimer’s Disease. PLoS ONE 2016, 11, e0157514. [Google Scholar] [CrossRef]
- Kaufmann, T.; Van Der Meer, D.; Doan, N.T.; Schwarz, E.; Lund, M.J.; Agartz, I.; Alnæs, D.; Barch, D.M.; Baur-Streubel, R.; Bertolino, A.; et al. Publisher Correction: Common brain disorders are associated with heritable patterns of apparent aging of the brain. Nat. Neurosci. 2019, 23, 295. [Google Scholar] [CrossRef] [PubMed]
- Cole, J.H. Multimodality neuroimaging brain-age in UK biobank: Relationship to biomedical, lifestyle, and cognitive factors. Neurobiol. Aging 2020, 92, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Wilcock, G.K.; Esiri, M.M.; Bowen, D.M.; Hughes, A.O. The differential involvement of subcortical nuclei in senile dementia of Alzheimer’s type. J. Neurol. Neurosurg. Psychiatry 1988, 51, 842–849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rüb, U.; Del Tredici, K.; Schultz, C.; Thal, D.; Braak, E.; Braak, H. The evolution of Alzheimer’s disease-related cytoskeletal pathology in the human raphe nuclei. Neuropathol. Appl. Neurobiol. 2000, 26, 553–567. [Google Scholar] [CrossRef]
- Rodríguez, J.J.; Noristani, H.; Verkhratsky, A. The serotonergic system in ageing and Alzheimer’s disease. Prog. Neurobiol. 2012, 99, 15–41. [Google Scholar] [CrossRef] [PubMed]
- Grinberg, L.T.; Rüb, U.; Ferretti, R.E.L.; Nitrini, R.; Farfel, J.M.; Polichiso, L.; Gierga, K.; Jacob-Filho, W.; Heinsen, H. The dorsal raphe nucleus shows phospho-tau neurofibrillary changes before the transentorhinal region in Alzheimer’s disease. A precocious onset? Neuropathol. Appl. Neurobiol. 2009, 35. [Google Scholar] [CrossRef]
- Michelsen, K.A.; Schmitz, C.; Steinbusch, H.W. The dorsal raphe nucleus—From silver stainings to a role in depression. Brain Res. Rev. 2007, 55, 329–342. [Google Scholar] [CrossRef]
- Aznar, S.; Qian, Z.-X.; Knudsen, G.M. Non-serotonergic dorsal and median raphe projection onto parvalbumin- and calbindin-containing neurons in hippocampus and septum. Neuroscience 2004, 124, 573–581. [Google Scholar] [CrossRef]
- Baker, K.G.; Halliday, G.M.; Hornung, J.P.; Geffen, L.B.; Cotton, R.G. Distribution, morphology and number of monoamine-synthesizing and substance P-containing neurons in the human dorsal raphe nucleus. Neuroscience 1991, 42, 757–775. [Google Scholar] [CrossRef]
- Kirby, L.; Pernar, L.; Valentino, R.; Beck, S. Distinguishing characteristics of serotonin and non-serotonin-containing cells in the dorsal raphe nucleus: Electrophysiological and immunohistochemical studies. Neuroscience 2003, 116, 669–683. [Google Scholar] [CrossRef] [Green Version]
- Ordway, G.A.; Stockmeier, C.A.; Cason, G.W.; Klimek, V. Pharmacology and Distribution of Norepinephrine Transporters in the Human Locus Coeruleus and Raphe Nuclei. J. Neurosci. 1997, 17, 1710–1719. [Google Scholar] [CrossRef]
- Farley, I.J.; Hornykiewicz, O. Noradrenaline distribution in subcortical areas of the human brain. Brain Res. 1977, 126, 53–62. [Google Scholar] [CrossRef]
- Steffener, J.; Habeck, C.; O’Shea, D.; Razlighi, Q.; Bherer, L.; Stern, Y. Differences between chronological and brain age are related to education and self-reported physical activity. Neurobiol. Aging 2016, 40, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Anatürk, M.; Kaufmann, T.; Cole, J.H.; Suri, S.; Griffanti, L.; Zsoldos, E.; Filippini, N.; Singh-Manoux, A.; Kivimäki, M.; Westlye, L.T.; et al. Prediction of brain age and cognitive age: Quantifying brain and cognitive maintenance in aging. Hum. Brain Mapp. 2021, 42, 1626–1640. [Google Scholar] [CrossRef]
- Edmonds, E.C.; Delano-Wood, L.; Galasko, D.R.; Salmon, D.P.; Bondi, M.W. Subjective Cognitive Complaints Contribute to Misdiagnosis of Mild Cognitive Impairment. J. Int. Neuropsychol. Soc. 2014, 20, 836–847. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, E.C.; Delano-Wood, L.; Clark, L.R.; Jak, A.J.; Nation, D.A.; McDonald, C.R.; Libon, D.J.; Au, R.; Galasko, D.; Salmon, D.P.; et al. Susceptibility of the conventional criteria for mild cognitive impairment to false-positive diagnostic errors. Alzheimer’s Dement. 2015, 11, 415–424. [Google Scholar] [CrossRef] [Green Version]
- Edmonds, E.C.; Eppig, J.; Bondi, M.W.; Leyden, K.M.; Goodwin, B.; Delano-Wood, L.; McDonald, C.R.; Alzheimer’s Disease Neuroimaging Initiative. Heterogeneous cortical atrophy patterns in MCI not captured by conventional diagnostic criteria. Neurology 2016, 87, 2108–2116. [Google Scholar] [CrossRef] [Green Version]
- Vuoksimaa, E.; McEvoy, L.K.; Holland, D.; Franz, C.E.; Kremen, W.S.; Alzheimer’s Disease Neuroimaging Initiative. Modifying the minimum criteria for diagnosing amnestic MCI to improve prediction of brain atrophy and progression to Alzheimer’s disease. Brain Imaging Behav. 2020, 14, 787–796. [Google Scholar] [CrossRef] [Green Version]
- Hölzel, B.K.; Carmody, J.; Vangel, M.; Congleton, C.; Yerramsetti, S.M.; Gard, T.; Lazar, S. Mindfulness practice leads to increases in regional brain gray matter density. Psychiatry Res. 2011, 191, 36–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, S.B.; Aslan, S.; Spence, J.S.; Keebler, M.W.; Defina, L.F.; Didehbani, N.; Perez, A.M.; Lu, H.; D’Esposito, M. Distinct Brain and Behavioral Benefits from Cognitive vs. Physical Training: A Randomized Trial in Aging Adults. Front. Hum. Neurosci. 2016, 10, 338. [Google Scholar] [CrossRef] [Green Version]
- Chalermpalanupap, T.; Kinkead, B.; Hu, W.T.; Kummer, M.P.; Hammerschmidt, T.; Heneka, M.T.; Weinshenker, D.; Levey, A.I. Targeting norepinephrine in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res. Ther. 2013, 5, 21–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, C.; Fahimi, A.; Das, D.; Mojabi, F.S.; Ponnusamy, R.; Salehi, A. Noradrenergic System in Down Syndrome and Alzheimer’s Disease A Target for Therapy. Curr. Alzheimer Res. 2015, 13, 68–83. [Google Scholar] [CrossRef] [PubMed]
- Dockree, P.M.; Barnes, J.J.; Matthews, N.; Dean, A.; Abe, R.; Nandam, L.S.; Kelly, S.P.; Bellgrove, M.A.; O’Connell, R.G. The Effects of Methylphenidate on the Neu-ral Signatures of Sustained Attention. Biol. Psychiatry 2017, 82, 687–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Segal, S.K.; Cotman, C.W.; Cahill, L.F. Exercise-Induced Noradrenergic Activation Enhances Memory Consolidation in Both Normal Aging and Patients with Amnestic Mild Cognitive Impairment. J. Alzheimer’s Dis. 2012, 32, 1011–1018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, P.; Pacholko, A.G.; Palaschuk, J.; Bekar, L.K. The locus coeruleus neurotoxin, DSP4, and/or a high sugar diet induce behavioral and biochemical alterations in wild-type mice consistent with Alzheimers related pathology. Metab. Brain Dis. 2018, 33, 1563–1571. [Google Scholar] [CrossRef]
- Pacholko, A.G.; Wotton, C.A.; Bekar, L.K. Poor Diet, Stress, and Inactivity Converge to Form a “Perfect Storm” That Drives Alzheimer’s Disease Pathogenesis. Neurodegener. Dis. 2019, 19, 60–77. [Google Scholar] [CrossRef]
- Engelborghs, S.; Gilles, C.; Ivanoiu, A.; Vandewoude, M. Rationale and clinical data supporting nutritional intervention in Alzheimer’s disease. Acta Clin. Belg. 2014, 69, 17–24. [Google Scholar] [CrossRef]
- Seidl, S.E.; Santiago, J.A.; Bilyk, H.; Potashkin, J.A. The emerging role of nutrition in Parkinson’s disease. Front. Aging Neurosci. 2014, 6, 36. [Google Scholar] [CrossRef] [Green Version]
- Scarmeas, N.; Luchsinger, J.A.; Mayeux, R.; Stern, Y. Mediterranean diet and Alzheimer disease mortality. Neurology 2007, 69, 1084–1093. [Google Scholar] [CrossRef] [Green Version]
- Barnard, N.D.; Bush, A.; Ceccarelli, A.; Cooper, J.; de Jager, C.A.; Erickson, K.I.; Fraser, G.; Kesler, S.; Levin, S.M.; Lucey, B.; et al. Dietary and lifestyle guidelines for the prevention of Alzheimer’s disease. Neurobiol. Aging 2014, 35, S74–S78. [Google Scholar] [CrossRef] [Green Version]
- Poly, C.; Massaro, J.; Seshadri, S.; Wolf, P.A.; Cho, E.; Krall, E.; Jacques, P.F.; Au, R. The relation of dietary choline to cognitive performance and white-matter hyperintensity in the Framingham Offspring Cohort. Am. J. Clin. Nutr. 2011, 94, 1584–1591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ylilauri, M.P.T.; Voutilainen, S.; Lönnroos, E.; Virtanen, H.E.K.; Tuomainen, T.-P.; Salonen, J.T.; Virtanen, J.K. Associations of dietary choline intake with risk of incident dementia and with cognitive performance: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2019, 110, 1416–1423. [Google Scholar] [CrossRef] [PubMed]
- Spiers, P.A.; Myers, D.; Hochanadel, G.S.; Lieberman, H.R.; Wurtman, R.J. Citicoline Improves Verbal Memory in Aging. Arch. Neurol. 1996, 53, 441–448. [Google Scholar] [CrossRef]
- Kühn, S.; Düzel, S.; Colzato, L.; Norman, K.; Gallinat, J.; Brandmaier, A.M.; Lindenberger, U.; Widaman, K.F. Food for thought: Association between dietary tyrosine and cognitive performance in younger and older adults. Psychol. Res. 2019, 83, 1097–1106. [Google Scholar] [CrossRef] [Green Version]
- Aliev, G.; Shahida, K.; Gan, S.H.; Firoz, C.; Khan, A.; Abuzenadah, A.; Kamal, W.; Kamal, M.; Tan, Y.; Qu, X.; et al. Alzheimer Disease and Type 2 Diabetes Mellitus: The Link to Tyrosine Hydroxylase and Probable Nutritional Strategies. CNS Neurol. Disord. Drug Targets 2014, 13, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Forssell, L.G.; Eklöf, R.; Winblad, B.; Forssell, L. Early Stages of Late Onset Alzheimer’s Disease. Acta Neurol. Scand. 1989, 79, 27–42. [Google Scholar] [CrossRef]
- Rasmussen, D.D.; Yen, S.S.C.; Ishizuka, B.; Quigley, M.E. Effects of Tyrosine and Tryptophan Ingestion on Plasma Catecholamine and 3,4-Dihydroxyphenylacetic Acid Concentrations. J. Clin. Endocrinol. Metab. 1983, 57, 760–763. [Google Scholar] [CrossRef]
- Agharanya, J.C.; Alonso, R.; Wurtman, R.J. Changes in catecholamine excretion after short-term tyrosine ingestion in normally fed human subjects. Am. J. Clin. Nutr. 1981, 34, 82–87. [Google Scholar] [CrossRef] [Green Version]
- Mahoney, C.R.; Castellani, J.; Kramer, F.M.; Young, A.; Lieberman, H.R. Tyrosine supplementation mitigates working memory decrements during cold exposure. Physiol. Behav. 2007, 92, 575–582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, J.R.; Lockwood, P.A.; Singh, A.; Deuster, P.A. Tyrosine Improves Working Memory in a Multitasking Environment. Pharmacol. Biochem. Behav. 1999, 64, 495–500. [Google Scholar] [CrossRef]
- Deijen, J.; Orlebeke, J. Effect of tyrosine on cognitive function and blood pressure under stress. Brain Res. Bull. 1994, 33, 319–323. [Google Scholar] [CrossRef]
- Deijen, J.; Wientjes, C.; Vullinghs, H.; Cloin, P.; Langefeld, J. Tyrosine improves cognitive performance and reduces blood pressure in cadets after one week of a combat training course. Brain Res. Bull. 1999, 48, 203–209. [Google Scholar] [CrossRef]
- Lieberman, H.R.; Corkin, S.; Spring, B.; Wurtman, R.J.; Growdon, J.H. The effects of dietary neurotransmitter precursors on human behavior. Am. J. Clin. Nutr. 1985, 42, 366–370. [Google Scholar] [CrossRef] [Green Version]
- Melnychuk, M.C.; Dockree, P.M.; O’Connell, R.; Murphy, P.R.; Balsters, J.H.; Robertson, I.H. Coupling of respiration and attention via the locus coeruleus: Effects of meditation and pranayama. Psychophysiology 2018, 55, e13091. [Google Scholar] [CrossRef] [PubMed]
- Craigmyle, N.A. The beneficial effects of meditation: Contribution of the anterior cingulate and locus coeruleus. Front. Psychol. 2013, 4, 731. [Google Scholar] [CrossRef] [Green Version]
- Luders, E.; Toga, A.W.; Lepore, N.; Gaser, C. The underlying anatomical correlates of long-term meditation: Larger hippocampal and frontal volumes of gray matter. NeuroImage 2009, 45, 672–678. [Google Scholar] [CrossRef] [Green Version]
- Vestergaard-Poulsen, P.; van Beek, M.; Skewes, J.; Bjarkam, C.; Stubberup, M.; Bertelsen, J.; Roepstorff, A. Long-term meditation is associated with increased gray matter density in the brain stem. Neuroreport 2009, 20, 170–174. [Google Scholar] [CrossRef]
- Singleton, O.; Hölzel, B.K.; Vangel, M.; Brach, N.; Carmody, J.; Lazar, S.W. Change in Brainstem Gray Matter Concentration Following a Mindfulness-Based Intervention is Correlated with Improvement in Psychological Well-Being. Front. Hum. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, K.C.; Nijeboer, S.; Dixon, M.L.; Floman, J.L.; Ellamil, M.; Rumak, S.P.; Christoff, K. Is meditation associ-ated with altered brain structure? A systematic review and meta-analysis of morphometric neuroimaging in meditation prac-titioners. Neurosci. Biobehav. Rev. 2014, 43, 48–73. [Google Scholar] [CrossRef]
- Tang, Y.-Y.; Lu, Q.; Geng, X.; Stein, E.A.; Yang, Y.; Posner, M.I. Short-term meditation induces white matter changes in the anterior cingulate. Proc. Natl. Acad. Sci. USA 2010, 107, 15649–15652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, R.; Friston, K.J.; Tang, Y.-Y. Brief Mindfulness Meditation Induces Gray Matter Changes in a Brain Hub. Neural Plast. 2020, 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]





| Sociodemographic | Neural Indices | Neuropsychological | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gender | Age *** | Education *** | Occupation * | TIV | BrainPAD *** | TMT-A *** | ||||||||
| Groups | M | F | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD |
| Ranges | min | max | min | max | min | max | min | max | min | max | min | max | ||
| HC (n = 395) | 168 | 227 | 73.45 | 7.21 | 16.96 | 2.21 | 6.21 | 1.72 | 1408.16 | 146.28 | 1.59 | 7.69 | 30.64 | 8.85 |
| 56 | 95 | 11 | 20 | 2 | 8 | 1082 | 1844 | −22.01 | 37.46 | 13 | 63 | |||
| MCI (n = 156) | 90 | 66 | 75.51 | 8.10 | 15.98 | 2.76 | 5.78 | 1.85 | 1426.12 | 142.78 | 5.45 | 9.00 | 39.07 | 17.86 |
| 55 | 97 | 8 | 20 | 2 | 8 | 1060 | 1884 | −21.23 | 26.21 | 18 | 150 | |||
| AD (n = 135) | 74 | 61 | 76.61 | 8.43 | 15.64 | 2.54 | 5.93 | 1.84 | 1385.79 | 167.47 | 13.32 | 9.13 | 61.61 | 36.59 |
| 55 | 95 | 11 | 20 | 2 | 8 | 1046 | 1785 | −6.46 | 42.33 | 19 | 150 | |||
| Locus Coeruleus | Dorsal Raphe | Median Raphe | Ventral Tegmental Area | Nucleus Basalis of Meynert | Control Pontine ROI | ||
|---|---|---|---|---|---|---|---|
| HC (n = 395) | |||||||
| BrainPAD | Pearson’s r | −0.197 *** | −0.292 *** | −0.107 | −0.074 | −0.107 | −0.109 |
| BF10 | 142.324 | 22.466 | 0.608 | 0.185 | 0.599 | 0.643 | |
| TMT-A | Pearson’s r | −0.082 | −0.158 | −0.025 | −0.045 | 0.085 | −0.042 |
| BF10 | 0.236 | 9.117 | 0.071 | 0.094 | 0.257 | 0.089 | |
| MCI (n = 156) | |||||||
| BrainPAD | Pearson’s r | −0.385 *** | −0.321 *** | −0.039 | 0.147 | −0.318 *** | 0.007 |
| BF10 | 19303.214 | 399.634 | 0.113 | 0.523 | 339.646 | 0.101 | |
| TMT-A | Pearson’s r | −0.326 *** | −0.219 | 0.001 | 0.019 | 0.029 | 0.007 |
| BF10 | 517.357 | 4.219 | 0.100 | 0.103 | 0.107 | 0.101 | |
| AD (n = 135) | |||||||
| BrainPAD | Pearson’s r | −0.297 ** | −0.249 | −0.217 | −0.305 | −0.132 | 0.100 |
| BF10 | 46.538 | 7.031 | 2.541 | 4.657 | 0.340 | 0.209 | |
| TMT-A | Pearson’s r | −0.384 *** | −0.119 | −0.041 | −0.319 *** | −0.315 *** | −0.5224 |
| BF10 | 3640.710 | 0.272 | 0.120 | 124.076 | 102.490 | 0.108 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Plini, E.R.G.; O’Hanlon, E.; Boyle, R.; Sibilia, F.; Rikhye, G.; Kenney, J.; Whelan, R.; Melnychuk, M.C.; Robertson, I.H.; Dockree, P.M. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells 2021, 10, 1829. https://doi.org/10.3390/cells10071829
Plini ERG, O’Hanlon E, Boyle R, Sibilia F, Rikhye G, Kenney J, Whelan R, Melnychuk MC, Robertson IH, Dockree PM. Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells. 2021; 10(7):1829. https://doi.org/10.3390/cells10071829
Chicago/Turabian StylePlini, Emanuele R. G., Erik O’Hanlon, Rory Boyle, Francesca Sibilia, Gaia Rikhye, Joanne Kenney, Robert Whelan, Michael C. Melnychuk, Ian H. Robertson, and Paul M. Dockree. 2021. "Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative" Cells 10, no. 7: 1829. https://doi.org/10.3390/cells10071829
APA StylePlini, E. R. G., O’Hanlon, E., Boyle, R., Sibilia, F., Rikhye, G., Kenney, J., Whelan, R., Melnychuk, M. C., Robertson, I. H., & Dockree, P. M. (2021). Examining the Role of the Noradrenergic Locus Coeruleus for Predicting Attention and Brain Maintenance in Healthy Old Age and Disease: An MRI Structural Study for the Alzheimer’s Disease Neuroimaging Initiative. Cells, 10(7), 1829. https://doi.org/10.3390/cells10071829