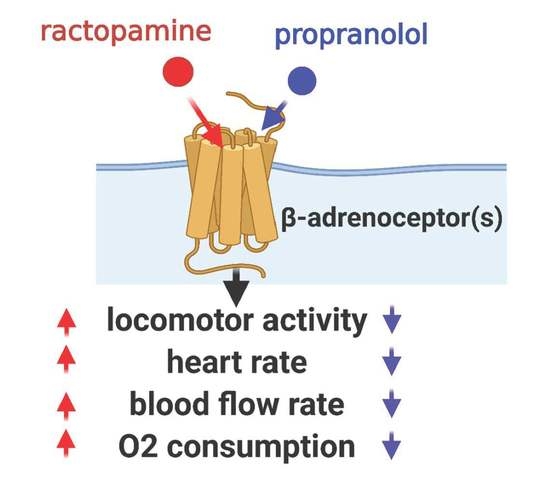
Evaluation of Effects of Ractopamine on Cardiovascular, Respiratory, and Locomotory Physiology in Animal Model Zebrafish Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Housing and Breeding of Zebrafish
2.2. Chemical Preparation
2.3. Automated Behavioral Analysis Using ZebraBox
2.4. Zebrafish Larvae Cardiac Physiology and Rhythm Assay
2.5. Zebrafish Oxygen Consumption Analysis
2.6. Blood Flow Measurement
2.7. Molecular Docking Analysis
2.8. Co-incubation of RAC and PROP Experiment
3. Results
3.1. Total Distance Traveled for Zebrafish Larvae after RAC Exposure
3.2. Total Rotation Movement for Zebrafish Larvae after RAC Exposure
3.3. Total Burst Count for Zebrafish Larvae after RAC Exposure
3.4. Respiratory Rate Analysis in Response to RAC Exposure in Zebrafish Larvae
3.5. Cardiovascular Performance Assay
3.6. Blood Flow Measurement after RAC Exposure
3.7. Molecular Docking for RAC and Endogenous β-adrenergic Receptor
3.8. Test Physiological Effects of β-Blocker ‘Propranolol’
3.9. Rescue Physiological Alterations Induced by RAC with PROP
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Behavior Endpoints | Propranolol Concentrations | ||||||||||||
| 0 ppm (control) | 0.1 ppm | 1 ppm | 2 ppm | 4 ppm | 8 ppm | ||||||||
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | ||||||||
| Average Total Distance (cm) | Light cycle | 7.35 ± 0.126 | a | 6.35 ± 0.128 | b | 7.79 ± 0.171 | c | 7.42 ± 0.293 | e | 10.09 ± 0.324 | d | 6.59 ± 0.163 | d |
| Dark cycle | 10.06 ± 0.106 | a | 11.05 ± 0.107 | b | 11.68 ± 0.162 | b | 9.88 ± 0.286 | c | 12.43 ± 0.336 | d | 7.49 ± 0.185 | d | |
| Average Rotation (count) | Light cycle | 0.032 ± 0.002 | a | 0.072 ± 0.005 | b | 0.041 ± 0.003 | c | 0.029 ± 0.003 | d | 0.023 ± 0.002 | e | 0.009 ± 0.002 | e |
| Dark cycle | 0.214 ± 0.006 | a | 0.422 ± 0.013 | b | 0.253 ± 0.008 | c | 0.147 ± 0.007 | d | 0.164 ± 0.008 | e | 0.034 ± 0.003 | e | |
| Average Burst Movement (count) | Light cycle | 0.654 ± 0.037 | a | 1.598 ± 0.091 | b | 1.485 ± 0.078 | c | 1.049 ± 0.064 | b | 1.124 ± 0.062 | a | 0.469 ± 0.025 | a |
| Dark cycle | 7.25 ± 0.125 | a | 29.74 ± 0.441 | b | 21.41 ± 0.320 | c | 11.77 ± 0.275 | a | 9.847 ± 0.257 | e | 1.797 ± 0.069 | e | |
| Cardiac Related Parameters | Propranolol Concentrations | |||||||
| 0 ppm (control) | 0.1 ppm | 4 ppm | 8 ppm | |||||
| Mean ± SEM | Mean ± SEM | Mean ± SEM | Mean ± SEM | |||||
| Heart Rate Ventricle (bpm) | 145.3 ± 2.956 | a | 140.6 ± 3.316 | ab | 134.7 ± 2.701 | bc | 130.8 ± 1.975 | c |
| Heart Rate Atrium (bpm) | 145.4 ± 2.888 | a | 140.4 ± 3.427 | ab | 134.9 ± 2.664 | bc | 130.7 ± 2.039 | c |
| Stroke Volume (pL/beat) | 80.94 ± 7.274 | a | 78.68 ± 7.275 | a | 71.37 ± 7.992 | a | 75.75 ± 4.338 | a |
| Cardiac Output (pL/min) | 11663 ± 101 | a | 10978 ± 983.0 | a | 9424 ± 993.9 | a | 9926 ± 568.2 | a |
| Ejection Fraction (%) | 37.36 ± 2.101 | a | 35.85 ± 2.148 | a | 32.37 ± 2.826 | a | 34.57 ± 1.508 | a |
| Shortening Fraction (%) | 14.68 ± 1.280 | a | 13.84 ± 1.078 | a | 12.36 ± 1.582 | a | 12.56 ± 0.728 | a |
| AV–Interval (s) | 0.1864 ± 0.022 | a | 0.161 ± 0.0206 | a | 0.149 ± 0.015 | a | 0.1785 ± 0.021 | a |
| VA–Interval (s) | 0.228 ± 0.0196 | a | 0.272 ± 0.023 | a | 0.299 ± 0.020 | b | 0.2821 ± 0.021 | a |
| SD1 Atrium (s) | 0.036 ± 0.006 | a | 0.034 ± 0.007 | a | 0.042 ± 0.006 | a | 0.041 ± 0.007 | a |
| SD2 Atrium (s) | 0.0212 ± 0.003 | a | 0.0214 ± 0.004 | ac | 0.027 ± 0.004 | a | 0.034 ± 0.005 | c |
| SD1 Ventricle (s) | 0.0219 ± 0.002 | a | 0.0319 ± 0.005 | ab | 0.033 ± 0.003 | b | 0.0274 ± 0.003 | ab |
| SD2 Ventricle (s) | 0.0136 ± 0.002 | a | 0.0204 ± 0.003 | b | 0.021 ± 0.002 | b | 0.017 ± 0.002 | ab |
| Max. Blood Flow Velocity (µm/s) | 856.2 ± 44.20 | a | 763.0 ± 71.85 | a | 769.1 ± 57.77 | a | 876.2 ± 51.92 | a |
| Avg. Blood Flow Velocity (µm/s) | 405.3 ± 15.55 | a | 348.5 ± 27.02 | a | 360.6 ± 24.48 | a | 400.6 ± 20.90 | a |
| Oxygen Consumption Rate (ppm) | 2.081 ± 0.182 | a | 2.485 ± 0.209 | a | 2.306 ± 0.171 | a | 2.685 ± 0.176 | b |
References
- Mennigen, J.A.; Stroud, P.; Zamora, J.M.; Moon, T.W.; Trudeau, V.L. Pharmaceuticals as neuroendocrine disruptors: Lessons learned from fish on Prozac. J. Toxicol. Environ. Health Part B 2011, 14, 387–412. [Google Scholar] [CrossRef]
- Houtman, C.J. Emerging contaminants in surface waters and their relevance for the production of drinking water in Europe. J. Integr. Environ. Sci. 2010, 7, 271–295. [Google Scholar] [CrossRef]
- Brumovský, M.; Bečanová, J.; Kohoutek, J.; Borghini, M.; Nizzetto, L. Contaminants of emerging concern in the open sea waters of the Western Mediterranean. Environ. Pollut. 2017, 229, 976–983. [Google Scholar] [CrossRef] [PubMed]
- Hakk, H.; Shelver, W.L.; Casey, F.X. Fate and transport of the β-adrenergic agonist ractopamine hydrochloride in soil–water systems. J. Environ. Sci. 2016, 45, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Kriewald, R.D. Effects of Ractopamine HCL on Physical and Reproductive Parameters in the Horse. PhD diss., A&M University, Houston, TX, USA, 2010. [Google Scholar]
- Ali, D.C.; Naveed, M.; Gordon, A.; Majeed, F.; Saeed, M.; Ogbuke, M.I.; Atif, M.; Zubair, H.M.; Changxing, L. β-Adrenergic receptor, an essential target in cardiovascular diseases. Hear. Fail. Rev. 2019, 25, 343–354. [Google Scholar] [CrossRef]
- Fent, K.; Weston, A.; Caminada, D. Ecotoxicology of human pharmaceuticals. Aquat. Toxicol. 2006, 76, 122–159. [Google Scholar] [CrossRef] [PubMed]
- Massarsky, A.; Trudeau, V.L.; Moon, T.W. β-blockers as endocrine disruptors: The potential effects of human β-blockers on aquatic organisms. J. Exp. Zool. A Ecol. Genet. Physiol. 2011, 315, 251–265. [Google Scholar] [CrossRef]
- de Almeida, V.V.; Nuñez, A.J.C.; Miyada, V.S. Ractopamine as a metabolic modifier feed additive for finishing pigs: A review. Braz. Arch. Biol. Technol. 2012, 55, 445–456. [Google Scholar] [CrossRef]
- Liu, X.; Grandy, D.K.; Janowsky, A. Ractopamine, a livestock feed additive, is a full agonist at trace amine–associated receptor 1. J. Pharmacol. Exp. Ther. 2014, 350, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Centner, T.J.; Alvey, J.C.; Stelzleni, A.M. Beta agonists in livestock feed: Status, health concerns, and international trade. J. Anim. Sci. 2014, 92, 4234–4240. [Google Scholar] [CrossRef]
- Lee, H.-C.; Chen, C.-M.; Wei, J.-T.; Chiu, H.-Y. Analysis of veterinary drug residue monitoring results for commercial livestock products in Taiwan between 2011 and 2015. J. Food Drug Anal. 2018, 26, 565–571. [Google Scholar] [CrossRef]
- Anderson, D.B.; Moody, D.E.; Hancock, D.L. Beta adrenergic agonists. In Encycl. Anim. Sci.; Marcel-Dekker Inc.: New York, NY, USA, 2004; pp. 104–108. [Google Scholar]
- Dalidowicz, J.; Thomson, T.; Babbitt, G. Ractopamine hydrochloride, a phenethanolamine repartitioning agent: Metabolism and tissue residues. In Xenobiotics and Food-Producing Animals; ACS Publications: Washington, DC, USA, 1992; pp. 234–243. [Google Scholar]
- Bartelt-Hunt, S.; Snow, D.; Damon-Powell, T.; Miesbach, D. Occurrence of steroid hormones and antibiotics in shallow groundwater impacted by livestock waste control facilities. J. Contam. Hydrol. 2011, 123, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Jaimes-Correa, J.C.; Snow, D.D.; Bartelt-Hunt, S.L. Seasonal occurrence of antibiotics and a beta agonist in an agriculturally-intensive watershed. Environ. Pollut. 2015, 205, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Sakai, N.; Sakai, M.; Haron, D.E.M.; Yoneda, M.; Mohd, M.A. Beta-agonist residues in cattle, chicken and swine livers at the wet market and the environmental impacts of wastewater from livestock farms in Selangor State, Malaysia. Chemosphere 2016, 165, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.-H.; Lin, A.; Wang, X.-H.; Lin, C.-F. Occurrence of β-blockers and β-agonists in hospital effluents and their receiving rivers in southern Taiwan. DESALINATION Water Treat. 2011, 32, 49–56. [Google Scholar] [CrossRef]
- Catalano, D.; Odore, R.; Amedeo, S.; Bellino, C.; Biasibetti, E.; Miniscalco, B.; Perona, G.; Pollicino, P.; Savarino, P.; Tomassone, L.; et al. Physiopathological changes related to the use of ractopamine in swine: Clinical and pathological investigations. Livest. Sci. 2012, 144, 74–81. [Google Scholar] [CrossRef]
- Yaeger, M.; Mullin, K.; Ensley, S.; Ware, W.; Slavin, R. Myocardial toxicity in a group of greyhounds administered ractopamine. Vet. Pathol. 2012, 49, 569–573. [Google Scholar] [CrossRef]
- Sun, L.; Wang, S.; Lin, X.; Tan, H.; Fu, Z. Early Life Exposure to Ractopamine Causes Endocrine-Disrupting Effects in Japanese Medaka (Oryzias latipes). Bull. Environ. Contam. Toxicol. 2015, 96, 150–155. [Google Scholar] [CrossRef]
- Sachett, A.; Bevilaqua, F.; Chitolina, R.; Garbinato, C.; Gasparetto, H.; Dal Magro, J.; Conterato, G.M.; Siebel, A.M. Ractopamine hydrochloride induces behavioral alterations and oxidative status imbalance in zebrafish. J. Toxicol. Environ. Health Part A 2018, 81, 194–201. [Google Scholar] [CrossRef]
- Garbinato, C.; Schneider, S.E.; Sachett, A.; Decui, L.; Conterato, G.M.; Müller, L.G.; Siebel, A.M. Exposure to ractopamine hydrochloride induces changes in heart rate and behavior in zebrafish embryos and larvae. Environ. Sci. Pollut. Res. 2020, 27, 21468–21475. [Google Scholar] [CrossRef]
- Ferreira, L.G.; Dos Santos, R.N.; Oliva, G.; Andricopulo, A.D. Molecular Docking and Structure-Based Drug Design Strategies. Molecules 2015, 20, 13384–13421. [Google Scholar] [CrossRef]
- Li, J.; Fu, A.; Zhang, L. An Overview of Scoring Functions Used for Protein–Ligand Interactions in Molecular Docking. Interdiscip. Sci. Comput. Life Sci. 2019, 11, 320–328. [Google Scholar] [CrossRef] [PubMed]
- Lohning, A.E.; Levonis, S.M.; Williams-Noonan, B.; Schweiker, S. A Practical Guide to Molecular Docking and Homology Modelling for Medicinal Chemists. Curr. Top. Med. Chem. 2017, 17, 2023–2040. [Google Scholar] [CrossRef]
- He, J.-H.; Gao, J.-M.; Huang, C.-J.; Li, C.-Q. Zebrafish models for assessing developmental and reproductive toxicity. Neurotoxicology Teratol. 2014, 42, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Avdesh, A.; Chen, M.; Martin-Iverson, M.; Mondal, A.; Ong, D.; Rainey-Smith, S.; Taddei, K.; Lardelli, M.; Groth, D.M.; Verdile, G.; et al. Regular Care and Maintenance of a Zebrafish (Danio rerio) Laboratory: An Introduction. J. Vis. Exp. 2012, e4196. [Google Scholar] [CrossRef]
- Hussain, A.; Audira, G.; Malhotra, N.; Uapipatanakul, B.; Chen, J.-R.; Lai, Y.-H.; Huang, J.-C.; Chen, K.H.-C.; Lai, H.-T.; Hsiao, C.-D. Multiple Screening of Pesticides Toxicity in Zebrafish and Daphnia Based on Locomotor Activity Alterations. Biomolecules 2020, 10, 1224. [Google Scholar] [CrossRef]
- Kristofco, L.A.; Cruz, L.C.; Haddad, S.P.; Behra, M.L.; Chambliss, C.K.; Brooks, B.W. Age matters: Developmental stage of Danio rerio larvae influences photomotor response thresholds to diazinion or diphenhydramine. Aquat. Toxicol. 2015, 170, 344–354. [Google Scholar] [CrossRef]
- Suryanto, M.E.; Audira, G.; Uapipatanakul, B.; Hussain, A.; Saputra, F.; Siregar, P.; Chen, K.H.-C.; Hsiao, C.-D. Antidepressant screening demonstrated non-monotonic responses to amitriptyline, amoxapine and sertraline in locomotor activity assay in larval zebrafish. Cells 2021, 10, 738. [Google Scholar] [CrossRef]
- Sampurna, B.P.; Audira, G.; Juniardi, S.; Lai, Y.-H.; Hsiao, C.-D. A simple image-based method to measure cardiac rhythm in zebrafish embryos. Inventions 2018, 3, 21. [Google Scholar] [CrossRef]
- Santoso, F.; Sampurna, B.P.; Lai, Y.-H.; Liang, S.-T.; Hao, E.; Chen, J.-R.; Hsiao, C.-D. Development of a simple image-based method for dynamic blood flow tracking in zebrafish embryos and its application in drug toxicity evaluation. Inventions 2019, 4, 65. [Google Scholar] [CrossRef]
- Tinevez, J.-Y.; Perry, N.; Schindelin, J.; Hoopes, G.; Reynolds, G.; Laplantine, E.; Bednarek, S.Y.; Shorte, S.; Eliceiri, K. TrackMate: An open and extensible platform for single-particle tracking. Methods 2017, 115, 80–90. [Google Scholar] [CrossRef]
- Rueden, C.; Schindelin, J.E.; Hiner, M.C.; Dezonia, B.E.; Walter, A.E.; Arena, E.T.; Eliceiri, K.W. ImageJ2: ImageJ for the next generation of scientific image data. BMC Bioinform. 2017, 18, 1–26. [Google Scholar] [CrossRef]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed]
- Webb, B.; Sali, A. Comparative protein structure modeling using modeller. Curr. Protoc. Bioinform. 2016, 54, 561–567. [Google Scholar] [CrossRef]
- Koska, J.; Spassov, V.Z.; Maynard, A.J.; Yan, L.; Austin, N.; Flook, P.; Venkatachalam, C.M. Fully Automated Molecular Mechanics Based Induced Fit Protein−Ligand Docking Method. J. Chem. Inf. Model. 2008, 48, 1965–1973. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, J.K.; Law, S.M.; Brooks, C.L. Flexible CDOCKER: Development and application of a pseudo-explicit structure-based docking method within CHARMM. J. Comput. Chem. 2016, 37, 753–762. [Google Scholar] [CrossRef]
- Shen, M.-Y.; Sali, A. Statistical potential for assessment and prediction of protein structures. Protein Sci. 2006, 15, 2507–2524. [Google Scholar] [CrossRef]
- John, B.; Sali, A. Comparative protein structure modeling by iterative alignment, model building and model assessment. Nucleic Acids Res. 2003, 31, 3982–3992. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Lai, L. Binding Site Detection and Druggability Prediction of Protein Targets for Structure- Based Drug Design. Curr. Pharm. Des. 2013, 19, 2326–2333. [Google Scholar] [CrossRef]
- Brooks, B.R.; Bruccoleri, R.E.; Olafson, B.D.; States, D.J.; Swaminathan, S.; Karplus, M. CHARMM: A program for macro-molecular energy, minimization, and dynamics calculations. J. Comput. Chem. 1983, 4, 187–217. [Google Scholar] [CrossRef]
- Bownik, A.; Sokołowska, N.; Ślaska, B. Effects of apomorphine, a dopamine agonist, on Daphnia magna: Imaging of swimming track density as a novel tool in the assessment of swimming activity. Sci. Total. Environ. 2018, 635, 249–258. [Google Scholar] [CrossRef]
- Mendes, J.; Pereira, J.; Pereira, T. Variability of Heart Rate in Athletes and Non Athletes. Eur. J. Public Heal. 2019, 29. [Google Scholar] [CrossRef]
- Chaswal, M.; Kapoor, R.; Batra, A.; Verma, S.; Yadav, B.S. Heart rate variability and cardiovascular reflex tests for assessment of autonomic functions in preeclampsia. Int. J. Hypertens. 2018, 2018, 8163824. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, C.-D.; Wu, H.-H.; Malhotra, N.; Liu, Y.-C.; Wu, Y.-H.; Lin, Y.-N.; Saputra, F.; Santoso, F.; Chen, K.H.-C. Expression and purification of recombinant ghk tripeptides are able to protect against acute cardiotoxicity from exposure to water-borne-copper in zebrafish. Biomolecules 2020, 10, 1202. [Google Scholar] [CrossRef] [PubMed]
- Cikes, M.; Solomon, S.D. Beyond ejection fraction: An integrative approach for assessment of cardiac structure and function in heart failure. Eur. Heart J. 2015, 37, 1642–1650. [Google Scholar] [CrossRef] [PubMed]
- van Dalen, E.C.; van den Brug, M.; Caron, H.N.; Kremer, L.C. Anthracycline-induced cardiotoxicity: Comparison of recommendations for monitoring cardiac function during therapy in paediatric oncology trials. Eur. J. Cancer 2006, 42, 3199–3205. [Google Scholar] [CrossRef] [PubMed]
- Chengode, S. Left ventricular global systolic function assessment by echocardiography. Ann. Card. Anaesth. 2016, 19, 26–34. [Google Scholar] [CrossRef]
- Tyrer, P.J.; Lader, M.H. Response to Propranolol and Diazepam in Somatic and Psychic Anxiety. BMJ 1974, 2, 14–16. [Google Scholar] [CrossRef]
- Gebauer, D.L.; Pagnussat, N.; Piato, Â.L.; Schaefer, I.C.; Bonan, C.D.; Lara, D.R. Effects of anxiolytics in zebrafish: Similarities and differences between benzodiazepines, buspirone and ethanol. Pharmacol. Biochem. Behav. 2011, 99, 480–486. [Google Scholar] [CrossRef]
- Finn, J.; Hui, M.; Li, V.W.T.; Lorenzi, V.; de la Paz, N.; Cheng, S.H.; Lai-Chan, L.; Schlenk, D. Effects of propranolol on heart rate and development in Japanese medaka (Oryzias latipes) and zebrafish (Danio rerio). Aquat. Toxicol. 2012, 122, 214–221. [Google Scholar] [CrossRef]
- Huggett, D.; Brooks, B.; Peterson, B.; Foran, C.; Schlenk, D. Toxicity of select beta adrenergic receptor-blocking pharmaceuticals (b-blockers) on aquatic organisms. Arch. Environ. Contam. Toxicol. 2002, 43, 229–235. [Google Scholar] [CrossRef]
- Sweetman, S.; Martindale, I. The complete drug reference pharmaceutical press. J. Med. Libr. Assoc. 2002, 100, 75–76. [Google Scholar]
- Jozefowski, S.J.; Plytycz, B. Characterization of β-adrenergic receptors in fish and amphibian lymphoid organs. Dev. Comp. Immunol. 1998, 22, 587–603. [Google Scholar] [CrossRef]
- Fraysse, B.; Mons, R.; Garric, J. Development of a zebrafish 4-day embryo-larval bioassay to assess toxicity of chemicals. Ecotoxicol. Environ. Saf. 2006, 63, 253–267. [Google Scholar] [CrossRef] [PubMed]
- Owen, S.F.; Giltrow, E.; Huggett, D.B.; Hutchinson, T.H.; Saye, J.; Winter, M.J.; Sumpter, J.P. Comparative physiology, pharmacology and toxicology of β-blockers: Mammals versus fish. Aquat. Toxicol. 2007, 82, 145–162. [Google Scholar] [CrossRef] [PubMed]
- Ternes, T.A. Occurrence of drugs in German sewage treatment plants and rivers. Water Res. 1998, 32, 3245–3260. [Google Scholar] [CrossRef]
- Santos, L.; Araujo, A.; Fachini, A.; Pena, A.; Delerue-Matos, C.; Montenegro, M. Ecotoxicological aspects related to the presence of pharmaceuticals in the aquatic environment. J. Hazard. Mater. 2010, 175, 45–95. [Google Scholar] [CrossRef] [PubMed]
- Black, J.W.; Crowther, A.F.; Shanks, R.G.; Smith, L.H.; Dornhorst, A.C. A new adrenergic betareceptor antagonist. Lancet 1964, 1, 1080–1081. [Google Scholar] [CrossRef]
- Margiotta-Casaluci, L.; Owen, S.F.; Rand-Weaver, M.; Winter, M.J. Testing the translational power of the zebrafish: An interspecies analysis of responses to cardiovascular drugs. Front. Pharmacol. 2019, 10, 893. [Google Scholar] [CrossRef]
- Marchant-Forde, J.; Lay, D., Jr.; Pajor, E.; Richert, B.; Schinckel, A. The effects of ractopamine on the behavior and physiology of finishing pigs. J. Anim. Sci. 2003, 81, 416–422. [Google Scholar] [CrossRef] [PubMed]
- Lonare, S.; Sole, S.; Umap, S.J. Exposure to ractopamine induces behavioural and reproductive alterations in zebrafish (danio rerio). Toxicol. Int. 2018, 25, 31–42. [Google Scholar]
- Lopes, E.; De Sousa, R.V.; Zangeronimo, M.; Pereira, A.N.D.J.; Coelho, M.D.R.; Ferreira, M.S.D.S.; Lima, R.R.; Marcondes, F.; Napimoga, M.H.; Pereira, L.J. Metabolic and behavioral effects of ractopamine at continuous low levels in rats under stress. Braz. Arch. Biol. Technol. 2015, 58, 406–413. [Google Scholar] [CrossRef]
- Srinivasan, A. Propranolol: A 50-year historical perspective. Ann. Indian Acad. Neurol. 2019, 22, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Prichard, B.N.C.; Gillam, P.M.C. Use of Propranolol (Inderal) in Treatment of Hypertension. BMJ 1964, 2, 725–727. [Google Scholar] [CrossRef]
- Hayes, P.E.; Schulz, S.C. Beta-blockers in anxiety disorders. J. Affect. Disord. 1987, 13, 119–130. [Google Scholar] [CrossRef]
- Frese, D.A.; Reinhardt, C.D.; Bartle, S.J.; Rethorst, D.N.; Bawa, B.; Thomason, J.D.; Loneragan, G.H.; Thomson, D.U. Effect of ractopamine hydrochloride and zilpaterol hydrochloride on cardiac electrophysiologic and hematologic variables in finishing steers. J. Am. Veter. Med Assoc. 2016, 249, 668–677. [Google Scholar] [CrossRef]
- Schwerte, T.; Prem, C.; Mairösl, A.; Pelster, B. Development of the sympatho-vagal balance in the cardiovascular system in zebrafish (Danio rerio) characterized by power spectrum and classical signal analysis. J. Exp. Biol. 2006, 209, 1093–1100. [Google Scholar] [CrossRef]
- Brown, D.; Ryan, K.; Daniel, Z.; Mareko, M.; Talbot, R.; Moreton, J.; Giles, T.C.B.; Emes, R.; Hodgman, C.; Parr, T.; et al. The Beta-adrenergic agonist, Ractopamine, increases skeletal muscle expression of Asparagine Synthetase as part of an integrated stress response gene program. Sci. Rep. 2018, 8, 15915. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Suman, S.; Li, S.; Nair, M.N.; Beach, C.M.; Edenburn, B.M.; Boler, D.D.; Dilger, A.C.; Felix, T.L. Ractopamine Influences Muscle Proteome Profile of Postmortem Beef Longissimus Lumborum. Meat Muscle Biol. 2017, 1, 140. [Google Scholar] [CrossRef]
- Barrett, A.; Cullum, V.A. The biological properties of the optical isomers of propranolol and their effects on cardiac arrhythmias. Br. J. Pharmacol. 1968, 34, 43–55. [Google Scholar] [CrossRef]
- Ruuskanen, J.O.; Xhaard, H.; Marjamäki, A.; Salaneck, E.; Salminen, T.; Yan, Y.-L.; Postlethwait, J.H.; Johnson, M.S.; Larhammar, D.; Scheinin, M. Identification of Duplicated Fourth α2-Adrenergic Receptor Subtype by Cloning and Mapping of Five Receptor Genes in Zebrafish. Mol. Biol. Evol. 2004, 21, 14–28. [Google Scholar] [CrossRef]
- Wang, Z.; Nishimura, Y.; Shimada, Y.; Umemoto, N.; Hirano, M.; Zang, L.; Oka, T.; Sakamoto, C.; Kuroyanagi, J.; Tanaka, T. Zebrafish β-adrenergic receptor mRNA expression and control of pigmentation. Gene 2009, 446, 18–27. [Google Scholar] [CrossRef]
- Johnston, S.; Staines, D.; Klein, A.; Marshall-Gradisnik, S. A targeted genome association study examining transient receptor potential ion channels, acetylcholine receptors, and adrenergic receptors in Chronic Fatigue Syndrome/Myalgic Encephalomyelitis. BMC Med. Genet. 2016, 17, 79. [Google Scholar] [CrossRef] [PubMed]
- Shappell, N.; Feil, V.; Smith, D.; Larsen, G.; McFarland, D. Response of c2c12 mouse and turkey skeletal muscle cells to the β-adrenergic agonist ractopamine. J. Anim. Sci. 2000, 78, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Maltin, C.A.; Delday, M.I.; Hay, S.M.; Smith, F.G.; Reeds, P.J. Propranolol apparently separates the physical and compositional characteristics of muscle growth induced by clenbuterol. Biosci. Rep. 1987, 7, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Goto, Y.; Ohgoshi, Y.; Yaku, H.; Kawaguchi, O.; Suga, H. Denopamine (beta1-selective adrenergic receptor agonist) and isoproterenol (non-selective beta-adrenergic receptor agonist) equally increase heart rate and myocardial oxygen consumption in dog heart. Jpn. Circ. J. 1991, 55, 972–982. [Google Scholar] [CrossRef] [PubMed]
- Kossack, M.; Hein, S.; Juergensen, L.; Siragusa, M.; Benz, A.; Katus, H.A.; Most, P.; Hassel, D. Induction of cardiac dysfunction in developing and adult zebrafish by chronic isoproterenol stimulation. J. Mol. Cell. Cardiol. 2017, 108, 95–105. [Google Scholar] [CrossRef]









| ZFIN Gene ID | Gene Name | Chromosomal Position | CDOCKER Score Ractopamine | CDOCKER Score Propranolol (R) | CDOCKER Score Propranolol (S) |
|---|---|---|---|---|---|
| ZDB-GENE-030131-2831 | adra1aa | 8 | 47.63 | 25.37 | 25.97 |
| ZDB-GENE-060503-384 | adra1ab | 10 | 44.77 | 22.98 | 22.40 |
| ZDB-GENE-120510-1 | adra1ba | 21 | 42.85 | 22.51 | 22.41 |
| ZDB-GENE-041114-51 | adra1bb | 14 | 44.83 | 25.58 | 27.33 |
| ZDB-GENE-090312-203 | adra1d | 1 | 42.50 | 23.17 | 23.37 |
| ZDB-GENE-021010-1 | adra2a | 22 | 43.68 | 21.53 | 23.25 |
| ZDB-GENE-021010-2 | adra2b | 8 | 46.91 | 24.93 | 24.48 |
| ZDB-GENE-021010-3 | adra2c | 1 | 41.90 | 24.20 | 23.57 |
| ZDB-GENE-021010-4 | adra2da | 14 | 44.27 | 24.42 | 22.44 |
| ZDB-GENE-021010-5 | adra2db | 21 | 42.74 | 25.17 | 22.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, K.; Saputra, F.; Suryanto, M.E.; Lai, Y.-H.; Huang, J.-C.; Yu, W.-H.; Chen, K.H.-C.; Lin, Y.-T.; Hsiao, C.-D. Evaluation of Effects of Ractopamine on Cardiovascular, Respiratory, and Locomotory Physiology in Animal Model Zebrafish Larvae. Cells 2021, 10, 2449. https://doi.org/10.3390/cells10092449
Abbas K, Saputra F, Suryanto ME, Lai Y-H, Huang J-C, Yu W-H, Chen KH-C, Lin Y-T, Hsiao C-D. Evaluation of Effects of Ractopamine on Cardiovascular, Respiratory, and Locomotory Physiology in Animal Model Zebrafish Larvae. Cells. 2021; 10(9):2449. https://doi.org/10.3390/cells10092449
Chicago/Turabian StyleAbbas, Kumail, Ferry Saputra, Michael Edbert Suryanto, Yu-Heng Lai, Jong-Chin Huang, Wen-Hao Yu, Kelvin H.-C. Chen, Ying-Ting Lin, and Chung-Der Hsiao. 2021. "Evaluation of Effects of Ractopamine on Cardiovascular, Respiratory, and Locomotory Physiology in Animal Model Zebrafish Larvae" Cells 10, no. 9: 2449. https://doi.org/10.3390/cells10092449
APA StyleAbbas, K., Saputra, F., Suryanto, M. E., Lai, Y. -H., Huang, J. -C., Yu, W. -H., Chen, K. H. -C., Lin, Y. -T., & Hsiao, C. -D. (2021). Evaluation of Effects of Ractopamine on Cardiovascular, Respiratory, and Locomotory Physiology in Animal Model Zebrafish Larvae. Cells, 10(9), 2449. https://doi.org/10.3390/cells10092449