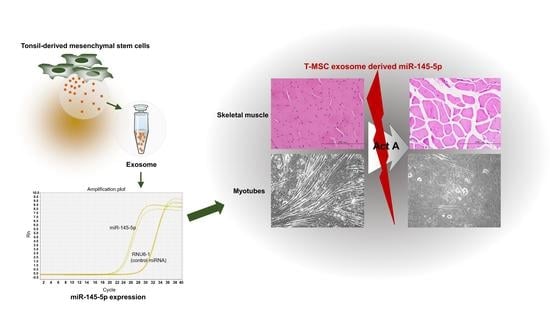
Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Cell Culture
2.3. Isolation of Exosomes from T-MSCs
2.4. Scanning Electron Microscopy (SEM)
2.5. Transmission Electron Microscopy (TEM)
2.6. Immunoblotting of Exosome Markers
2.7. Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
2.8. Prediction of Target Genes
2.9. MTT Assay
2.10. Transfection
2.11. Immunofluorescence Staining
2.12. Chemotherapy in Mice
2.13. Histology
2.14. ELISA
2.15. Statistics
3. Results
3.1. T-MSC Exosomes Recovered Weight and Muscle Loss in Bu-Cy Treated Mice
3.2. Activin A Disturbs Myotube Differentiation
3.3. T-MSC Exosomes Highly Express Activin A Receptor Targeting has-miR-145-5p
3.4. T-MSC Exosomes Restored Muscular Differentiation Impairment Induced by Activin A
3.5. T-MSC Exosomes Regulate Muscular Response to Activin A via has-miR-145-5p Activity
3.6. T-MSC Exosomes Rescue Body Weight Loss and Skeletal Muscle Loss via has-miR-145-5p Activity In Vivo
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dantzer, R.; Meagher, M.W.; Cleeland, C.S. Translational approaches to treatment-induced symptoms in cancer patients. Nat. Rev. Clin. Oncol. 2012, 9, 414–426. [Google Scholar] [CrossRef] [Green Version]
- Baracos, V.E.; Mazurak, V.C.; Bhullar, A.S. Cancer cachexia is defined by an ongoing loss of skeletal muscle mass. Ann. Palliat. Med. 2019, 8, 3–12. [Google Scholar] [CrossRef]
- Guigni, B.A.; Callahan, D.M.; Tourville, T.W.; Miller, M.S.; Fiske, B.; Voigt, T.; Korwin-Mihavics, B.; Anathy, V.; Dittus, K.; Toth, M.J. Skeletal muscle atrophy and dysfunction in breast cancer patients: Role for chemotherapy-derived oxidant stress. Am. J. Physiol. Cell Physiol. 2018, 315, C744–C756. [Google Scholar] [CrossRef]
- Coletti, D. Chemotherapy-induced muscle wasting: An update. Eur. J. Transl. Myol. 2018, 28, 7587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, K.V.; Chen, J.D.; Wu, W.T.; Huang, K.C.; Hsu, C.T.; Han, D.S. Association between Loss of Skeletal Muscle Mass and Mortality and Tumor Recurrence in Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Liver Cancer 2018, 7, 90–103. [Google Scholar] [CrossRef]
- Reisinger, K.W.; Bosmans, J.W.; Uittenbogaart, M.; Alsoumali, A.; Poeze, M.; Sosef, M.N.; Derikx, J.P. Loss of Skeletal Muscle Mass During Neoadjuvant Chemoradiotherapy Predicts Postoperative Mortality in Esophageal Cancer Surgery. Ann. Surg. Oncol. 2015, 22, 4445–4452. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ma, J.; Li, L.; Zhu, X.D. Severe muscle loss during radical chemoradiotherapy for non-metastatic nasopharyngeal carcinoma predicts poor survival. Cancer Med. 2019, 8, 6604–6613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Latres, E.; Mastaitis, J.; Fury, W.; Miloscio, L.; Trejos, J.; Pangilinan, J.; Okamoto, H.; Cavino, K.; Na, E.; Papatheodorou, A.; et al. Activin A more prominently regulates muscle mass in primates than does GDF8. Nat. Commun. 2017, 8, 15153. [Google Scholar] [CrossRef] [Green Version]
- Attisano, L.; Wrana, J.L.; Montalvo, E.; Massague, J. Activation of signalling by the activin receptor complex. Mol. Cell. Biol. 1996, 16, 1066–1073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sartori, R.; Milan, G.; Patron, M.; Mammucari, C.; Blaauw, B.; Abraham, R.; Sandri, M. Smad2 and 3 transcription factors control muscle mass in adulthood. Am. J. Physiol. Cell Physiol. 2009, 296, C1248–C1257. [Google Scholar] [CrossRef] [Green Version]
- Paajanen, J.; Ilonen, I.; Lauri, H.; Jarvinen, T.; Sutinen, E.; Ollila, H.; Rouvinen, E.; Lemstrom, K.; Rasanen, J.; Ritvos, O.; et al. Elevated Circulating Activin A Levels in Patients With Malignant Pleural Mesothelioma Are Related to Cancer Cachexia and Reduced Response to Platinum-based Chemotherapy. Clin. Lung Cancer 2020, 21, e142–e150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressel, D.; Ritter, C.A.; Sperker, B.; Grube, M.; Maier, T.; Klingebiel, T.; Siegmund, W.; Beck, J.F.; Kroemer, H.K. Busulfan induces activin A expression in vitro and in vivo: A possible link to venous occlusive disease. Clin. Pharmacol. Ther. 2003, 74, 264–274. [Google Scholar] [CrossRef]
- Choi, D.W.; Cho, K.A.; Lee, H.J.; Kim, Y.H.; Woo, K.J.; Park, J.W.; Ryu, K.H.; Woo, S.Y. Cotransplantation of tonsilderived mesenchymal stromal cells in bone marrow transplantation promotes thymus regeneration and T cell diversity following cytotoxic conditioning. Int. J. Mol. Med. 2020, 46, 1166–1174. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Cho, K.A.; Lee, H.J.; Park, M.; Shin, S.J.; Park, J.W.; Woo, S.Y.; Ryu, K.H. Conditioned Medium from Human Tonsil-Derived Mesenchymal Stem Cells Enhances Bone Marrow Engraftment via Endothelial Cell Restoration by Pleiotrophin. Cells 2020, 9, 221. [Google Scholar] [CrossRef] [Green Version]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Vainshtein, A.; Sandri, M. Signaling Pathways That Control Muscle Mass. Int. J. Mol. Sci. 2020, 21, 4759. [Google Scholar] [CrossRef]
- Han, H.Q.; Zhou, X.; Mitch, W.E.; Goldberg, A.L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 2013, 45, 2333–2347. [Google Scholar] [CrossRef]
- Zhou, G.; Gui, X.; Chen, R.; Fu, X.; Ji, X.; Ding, H. Elevated serum Activin A in chronic obstructive pulmonary disease with skeletal muscle wasting. Clinics 2019, 74, e981. [Google Scholar] [CrossRef]
- Matzuk, M.M.; Finegold, M.J.; Mather, J.P.; Krummen, L.; Lu, H.; Bradley, A. Development of cancer cachexia-like syndrome and adrenal tumors in inhibin-deficient mice. Proc. Natl. Acad. Sci. USA 1994, 91, 8817–8821. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.L.; Walton, K.L.; Winbanks, C.E.; Murphy, K.T.; Thomson, R.E.; Makanji, Y.; Qian, H.; Lynch, G.S.; Harrison, C.A.; Gregorevic, P. Elevated expression of activins promotes muscle wasting and cachexia. FASEB J. 2014, 28, 1711–1723. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, J.L.; Lu, J.; Song, Y.; Kwak, K.S.; Jiao, Q.; Rosenfeld, R.; Chen, Q.; Boone, T.; Simonet, W.S.; et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 2010, 142, 531–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loumaye, A.; de Barsy, M.; Nachit, M.; Lause, P.; Frateur, L.; van Maanen, A.; Trefois, P.; Gruson, D.; Thissen, J.P. Role of Activin A and myostatin in human cancer cachexia. J. Clin. Endocrinol. Metab. 2015, 100, 2030–2038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anastasilakis, A.D.; Polyzos, S.A.; Makras, P.; Gkiomisi, A.; Savvides, M.; Papatheodorou, A.; Terpos, E. Circulating activin-A is elevated in postmenopausal women with low bone mass: The three-month effect of zoledronic acid treatment. Osteoporos. Int. 2013, 24, 2127–2132. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.J.; Zhang, X.; Kumar, A.; Atkinson, E.J.; Zhu, Y.; Jachim, S.; Mazula, D.L.; Brown, A.K.; Berning, M.; Aversa, Z.; et al. The senescence-associated secretome as an indicator of age and medical risk. JCI Insight 2020, 5, e133668. [Google Scholar] [CrossRef]
- Bodine, S.C.; Latres, E.; Baumhueter, S.; Lai, V.K.; Nunez, L.; Clarke, B.A.; Poueymirou, W.T.; Panaro, F.J.; Na, E.; Dharmarajan, K.; et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 2001, 294, 1704–1708. [Google Scholar] [CrossRef]
- Gomes, M.D.; Lecker, S.H.; Jagoe, R.T.; Navon, A.; Goldberg, A.L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl. Acad. Sci. USA 2001, 98, 14440–14445. [Google Scholar] [CrossRef] [Green Version]
- Bodine, S.C.; Baehr, L.M. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am. J. Physiol. Endocrinol. Metab. 2014, 307, E469–E484. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Choi, Y.; Jung, N.; Yu, Y.; Ryu, K.H.; Kim, H.S.; Jo, I.; Choi, B.O.; Jung, S.C. Myogenic differentiation potential of human tonsil-derived mesenchymal stem cells and their potential for use to promote skeletal muscle regeneration. Int. J. Mol. Med. 2016, 37, 1209–1220. [Google Scholar] [CrossRef] [Green Version]
- Park, S.; Choi, Y.; Kwak, G.; Hong, Y.B.; Jung, N.; Kim, J.; Choi, B.O.; Jung, S.C. Application of differentiated human tonsil-derived stem cells to trembler-J mice. Muscle Nerve 2018, 57, 478–486. [Google Scholar] [CrossRef]
- Volarevic, V.; Markovic, B.S.; Gazdic, M.; Volarevic, A.; Jovicic, N.; Arsenijevic, N.; Armstrong, L.; Djonov, V.; Lako, M.; Stojkovic, M. Ethical and Safety Issues of Stem Cell-Based Therapy. Int. J. Med. Sci. 2018, 15, 36–45. [Google Scholar] [CrossRef] [Green Version]
- Gazdic, M.; Volarevic, V.; Arsenijevic, N.; Stojkovic, M. Mesenchymal stem cells: A friend or foe in immune-mediated diseases. Stem. Cell Rev. Rep. 2015, 11, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Zhang, B.; Shi, H.; Qian, H.; Xu, W. MSC-exosome: A novel cell-free therapy for cutaneous regeneration. Cytotherapy 2018, 20, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef] [Green Version]
- Raposo, G.; Stoorvogel, W. Extracellular vesicles: Exosomes, microvesicles, and friends. J. Cell Biol. 2013, 200, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Locci, M.; Wu, J.E.; Arumemi, F.; Mikulski, Z.; Dahlberg, C.; Miller, A.T.; Crotty, S. Activin A programs the differentiation of human TFH cells. Nat. Immunol. 2016, 17, 976–984. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Martinez, G.; Molina-Hernandez, A.; Velasco, I. Activin A promotes neuronal differentiation of cerebrocortical neural progenitor cells. PLoS ONE 2012, 7, e43797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrigo, J.; Rivera, J.C.; Aravena, J.; Cabrera, D.; Simon, F.; Ezquer, F.; Ezquer, M.; Cabello-Verrugio, C. High Fat Diet-Induced Skeletal Muscle Wasting Is Decreased by Mesenchymal Stem Cells Administration: Implications on Oxidative Stress, Ubiquitin Proteasome Pathway Activation, and Myonuclear Apoptosis. Oxid. Med. Cell. Longev. 2016, 2016, 9047821. [Google Scholar] [CrossRef] [Green Version]






| Primers | Sequences | Product Size (bp) |
|---|---|---|
| MyoD | F: 5′- CCACTCCGGGACATAGACTTG -3′ R: 5′- AAAAGCGCAGGTCTGGTGAG -3′ | 109 |
| Myh1 | F: 5′- GCGAATCGAGGCTCAGAACAA -3′ R: 5′- GTAGTTCCGCCTTCGGTCTTG -3′ | 138 |
| Acvr2a | F: 5′- ATAAACGGCGACATTGTTTTGC -3′ R: 5′- TCGGTGTAACAGGATTTGAAGTG -3′ | 234 |
| Acvr1b | F: 5′- CTGCCTACAGACCAACTACACC -3′ R: 5′- GCAGAAGTCAATATAGCAGCAGT -3′ | 190 |
| Murf1 | F: 5′- GTGTGAGGTGCCTACTTGCTC -3′ R: 5′- TGAGAGATGATCGTCTGCACT -3′ | 162 |
| Atrogin1 | F: 5′- CAGCTTCGTGAGCGACCTC -3′ R: 5′- GGCAGTCGAGAAGTCCAGTC -3′ | 244 |
| Gapdh | F: 5′- GGTAAAGTGGATATTGTTGCCATCAATG -3′ R: 5′- GGAGGGATCTCGCTCCTGGAAGATGGTG -3′ | 173 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cho, K.-A.; Choi, D.-W.; Kim, Y.-H.; Kim, J.; Ryu, K.-H.; Woo, S.-Y. Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors. Cells 2021, 10, 2169. https://doi.org/10.3390/cells10082169
Cho K-A, Choi D-W, Kim Y-H, Kim J, Ryu K-H, Woo S-Y. Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors. Cells. 2021; 10(8):2169. https://doi.org/10.3390/cells10082169
Chicago/Turabian StyleCho, Kyung-Ah, Da-Won Choi, Yu-Hee Kim, Jungwoo Kim, Kyung-Ha Ryu, and So-Youn Woo. 2021. "Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors" Cells 10, no. 8: 2169. https://doi.org/10.3390/cells10082169
APA StyleCho, K. -A., Choi, D. -W., Kim, Y. -H., Kim, J., Ryu, K. -H., & Woo, S. -Y. (2021). Mesenchymal Stem Cell-Derived Exosomes Protect Muscle Loss by miR-145-5p Activity Targeting Activin A Receptors. Cells, 10(8), 2169. https://doi.org/10.3390/cells10082169