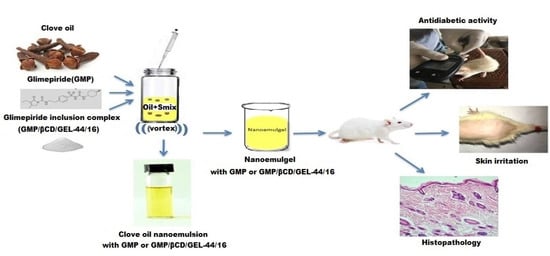
Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Solubility-Studies
2.3. Construction of Pseudo-Ternary Phase Diagram
2.4. Preparation of Nanoemulsion Formulations
2.5. Preparation of Drug-Loaded Nanoemulsion and Nanoemulsion Gel Formulations
2.6. Characterization of Nanoemulsion and Nanoemulgel Formulations
2.7. Ex Vivo Permeation Study
2.8. In Vivo Antidiabetic Study
2.9. Skin Irritation Study
2.10. Statistical Analysis
3. Results and Discussion
3.1. Solubility Studies
3.2. Pseudo Ternary Phase Diagram
3.3. Selection of Formulae
3.4. Characterization of Nanoemulsion and Nanoemulsion Based Gels
3.4.1. Visual Inspection
3.4.2. Droplet Size and Polydispersity Index Measurement (PDI)
3.4.3. Measurement of Zeta Potential
3.4.4. Determination of Viscosity
3.4.5. pH and Conductivity Measurements
3.4.6. Ex Vivo Permeation Study
3.4.7. In Vivo Anti-Diabetic Studies
3.4.8. Skin Irritation Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IDF Diabetes Atlas 2019, 9th ed.; International Diabetes Federation: Brussels, Belgium, 2019.
- Tao, Z.; Shi, A.; Zhao, J. Epidemiological perspectives of diabetes. Cell Biochem. Biophys. 2015, 73, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Jessup, A.N. Diabetes Mellitus: A Nursing Perspective. In Nutritional and Therapeutic Interventions for Diabetes and Metabolic Syndrome; Elsevier: Amsterdam, The Netherlands, 2012; pp. 103–110. [Google Scholar]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to diabetes mellitus. In Diabetes; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1–11. [Google Scholar]
- Piechota, G.; Małkiewicz, J.; Karwat, I.D. Type-2 diabetes mellitus as a cause of disability. Przeglad Epidemiol. 2004, 58, 677. [Google Scholar]
- Cooke, W.D.; Plotnick, L. Type 1 diabetes mellitus in pediatrics. Pediatr. Rev. 2008, 29, 374–384. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Sun, J.; Han, X.; Wu, Y.; Yan, Z.; Han, J.; He, Z. Strategies to improve dissolution and oral absorption of glimepiride tablets: Solid dispersion versus micronization techniques. Drug Dev. Ind. Pharm. 2011, 37, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Ammar, H.; Salama, H.; El-Nahhas, S.; Elmotasem, H. Design and evaluation of chitosan films for transdermal delivery of glimepiride. Curr. Drug Deliv. 2008, 5, 290–298. [Google Scholar] [CrossRef]
- Ammar, H.; Salama, H.; Ghorab, M.; Mahmoud, A. Implication of inclusion complexation of glimepiride in cyclodextrin–polymer systems on its dissolution, stability and therapeutic efficacy. Int. J. Pharm. 2006, 320, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, E.A.; Meshali, M.M.; Foda, A.M.M.; Borg, T.M. Improvement of dissolution and hypoglycemic efficacy of glimepiride by different carriers. AAPS PharmSciTech 2012, 13, 1013–1023. [Google Scholar] [CrossRef] [Green Version]
- Irfan, M.; Khan, I.U.; Shah, P.A.; Khalid, I. Investigation of the Co-Effect of Gelucire and ß-Cyclodextrin on Glimepiride Solubilization. Lat. Am. J. Pharm. 2019, 38, 1733–1740. [Google Scholar]
- Ahad, A.; Al-Saleh, A.A.; Akhtar, N.; Al-Mohizea, A.M.; Al-Jenoobi, F.I. Transdermal delivery of antidiabetic drugs: Formulation and delivery strategies. Drug Discov. Today 2015, 20, 1217–1227. [Google Scholar] [CrossRef]
- Tanwar, H.; Sachdeva, R. Transdermal drug delivery system: A review. Int. J. Pharm. Sci. Res. 2016, 7, 2274. [Google Scholar]
- Touitou, E. Drug delivery across the skin. Expert Opin. Biol. Ther. 2002, 2, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv 2010, 1, 109–131. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, T.A.; Khalid, M.; Aljaeid, B.M.; Fahmy, U.A.; Abd-Allah, F.I. Transdermal glimepiride delivery system based on optimized ethosomal nano-vesicles: Preparation, characterization, in vitro, ex vivo and clinical evaluation. Int. J. Pharm. 2016, 500, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.A.; Kurakula, M.; Banjar, Z.M.; Afouna, M.I.; Zidan, A.S. Quality by design coupled with near infrared in formulation of transdermal glimepiride liposomal films. J. Pharm. Sci. 2015, 104, 2062–2075. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.A.; Afouna, M.I.; El-Say, K.M.; Abdel-Naim, A.B.; Khedr, A.; Banjar, Z.M. Optimization of self-nanoemulsifying systems for the enhancement of in vivo hypoglycemic efficacy of glimepiride transdermal patches. Expert Opin. Drug Deliv. 2014, 11, 1005–1013. [Google Scholar] [CrossRef] [PubMed]
- Gashlan, M.H.; Al-Beladi, A.B. Effects of clove oil on liver and antioxidant status of streptozotocin-induced diabetic rats. GARJMMS 2017, 6, 103–110. [Google Scholar]
- Topal, F. Anticholinergic and antidiabetic effects of isoeugenol from clove (Eugenia caryophylata) oil. Int. J. Food Prop. 2019, 22, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Salim, B.; Said, G.; Noureddine, M.; Hocine, A.; Angelika, B.A. A note study on antidiabetic effect of main molecules contained in clove using molecular modeling interactions with DPP-4 enzyme. Int. J. Comput. Theor. Chem. 2017, 5, 9. [Google Scholar] [CrossRef] [Green Version]
- Parhi, R.; Suresh, P.; Mondal, S.; Mahesh Kumar, P. Novel penetration enhancers for skin applications: A review. Curr. Drug Deliv. 2012, 9, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, V.K.; Singh, O.P.; Shafaat, K.; Kumar, S.; Ahmad, F.J. Formulation and optimization of nanoemulsion using antifungal lipid and surfactant for accentuated topical delivery of Amphotericin B. Drug Deliv. 2016, 23, 3101–3110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, K.H.; Peh, K.K. Identification of phases of various oil, surfactant/co-surfactants and water system by ternary phase diagram. Acta Pol. Pharm. 2014, 71, 301–309. [Google Scholar] [PubMed]
- Algahtani, S.M.; Ahmad, M.Z.; Ahmad, J. Nanoemulgel for improved topical delivery of retinyl palmitate: Formulation design and stability evaluation. Nanomaterials 2020, 10, 848. [Google Scholar] [CrossRef] [PubMed]
- Samia, O.; Hanan, R.; Kamal, E.T. Carbamazepine mucoadhesive nanoemulgel (MNEG) as brain targeting delivery system via the olfactory mucosa. Drug Deliv. 2012, 19, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; da Silva Almeida, J.R.G. Development and evaluation of stability of a gel formulation containing the monoterpene borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef] [Green Version]
- Jeengar, M.K.; Rompicharla, S.V.K.; Shrivastava, S.; Chella, N.; Shastri, N.R.; Naidu, V.; Sistla, R. Emu oil based nano-emulgel for topical delivery of curcumin. Int. J. Pharm. 2016, 506, 222–236. [Google Scholar] [CrossRef]
- Ladrière, L.; Malaisse-Lagae, F.; Fuhlendorff, J.; Malaisse, W.J. Repaglinide, glibenclamide and glimepiride administration to normal and hereditarily diabetic rats. Eur. J. Pharmacol. 1997, 335, 227–234. [Google Scholar] [CrossRef]
- Morsy, M.A.; Abdel-Latif, R.G.; Nair, A.B.; Venugopala, K.N.; Ahmed, A.F.; Elsewedy, H.S.; Shehata, T.M. Preparation and evaluation of atorvastatin-loaded nanoemulgel on wound-healing efficacy. Pharmaceutics 2019, 11, 609. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.S.; Alam, M.S.; Alam, N.; Siddiqui, M.R. Preparation, characterization and stability study of dutasteride loaded nanoemulsion for treatment of benign prostatic hypertrophy. Iran. J. Pharm. Res. IJPR 2014, 13, 1125. [Google Scholar]
- Kumar, P.G.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. B 2011, 1, 208–219. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.; Bishnoi, R.S.; Shukla, A.K.; Jain, C.P. Techniques for formulation of nanoemulsion drug delivery system: A review. Prev. Nutr. Food Sci. 2019, 24, 225. [Google Scholar] [CrossRef] [PubMed]
- Elnaggar, S.Y.; El-Massik, M.A.; Abdallah, O.Y. Self-nanoemulsifying drug delivery systems of tamoxifen citrate: Design and optimization. Int. J. Pharm. 2009, 380, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Shafiq-un-Nabi, S.; Shakeel, F.; Talegaonkar, S.; Ali, J.; Baboota, S.; Ahuja, A.; Khar, R.K.; Ali, M. Formulation development and optimization using nanoemulsion technique: A technical note. AAPS PharmSciTech 2007, 8, E12–E17. [Google Scholar] [CrossRef]
- Lala, R.; Awari, N. Nanoemulsion-based gel formulations of COX-2 inhibitors for enhanced efficacy in inflammatory conditions. Appl. Nanosci. 2014, 4, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Rizwan, M.; Aqil, M.; Azeem, A.; Talegaonkar, S.; Sultana, Y.; Ali, A. Enhanced transdermal delivery of carvedilol using nanoemulsion as a vehicle. J. Exp. Nanosci. 2010, 5, 390–411. [Google Scholar] [CrossRef] [Green Version]
- Elmataeeshy, M.E.; Sokar, M.S.; Bahey-El-Din, M.; Shaker, D.S. Enhanced transdermal permeability of Terbinafine through novel nanoemulgel formulation; Development, in vitro and in vivo characterization. Future J. Pharm. Sci. 2018, 4, 18–28. [Google Scholar] [CrossRef]
- Jaiswal, M.; Dudhe, R.; Sharma, P. Nanoemulsion: An advanced mode of drug delivery system. 3 Biotech 2015, 5, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Aboofazeli, R. Nanometric-scaled emulsions (nanoemulsions). Iran. J. Pharm. Res. IJPR 2010, 9, 325. [Google Scholar]
- Shakeel, F.; Baboota, S.; Ahuja, A.; Ali, J.; Aqil, M.; Shafiq, S. Nanoemulsions as vehicles for transdermal delivery of aceclofenac. Aaps PharmSciTech 2007, 8, 191. [Google Scholar] [CrossRef] [Green Version]
- Negussie, A.H.; Miller, J.L.; Reddy, G.; Drake, S.K.; Wood, B.J.; Dreher, M.R. Synthesis and in vitro evaluation of cyclic NGR peptide targeted thermally sensitive liposome. J. Control. Release 2010, 143, 265–273. [Google Scholar] [CrossRef] [Green Version]
- Wissing, S.; Kayser, O.; Müller, R. Solid lipid nanoparticles for parenteral drug delivery. Adv. Drug Deliv. Rev. 2004, 56, 1257–1272. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of Micro-and Nanoemulsions as Vehicles for Essential Oils: Formulation, Preparation and Stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef] [Green Version]
- Nikolakakis, I.; Panagopoulou, A.; Salis, A.; Malamataris, S. Relationships between the properties of self-emulsifying pellets and of the emulsions used as massing liquids for their preparation. AAPS PharmSciTech 2015, 16, 129–139. [Google Scholar] [CrossRef] [Green Version]
- Fanun, M. Formulation and characterization of microemulsions based on mixed nonionic surfactants and peppermint oil. J. Colloid Interface Sci. 2010, 343, 496–503. [Google Scholar] [CrossRef]
- Chime, S.; Kenechukwu, F.; Attama, A. Nanoemulsions—Advances in formulation, characterization and applications in drug delivery. In Application of Nanotechnology in Drug Delivery; IntechOpen: London, UK, 2014; Volume 3, Chapter 3. [Google Scholar]
- Chiesa, M.; Garg, J.; Kang, Y.T.; Chen, G. Thermal conductivity and viscosity of water-in-oil nanoemulsions. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 67–72. [Google Scholar] [CrossRef]
- Saraf, S.; Sahu, S.; Kaur, C.D.; Saraf, S. Comparative measurement of hydration effects of herbal moisturizers. Pharmacogn. Res. 2010, 2, 146. [Google Scholar] [CrossRef] [Green Version]
- Gurpreet, K.; Singh, S. Review of nanoemulsion formulation and characterization techniques. Indian J. Pharm. Sci. 2018, 80, 781–789. [Google Scholar] [CrossRef]
- Mahtab, A.; Anwar, M.; Mallick, N.; Naz, Z.; Jain, G.K.; Ahmad, F.J. Transungual delivery of ketoconazole nanoemulgel for the effective management of onychomycosis. AAPS PharmSciTech 2016, 17, 1477–1490. [Google Scholar] [CrossRef]
- Djordjevic, L.; Primorac, M.; Stupar, M. In vitro release of diclofenac diethylamine from caprylocaproylmacrogolglycerides based microemulsions. Int. J. Pharm. 2005, 296, 73–79. [Google Scholar] [CrossRef]
- Podlogar, F.; Rogač, M.B.; Gašperlin, M. The effect of internal structure of selected water–Tween 40®–Imwitor 308®–IPM microemulsions on ketoprofene release. Int. J. Pharm. 2005, 302, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Rhee, Y.-S.; Choi, J.-G.; Park, E.-S.; Chi, S.-C. Transdermal delivery of ketoprofen using microemulsions. Int. J. Pharm. 2001, 228, 161–170. [Google Scholar] [CrossRef]
- Azeem, A.; Ahmad, F.J.; Khar, R.K.; Talegaonkar, S. Nanocarrier for the transdermal delivery of an antiparkinsonian drug. AAPS PharmSciTech 2009, 10, 1093–1103. [Google Scholar] [CrossRef]
- Rachmawati, H.; Edityaningrum, C.A.; Mauludin, R. Molecular inclusion complex of curcumin–β-cyclodextrin nanoparticle to enhance curcumin skin permeability from hydrophilic matrix gel. AAPS PharmSciTech 2013, 14, 1303–1312. [Google Scholar] [CrossRef] [Green Version]
- Loftsson, T.; Masson, M. Cyclodextrins in topical drug formulations: Theory and practice. Int. J. Pharm. 2001, 225, 15–30. [Google Scholar] [CrossRef]
- Dalmora, M.; Dalmora, S.; Oliveira, A.G.d. Inclusion complex of piroxicam with β-cyclodextrin and incorporation in cationic microemulsion. In vitro drug release and in vivo topical anti-inflammatory effect. Int. J. Pharm. 2001, 222, 45–55. [Google Scholar] [CrossRef]





| Formulation Code | Composition (% w/w) | ||
|---|---|---|---|
| Oil | Smix | Water | |
| NE-1 | 10% | 35% | 55% |
| NE-2 | 15% | 40% | 45% |
| NE-3 | 15% | 45% | 35% |
| Formulation Code | Particle Size (nm) | Polydispersity Index (PDI) | ||||
|---|---|---|---|---|---|---|
| Blank | GMP-Loaded | GMP/βCD/GEL-44/16-Loaded | Blank | GMP-Loaded | GMP/βCD/GEL-44/16-Loaded | |
| NE-1 | 133.4 ± 2.01 | 141.9 ±3.35 | 143.1 ± 2.60 | 0.269 ± 0.006 | 0.260± 0.004 | 0.315± 0.008 |
| NE-2 | 157.9 ± 2.40 | 213.3 ± 2.55 | 233.3 ± 3.60 | 0.260 ± 0.006 | 0.428± 0.006 | 0.421± 0.007 |
| NE-3 | 137.8 ± 2.60 | 151.9 ± 3.20 | 168.4 ± 2.20 | 0.230 ± 0.004 | 0.225± 0.008 | 0.383± 0.008 |
| Formulation Code | Zeta Potential (mV) | ||
|---|---|---|---|
| Blank | GMP-Loaded | GMP/βCD/GEL-44/16-Loaded | |
| NE-1 | −17.5 ± 0.4 | −20.5 ± 0.2 | −14.2 ± 0.45 |
| NE-2 | −17.8 ± 0.3 | −20.7 ± 0.35 | −17.1 ± 0.35 |
| NE-3 | −16.5 ± 0.4 | −15.4 ± 0.30 | −12.8 ± 0.25 |
| Formulation Code | Viscosity (cP) | ||
|---|---|---|---|
| Blank | GMP-Loaded | GMP/ΒCD/GEL-44/16-Loaded | |
| NE-1 | 30.4 ± 1.85 | 32.8 ± 1.80 | 34.7 ± 1.53 |
| NE-2 | 43.7 ± 1.50 | 45.2 ± 1.51 | 46.4 ± 1.12 |
| NE-3 | 55.1 ± 2.20 | 58.1 ± 1.60 | 58.7 ± 1.15 |
| Formulation Code | pH | Conductivity (µS/cm) | ||||
|---|---|---|---|---|---|---|
| Blank | GMP-Loaded | GMP/βCD/GEL-44/16-Loaded | Blank | GMP-Loaded | GMP/βCD/GEL-44/16-Loaded | |
| NE-1 | 5.19 ± 0.22 | 5.10 ± 0.23 | 5.06 ± 0.27 | 63.38 ± 3.22 | 62.50 ± 3.31 | 60.7 ± 2.20 |
| NE-2 | 5.49 ± 0.12 | 5.32 ± 0.16 | 5.27 ± 0.12 | 51.04 ± 2.65 | 50.57 ± 2.99 | 48.9 ± 3.01 |
| NE-3 | 5.59 ± 0.14 | 5.51 ± 0.17 | 5.45 ± 0.22 | 35.72 ± 2.70 | 34.01 ± 2.69 | 34.19 ± 2.01 |
| Parameters | GMP-Loaded Nanoemulgel | GMP/βCD/GEL-44/16-Loaded Nanoemulgel | ||||
|---|---|---|---|---|---|---|
| NEG-1 | NEG-2 | NEG-3 | NEG-1 | NEG-2 | NEG-3 | |
| pH | 6.20 ± 0.10 | 6.49 ± 0.09 | 6.65 ± 0.17 | 6.16 ± 0.11 | 6.41 ± 0.16 | 6.62 ± 0.18 |
| Viscosity (cp) | 15,118.71 ± 193.21 | 15,229.04 ± 137.0 | 15,375.12 ± 175.6 | 15,250.9 ± 148.6 | 15,393.06 ± 177.3 | 15,475.21 ± 178.2 |
| Spreadability (cm2/g) | 1.47 ± 0.08 | 1.43 ± 0.08 | 1.38 ± 0.05 | 1.41 ± 0.04 | 1.37 ± 0.07 | 1.35 ± 0.08 |
| Formulation Code | Flux (J) (µg/cm2/h) | Permeability Constant (Kp) (cm/h) |
|---|---|---|
| NEG-1 | 57.16 ± 7.49 abc | 0.028 abc |
| NEG-2 | 51.55 ± 3.80 abc | 0.025 abc |
| NEG-3 | 54.48 ± 6.59 abc | 0.027 abc |
| NEG-1(IC) | 70.06 ± 6.60 abcdef | 0.035 abcdef |
| NEG-2(IC) | 58.80 ± 3.62 abc | 0.029 abc |
| NEG-3(IC) | 62.80 ± 6.66 abc | 0.031 abc |
| GMP in clove oil | 10.29 ± 1.25 | 0.005 |
| GMP/βCD/GEL-44/16 in clove oil | 13.86 ± 3.59 | 0.006 |
| GMP aqueous suspension | 3.41 ± 3.25 | 0.002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Razzaq, F.A.; Asif, M.; Asghar, S.; Iqbal, M.S.; Khan, I.U.; Khan, S.-U.-D.; Irfan, M.; Syed, H.K.; Khames, A.; Mahmood, H.; et al. Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation. Cells 2021, 10, 2404. https://doi.org/10.3390/cells10092404
Razzaq FA, Asif M, Asghar S, Iqbal MS, Khan IU, Khan S-U-D, Irfan M, Syed HK, Khames A, Mahmood H, et al. Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation. Cells. 2021; 10(9):2404. https://doi.org/10.3390/cells10092404
Chicago/Turabian StyleRazzaq, Fizza Abdul, Muhammad Asif, Sajid Asghar, Muhammad Shahid Iqbal, Ikram Ullah Khan, Salah-Ud-Din Khan, Muhammad Irfan, Haroon Khalid Syed, Ahmed Khames, Hira Mahmood, and et al. 2021. "Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation" Cells 10, no. 9: 2404. https://doi.org/10.3390/cells10092404
APA StyleRazzaq, F. A., Asif, M., Asghar, S., Iqbal, M. S., Khan, I. U., Khan, S. -U. -D., Irfan, M., Syed, H. K., Khames, A., Mahmood, H., Ibrahim, A. Y., & El Sisi, A. M. (2021). Glimepiride-Loaded Nanoemulgel; Development, In Vitro Characterization, Ex Vivo Permeation and In Vivo Antidiabetic Evaluation. Cells, 10(9), 2404. https://doi.org/10.3390/cells10092404