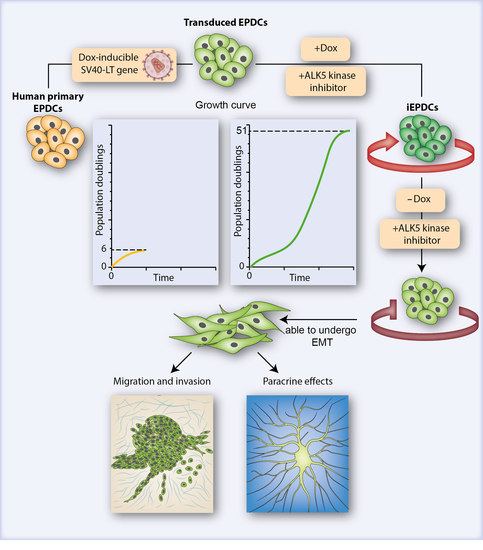
Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells
Abstract
:1. Introduction
2. Methods
2.1. LV Production
2.2. Isolation and Culture of Primary Human EPDCs
2.3. Transduction of Human EPDCs
2.4. Analysis of Cell Growth of Primary and Transduced EPDCs
2.5. Inducible LT Expression upon Dox Addition
2.6. EMT Assay
2.7. Scratch Wound Healing Assay
2.8. Invasion Assay
2.9. Co-Culture of Primary EPDCs and iEPDCs with Sympathetic Ganglia
2.10. Immunofluorescence Staining
2.11. Statistics
3. Results
3.1. Generation of iEPDCs
3.2. IEPDCs Undergo EMT and Show a Mesenchymal Phenotype upon TGFβ3 Stimulation

3.3. Proliferating iEPDCs Show a Reduced Propensity to Undergo EMT upon TGFβ3 Stimulation
3.4. Mesenchymal iEPDCs Show Robust Migration and Invasion Ability
3.5. Mesenchymal iEPDCs Promote Neurite Outgrowth from Sympathetic Ganglia
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, B.; Ma, Q.; Rajagopal, S.; Wu, S.M.; Domian, I.; Rivera-Feliciano, J.; Jiang, D.; von Gise, A.; Ikeda, S.; Chien, K.R.; et al. Epicardial progenitors contribute to the cardiomyocyte lineage in the developing heart. Nature 2008, 454, 109–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolditz, D.P.; Wijffels, M.C.; Blom, N.A.; van der Laarse, A.; Hahurij, N.D.; Lie-Venema, H.; Markwald, R.R.; Poelmann, R.E.; Schalij, M.J.; Gittenberger-de Groot, A.C. Epicardium-derived cells in development of annulus fibrosis and persistence of accessory pathways. Circulation 2008, 117, 1508–1517. [Google Scholar] [CrossRef] [Green Version]
- Gittenberger-de Groot, A.C.; Vrancken Peeters, M.P.; Mentink, M.M.; Gourdie, R.G.; Poelmann, R.E. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ. Res. 1998, 82, 1043–1052. [Google Scholar] [CrossRef] [Green Version]
- von Gise, A.; Pu, W.T. Endocardial and epicardial epithelial to mesenchymal transitions in heart development and disease. Circ. Res. 2012, 110, 1628–1645. [Google Scholar] [CrossRef] [PubMed]
- Smits, A.M.; Riley, P.R. Epicardium-derived heart repair. J. Dev. Biol. 2014, 2, 84–100. [Google Scholar] [CrossRef]
- Duan, J.; Gherghe, C.; Liu, D.; Hamlett, E.; Srikantha, L.; Rodgers, L.; Regan, J.N.; Rojas, M.; Willis, M.; Leask, A.; et al. Wnt1/βcatenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. EMBO J. 2012, 31, 429–442. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Wijk, B.; Gunst, Q.D.; Moorman, A.F.; van den Hoff, M.J. Cardiac regeneration from activated epicardium. PLoS ONE 2012, 7, e44692. [Google Scholar] [CrossRef] [Green Version]
- Zhou, B.; Honor, L.B.; He, H.; Ma, Q.; Oh, J.H.; Butterfield, C.; Lin, R.Z.; Melero-Martin, J.M.; Dolmatova, E.; Duffy, H.S.; et al. Adult mouse epicardium modulates myocardial injury by secreting paracrine factors. J. Clin. Investig. 2011, 121, 1894–1904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gittenberger-de Groot, A.C.; Winter, E.M.; Goumans, M.J.; Bartelings, M.M.; Poelmann, R.E. The arterial epicardium: A developmental approach to cardiac disease and repair. In Etiology and Morphogenesis of Congenital Heart Disease: From Gene Function and Cellular Interaction to Morphology; Nakanishi, T., Markwald, R.R., Baldwin, H.S., Keller, B.B., Srivastava, D., Yamagishi, H., Eds.; Springer: Tokyo, Japan, 2016; pp. 11–18. [Google Scholar]
- Quijada, P.; Trembley, M.A.; Small, E.M. The role of the epicardium during heart development and repair. Circ. Res. 2020, 126, 377–394. [Google Scholar] [CrossRef]
- Van Tuyn, J.; Atsma, D.E.; Winter, E.M.; van der Velde-van Dijke, I.; Pijnappels, D.A.; Bax, N.A.M.; Knaän-Shanzer, S.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van der Laarse, A.; et al. Epicardial cells of human adults can undergo an epithelial-to-mesenchymal transition and obtain characteristics of smooth muscle cells In Vitro. Stem Cells 2007, 25, 271–278. [Google Scholar] [CrossRef]
- Moerkamp, A.T.; Lodder, K.; van Herwaarden, T.; Dronkers, E.; Dingenouts, C.K.E.; Tengstrom, F.C.; van Brakel, T.J.; Goumans, M.J.; Smits, A.M. Human fetal and adult epicardial-derived cells: A novel model to study their activation. Stem Cell Res. Ther. 2016, 7, 174. [Google Scholar] [CrossRef] [Green Version]
- Dronkers, E.; Wauters, M.M.M.; Goumans, M.J.; Smits, A.M. Epicardial TGFβ and BMP signaling in cardiac regeneration: What lesson can we learn from the developing heart? Biomolecules 2020, 10, 404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, E.M.; Grauss, R.W.; Hogers, B.; van Tuyn, J.; van der Geest, R.; Lie-Venema, H.; Steijn, R.V.; Maas, S.; DeRuiter, M.C.; deVries, A.A.; et al. Preservation of left ventricular function and attenuation of remodeling after transplantation of human epicardium-derived cells into the infarcted mouse heart. Circulation 2007, 116, 917–927. [Google Scholar] [CrossRef] [Green Version]
- Smits, A.M.; Dronkers, E.; Goumans, M.-J. The epicardium as a source of multipotent adult cardiac progenitor cells: Their origin, role and fate. Pharmacol. Res. 2018, 127, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Smits, A.M.; van Munsteren, J.C.; Gittenberger-de Groot, A.C.; Poelmann, R.E.; van Brakel, T.J.; Schalij, M.J.; Goumans, M.J.; DeRuiter, M.C.; Jongbloed, M.R.M. Human epicardium-derived cells reinforce cardiac sympathetic innervation. J. Mol. Cell. Cardiol. 2020, 143, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Dronkers, E.; Moerkamp, A.T.; van Herwaarden, T.; Goumans, M.-J.; Smits, A.M. The isolation and culture of primary epicardial cells derived from human adult and fetal heart specimens. J. Vis. Exp. 2018, 134, 57370. [Google Scholar] [CrossRef]
- Jat, P.S.; Noble, M.D.; Ataliotis, P.; Tanaka, Y.; Yannoutsos, N.; Larsen, L.; Kioussis, D. Direct derivation of conditionally immortal cell lines from an H-2Kb-tsA58 transgenic mouse. Proc. Natl. Acad. Sci. USA 1991, 88, 5096–5100. [Google Scholar] [CrossRef] [Green Version]
- Austin, A.F.; Compton, L.A.; Love, J.D.; Barnett, J.V. Immortalized mouse epicardial cells undergo differentiation in response to Transforming Growth Factor-β. FASEB J. 2007, 21, A973. [Google Scholar] [CrossRef]
- Zhang, J.C.; Kim, S.; Helmke, B.P.; Yu, W.W.; Du, K.L.; Lu, M.M.; Strobeck, M.; Yu, Q.; Parmacek, M.S. Analysis of SM22alpha-deficient mice reveals unanticipated insights into smooth muscle cell differentiation and function. Mol. Cell. Biol. 2001, 21, 1336–1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Kuipers, E.N.; Sips, H.C.M.; Dorleijn, J.C.; van Dam, A.D.; Christodoulides, C.; Karpe, F.; Zhou, G.Q.; Boon, M.R.; Rensen, P.C.N.; et al. Conditionally immortalized brown preadipocytes can switch between proliferative and differentiated states. BBA-Mol. Cell Biol. Lipids 2019, 1864, 158511. [Google Scholar] [CrossRef]
- Liu, J.; Volkers, L.; Jangsangthong, W.; Bart, C.I.; Engels, M.C.; Zhou, G.; Schalij, M.J.; Ypey, D.L.; Pijnappels, D.A.; de Vries, A.A.F. Generation and primary characterization of iAM-1, a versatile new line of conditionally immortalized atrial myocytes with preserved cardiomyogenic differentiation capacity. Cardiovasc. Res. 2018, 114, 1848–1859. [Google Scholar] [CrossRef] [PubMed]
- Denning, W.; Das, S.; Guo, S.; Xu, J.; Kappes, J.C.; Hel, Z. Optimization of the transductional efficiency of lentiviral vectors: Effect of sera and polycations. Mol. Biotechnol. 2013, 53, 308–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.0031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hellemans, J.; Mortier, G.; De Paepe, A.; Speleman, F.; Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol. 2007, 8, R19. [Google Scholar] [CrossRef] [Green Version]
- Bax, N.A.; van Oorschot, A.A.; Maas, S.; Braun, J.; van Tuyn, J.; de Vries, A.A.; Groot, A.C.; Goumans, M.J. In Vitro epithelial-to-mesenchymal transformation in human adult epicardial cells is regulated by TGFβ-signaling and WT1. Basic Res. Cardiol. 2011, 106, 829–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Gise, A.; Zhou, B.; Honor, L.B.; Ma, Q.; Petryk, A.; Pu, W.T. WT1 regulates epicardial epithelial to mesenchymal transition through β-catenin and retinoic acid signaling pathways. Dev. Biol. 2011, 356, 421–431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Estrada, O.M.; Lettice, L.A.; Essafi, A.; Guadix, J.A.; Slight, J.; Velecela, V.; Hall, E.; Reichmann, J.; Devenney, P.S.; Hohenstein, P.; et al. Wt1 is required for cardiovascular progenitor cell formation through transcriptional control of Snail and E-cadherin. Nat. Genet. 2010, 42, 89–93. [Google Scholar] [CrossRef] [Green Version]
- Velecela, V.; Torres-Cano, A.; Garcia-Melero, A.; Ramiro-Pareta, M.; Muller-Sanchez, C.; Segarra-Mondejar, M.; Chau, Y.Y.; Campos-Bonilla, B.; Reina, M.; Soriano, F.X.; et al. Epicardial cell shape and maturation are regulated by Wt1 via transcriptional control of Bmp4. Development 2019, 146, dev178723. [Google Scholar] [CrossRef] [Green Version]
- Bochmann, L.; Sarathchandra, P.; Mori, F.; Lara-Pezzi, E.; Lazzaro, D.; Rosenthal, N. Revealing new mouse epicardial cell markers through transcriptomics. PLoS ONE 2010, 5, e11429. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, L.; McManus, S.A.; Moignard, V.; Sebukhan, D.; Delaune, A.; Andrews, S.; Bernard, W.G.; Morrison, M.A.; Riley, P.R.; Gottgens, B.; et al. BNC1 regulates cell heterogeneity in human pluripotent stem cell-derived epicardium. Development 2019, 146, dev174441. [Google Scholar] [CrossRef] [Green Version]
- Moss, J.B.; Xavier-Neto, J.; Shapiro, M.D.; Nayeem, S.M.; McCaffery, P.; Drager, U.C.; Rosenthal, N. Dynamic patterns of retinoic acid synthesis and response in the developing mammalian heart. Dev. Biol. 1998, 199, 55–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xavier-Neto, J.; Shapiro, M.D.; Houghton, L.; Rosenthal, N. Sequential programs of retinoic acid synthesis in the myocardial and epicardial layers of the developing avian heart. Dev. Biol. 2000, 219, 129–141. [Google Scholar] [CrossRef] [Green Version]
- Wilm, B.; Ipenberg, A.; Hastie, N.D.; Burch, J.B.; Bader, D.M. The serosal mesothelium is a major source of smooth muscle cells of the gut vasculature. Development 2005, 132, 5317–5328. [Google Scholar] [CrossRef] [Green Version]
- Smith, C.L.; Baek, S.T.; Sung, C.Y.; Tallquist, M.D. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require PDGF receptor signaling. Circ. Res. 2011, 108, e15–e26. [Google Scholar] [CrossRef]
- Katz, T.C.; Singh, M.K.; Degenhardt, K.; Rivera-Feliciano, J.; Johnson, R.L.; Epstein, J.A.; Tabin, C.J. Distinct compartments of the proepicardial organ give rise to coronary vascular endothelial cells. Dev. Cell 2012, 22, 639–650. [Google Scholar] [CrossRef] [Green Version]
- Compton, L.A.; Potash, D.A.; Mundell, N.A.; Barnett, J.V. Transforming growth factor-beta induces loss of epithelial character and smooth muscle cell differentiation in epicardial cells. Dev. Dyn. Off. Publ. Am. Assoc. Anat. 2006, 235, 82–93. [Google Scholar] [CrossRef]
- Saifi, O.; Ghandour, B.; Jaalouk, D.; Refaat, M.; Mahfouz, R. Myocardial regeneration: Role of epicardium and implicated genes. Mol. Biol. Rep. 2019, 46, 6661–6674. [Google Scholar] [CrossRef] [PubMed]
- Maqsood, M.I.; Matin, M.M.; Bahrami, A.R.; Ghasroldasht, M.M. Immortality of cell lines: Challenges and advantages of establishment. Cell Biol. Int. 2013, 37, 1038–1045. [Google Scholar] [CrossRef] [PubMed]
- Wall, I.; Toledo, G.S.; Jat, P. Recent advances in conditional cell immortalization technology. Cell Gene Ther. Insights 2016, 2, 339–355. [Google Scholar] [CrossRef]
- Stepanenko, A.A.; Kavsan, V.M. Immortalization and malignant transformation of Eukaryotic cells. Cytol. Genet. 2012, 46, 96–129. [Google Scholar] [CrossRef]
- Rahman, N.A.; Rasil, A.N.H.M.; Meyding-Lamade, U.; Craemer, E.M.; Diah, S.; Tuah, A.A.; Muharram, S.H. Immortalized endothelial cell lines for in vitro blood-brain barrier models: A systematic review. Brain Res. 2016, 1642, 532–545. [Google Scholar] [CrossRef]
- Ramboer, E.; De Craene, B.; De Kock, J.; Vanhaecke, T.; Berx, G.; Rogiers, V.; Vinken, M. Strategies for immortalization of primary hepatocytes. J. Hepatol. 2014, 61, 925–943. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Shay, J.W.; Minna, J.D. Immortalized normal human lung epithelial cell models for studying lung cancer biology. Respir. Investig. 2020, 58, 344–354. [Google Scholar] [CrossRef]
- Choi, M.; Lee, C. Immortalization of primary keratinocytes and its application to skin research. Biomol. Ther. 2015, 23, 391–399. [Google Scholar] [CrossRef] [Green Version]
- Ke, Y.; Reddel, R.R.; Gerwin, B.I.; Reddel, H.K.; Somers, A.N.; McMenamin, M.G.; LaVeck, M.A.; Stahel, R.A.; Lechner, J.F.; Harris, C.C. Establishment of a human in vitro mesothelial cell model system for investigating mechanisms of asbestos-induced mesothelioma. Am. J. Pathol. 1989, 134, 979–991. [Google Scholar] [PubMed]
- Fischereder, M.; Luckow, B.; Sitter, T.; Schroppel, B.; Banas, B.; Schlondorff, D. Immortalization and characterization of human peritoneal mesothelial cells. Kidney Int. 1997, 51, 2006–2012. [Google Scholar] [CrossRef] [Green Version]
- Pruett, N.; Singh, A.; Shankar, A.; Schrump, D.S.; Hoang, C.D. Normal mesothelial cell lines newly derived from human pleural biopsy explants. Am. J. Physiol. Lung Cell. 2020, 319, L652–L660. [Google Scholar] [CrossRef] [PubMed]
- Lamouille, S.; Xu, J.; Derynck, R. Molecular mechanisms of epithelial-mesenchymal transition. Nat. Rev. Mol. Cell Biol. 2014, 15, 178–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrero, M.J.; Boue, S.; Belmonte, J.C.I. Epigenetic mechanisms that regulate cell identity. Cell Stem Cell 2010, 7, 565–570. [Google Scholar] [CrossRef] [Green Version]
- Thiagalingam, S. Epigenetic memory in development and disease: Unraveling the mechanism. BBA Rev. Cancer 2020, 1873, 188349. [Google Scholar] [CrossRef] [PubMed]
- Li, V.C.; Kirschner, M.W. Molecular ties between the cell cycle and differentiation in embryonic stem cells. Proc. Natl. Acad. Sci. USA 2014, 111, 9503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, G.; Hughes, P.J.; Michell, R.H. Cell differentiation and proliferation—Simultaneous but independent? Exp. Cell Res. 2003, 291, 282–288. [Google Scholar] [CrossRef]
- Hardwick, L.J.A.; Ali, F.R.; Azzarelli, R.; Philpott, A. Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res. 2015, 359, 187–200. [Google Scholar] [CrossRef] [Green Version]
- Dong, C.Y.; Zhang, J.W.; Fang, S.; Liu, F.S. IGFBP5 increases cell invasion and inhibits cell proliferation by EMT and Akt signaling pathway in Glioblastoma multiforme cells. Cell Div. 2020, 15, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Buel, G.R.; Nagiec, M.J.; Han, M.J.; Roux, P.P.; Blenis, J.; Yoon, S.O. ERK2 regulates epithelial-to-mesenchymal plasticity through DOCK10-dependent Rac1/FoxO1 activation. Proc. Natl. Acad. Sci. USA 2019, 116, 2967–2976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Testa, J.R.; Berns, A. Preclinical models of malignant mesothelioma. Front. Oncol. 2020, 10, 101. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ge, Y.; Smits, A.M.; Liu, J.; Zhang, J.; van Brakel, T.J.; Goumans, M.J.T.H.; Jongbloed, M.R.M.; de Vries, A.A.F. Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells. Cells 2021, 10, 2064. https://doi.org/10.3390/cells10082064
Ge Y, Smits AM, Liu J, Zhang J, van Brakel TJ, Goumans MJTH, Jongbloed MRM, de Vries AAF. Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells. Cells. 2021; 10(8):2064. https://doi.org/10.3390/cells10082064
Chicago/Turabian StyleGe, Yang, Anke M. Smits, Jia Liu, Juan Zhang, Thomas J. van Brakel, Marie José T. H. Goumans, Monique R. M. Jongbloed, and Antoine A. F. de Vries. 2021. "Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells" Cells 10, no. 8: 2064. https://doi.org/10.3390/cells10082064
APA StyleGe, Y., Smits, A. M., Liu, J., Zhang, J., van Brakel, T. J., Goumans, M. J. T. H., Jongbloed, M. R. M., & de Vries, A. A. F. (2021). Generation, Characterization, and Application of Inducible Proliferative Adult Human Epicardium-Derived Cells. Cells, 10(8), 2064. https://doi.org/10.3390/cells10082064