Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms
Abstract
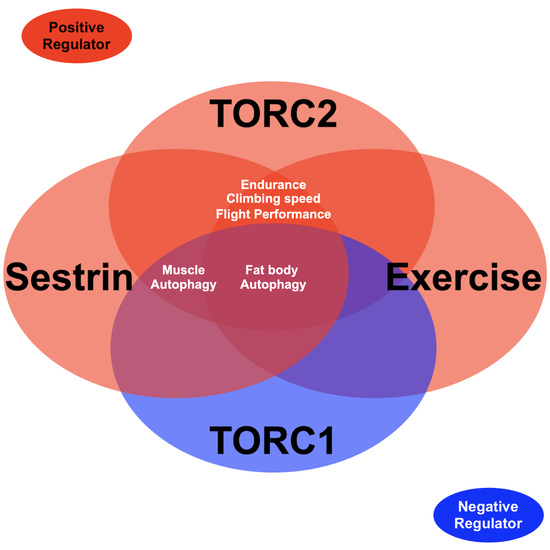
:1. Introduction
2. Materials and Methods
2.1. Fly Stocks and Maintenance
2.2. Exercise Training
2.3. Genetic Controls
2.4. Climbing Speed
2.5. qRT PCR
2.6. Lysotracker
3. Results
3.1. Sestrin Drives Exercise-Induced Increases to Climbing Speed and Atg8a Expression
3.2. Dsesn Induces Lysosomal Activity in Parallel with Exercise
3.3. Exercise-Induced Adaptations to Climbing Speed and Lysosomal Activity Require both Oxidoreductase and TOR-Modulating Functions of Sestrin
3.4. AKT Is Critical for Sestrin to Improve Climbing Speed and Enhance Lysosomal Activity
3.5. The TORC1 Axis Is Dispensable for Sestrin’s Effects on Climbing Speed and Lysosomal Activity
3.6. PGC1α Is Essential for the Beneficial Effects of Sestrin
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Houde, S.C.; Melillo, K.D. Caring for an aging population: Review of policy initiatives. J. Gerontol. Nurs. 2009, 35, 8–13. [Google Scholar] [CrossRef]
- Yazdanyar, A.; Newman, A.B. The burden of cardiovascular disease in the elderly: Morbidity, mortality, and costs. Clin. Geriatr. Med. 2009, 25, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartwright, T. ‘Getting on with life’: The experiences of older people using complementary health care. Soc. Sci. Med. 2007, 64, 1692–1703. [Google Scholar] [CrossRef] [PubMed]
- Finlayson, M.L.; Peterson, E.W. Falls, aging, and disability. Phys. Med. Rehabil. Clin. N. Am. 2010, 21, 357–373. [Google Scholar] [CrossRef]
- Nagatomo, I.; Kita, K.; Takigawa, M.; Nomaguchi, M.; Sameshima, K. A study of the quality of life in elderly people using psychological testing. Int. J. Geriatr. Psychiatry 1997, 12, 599–608. [Google Scholar] [CrossRef]
- Cutler, R.G.; Mattson, M.P. The adversities of aging. Ageing Res. Rev. 2006, 5, 221–238. [Google Scholar] [CrossRef] [PubMed]
- Bo, H.; Zhang, Y.; Ji, L.L. Redefining the role of mitochondria in exercise: A dynamic remodeling. Ann. N. Y. Acad. Sci. 2010, 1201, 121–128. [Google Scholar] [CrossRef]
- Gibala, M. Molecular responses to high-intensity interval exercise. Appl. Physiol. Nutr. Metab. 2009, 34, 428–432. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Mettauer, B.; Bigard, X. Beneficial effects of endurance training on cardiac and skeletal muscle energy metabolism in heart failure. Cardiovasc. Res. 2007, 73, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Ascensao, A.; Lumini-Oliveira, J.; Oliveira, P.J.; Magalhaes, J. Mitochondria as a target for exercise-induced cardioprotection. Curr. Drug Targets 2011, 12, 860–871. [Google Scholar] [CrossRef]
- Laker, R.C.; Xu, P.; Ryall, K.A.; Sujkowski, A.; Kenwood, B.M.; Chain, K.H.; Zhang, M.; Royal, M.A.; Hoehn, K.L.; Driscoll, M.; et al. A novel MitoTimer reporter gene for mitochondrial content, structure, stress, and damage in vivo. J. Biol. Chem. 2014, 289, 12005–12015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kennedy, R.L.; Chokkalingham, K.; Srinivasan, R. Obesity in the elderly: Who should we be treating, and why, and how? Curr. Opin. Clin. Nutr. Metab. Care 2004, 7, 3–9. [Google Scholar] [CrossRef]
- Sujkowski, A.; Saunders, S.; Tinkerhess, M.; Piazza, N.; Jennens, J.; Healy, L.; Zheng, L.; Wessells, R. dFatp regulates nutrient distribution and long-term physiology in Drosophila. Aging Cell 2012. [Google Scholar] [CrossRef] [Green Version]
- Mittendorfer, B.; Horowitz, J.F.; Klein, S. Gender differences in lipid and glucose kinetics during short-term fasting. Am. J. Physiol. Endocrinol. Metab. 2001, 281, E1333–E1339. [Google Scholar] [CrossRef] [PubMed]
- Corcoran, M.P.; Lamon-Fava, S.; Fielding, R.A. Skeletal muscle lipid deposition and insulin resistance: Effect of dietary fatty acids and exercise. Am. J. Clin. Nutr. 2007, 85, 662–677. [Google Scholar] [PubMed]
- Rafalski, V.A.; Brunet, A. Energy metabolism in adult neural stem cell fate. Prog. Neurobiol. 2011, 93, 182–203. [Google Scholar] [CrossRef]
- Sujkowski, A.; Bazzell, B.; Carpenter, K.; Arking, R.; Wessells, R.J. Endurance exercise and selective breeding for longevity extend Drosophila healthspan by overlapping mechanisms. Aging (Albany N. Y.) 2015, 7, 535–552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laranjeiro, R.; Harinath, G.; Hewitt, J.E.; Hartman, J.H.; Royal, M.A.; Meyer, J.N.; Vanapalli, S.A.; Driscoll, M. Swim exercise in Caenorhabditis elegans extends neuromuscular and gut healthspan, enhances learning ability, and protects against neurodegeneration. Proc. Natl. Acad. Sci. USA 2019, 116, 23829–23839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujkowski, A.; Gretzinger, A.; Soave, N.; Todi, S.V.; Wessells, R. Alpha- and beta-adrenergic octopamine receptors in muscle and heart are required for Drosophila exercise adaptations. PLoS Genet. 2020, 16, e1008778. [Google Scholar] [CrossRef] [PubMed]
- Buchner, D.M.; Wagner, E.H. Preventing frail health. Clin. Geriatr. Med. 1992, 8, 1–17. [Google Scholar] [CrossRef]
- Phillips, E.M.; Schneider, J.C.; Mercer, G.R. Motivating elders to initiate and maintain exercise. Arch. Phys. Med. Rehabil. 2004, 85, S52–S57. [Google Scholar] [CrossRef]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Budanov, A.V.; Park, E.J.; Birse, R.; Kim, T.E.; Perkins, G.A.; Ocorr, K.; Ellisman, M.H.; Bodmer, R.; Bier, E.; et al. Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies. Science 2010, 327, 1223–1228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Budanov, A.V.; Lee, J.H.; Karin, M. Stressin’ Sestrins take an aging fight. EMBO Mol. Med. 2010, 2, 388–400. [Google Scholar] [CrossRef]
- Peeters, H.; Debeer, P.; Bairoch, A.; Wilquet, V.; Huysmans, C.; Parthoens, E.; Fryns, J.P.; Gewillig, M.; Nakamura, Y.; Niikawa, N.; et al. PA26 is a candidate gene for heterotaxia in humans: Identification of a novel PA26-related gene family in human and mouse. Hum. Genet. 2003, 112, 573–580. [Google Scholar] [CrossRef]
- Matheu, A.; Maraver, A.; Klatt, P.; Flores, I.; Garcia-Cao, I.; Borras, C.; Flores, J.M.; Vina, J.; Blasco, M.A.; Serrano, M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature 2007, 448, 375–379. [Google Scholar] [CrossRef] [PubMed]
- Budanov, A.V.; Karin, M. p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling. Cell 2008, 134, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, A.; Cho, C.S.; Namkoong, S.; Cho, U.S.; Lee, J.H. Biochemical Basis of Sestrin Physiological Activities. Trends Biochem. Sci. 2016, 41, 621–632. [Google Scholar] [CrossRef] [Green Version]
- Maiuri, M.C.; Malik, S.A.; Morselli, E.; Kepp, O.; Criollo, A.; Mouchel, P.L.; Carnuccio, R.; Kroemer, G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 2009, 8, 1571–1576. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sujkowski, A.; Namkoong, S.; Gu, B.; Cobb, T.; Kim, B.; Kowalsky, A.H.; Cho, C.S.; Semple, I.; Ro, S.H.; et al. Sestrins are evolutionarily conserved mediators of exercise benefits. Nat. Commun. 2020, 11, 190. [Google Scholar] [CrossRef]
- Piazza, N.; Gosangi, B.; Devilla, S.; Arking, R.; Wessells, R. Exercise-training in young Drosophila melanogaster reduces age-related decline in mobility and cardiac performance. PLoS ONE 2009, 4, e5886. [Google Scholar] [CrossRef]
- Damschroder, D.; Cobb, T.; Sujkowski, A.; Wessells, R. Drosophila Endurance Training and Assessment and Its Effects on Systemic Adaptations. BioProtocols 2017. [Google Scholar] [CrossRef]
- Sujkowski, A.; Ramesh, D.; Brockmann, A.; Wessells, R. Octopamine Drives Endurance Exercise Adaptations in Drosophila. Cell Rep. 2017, 21, 1809–1823. [Google Scholar] [CrossRef] [Green Version]
- Bai, H.; Kang, P.; Hernandez, A.M.; Tatar, M. Activin signaling targeted by insulin/dFOXO regulates aging and muscle proteostasis in Drosophila. PLoS Genet. 2013, 9, e1003941. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.L.; Kang, C.; Zhang, Y. Exercise-induced hormesis and skeletal muscle health. Free. Radic. Biol. Med. 2016. [Google Scholar] [CrossRef] [PubMed]
- Lira, V.A.; Okutsu, M.; Zhang, M.; Greene, N.P.; Laker, R.C.; Breen, D.S.; Hoehn, K.L.; Yan, Z. Autophagy is required for exercise training-induced skeletal muscle adaptation and improvement of physical performance. FASEB J. 2013, 27, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- Ogura, Y.; Iemitsu, M.; Naito, H.; Kakigi, R.; Kakehashi, C.; Maeda, S.; Akema, T. Single bout of running exercise changes LC3-II expression in rat cardiac muscle. Biochem. Biophys. Res. Commun. 2011, 414, 756–760. [Google Scholar] [CrossRef]
- Kim, J.S.; Ro, S.H.; Kim, M.; Park, H.W.; Semple, I.A.; Park, H.; Cho, U.S.; Wang, W.; Guan, K.L.; Karin, M.; et al. Sestrin2 inhibits mTORC1 through modulation of GATOR complexes. Sci. Rep. 2015, 5, 9502. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Chowdhuri, D.K. Modulation of sestrin confers protection to Cr(VI) induced neuronal cell death in Drosophila melanogaster. Chemosphere 2018, 191, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Dowling, R.J.O.; Topisirovic, I.; Alain, T.; Bidinosti, M.; Fonseca, B.D.; Petroulakis, E.; Wang, X.S.; Larsson, O.; Selvaraj, A.; Liu, Y.; et al. mTORC1-Mediated Cell Proliferation, But Not Cell Growth, Controlled by the 4E-BPs. Science 2010, 328, 1172–1176. [Google Scholar] [CrossRef] [Green Version]
- Hollmann, W.; Struder, H.K.; Tagarakis, C.V.; King, G. Physical activity and the elderly. Eur. J. Cardiovasc. Prev. Rehabil. 2007, 14, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Zettel-Watson, L.; Suen, M.; Wehbe, L.; Rutledge, D.N.; Cherry, B.J. Aging well: Processing speed inhibition and working memory related to balance and aerobic endurance. Geriatr. Gerontol. Int. 2017, 17, 108–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.A.; DuMontier, C.; Murillo, A.; Hshieh, T.T.; Bean, J.F.; Soiffer, R.J.; Stone, R.M.; Abel, G.A.; Driver, J.A. Gait speed, grip strength, and clinical outcomes in older patients with hematologic malignancies. Blood 2019, 134, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Rijnhart, J.J.M.; Skoog, J.; Robitaille, A.; van den Hout, A.; Ferrucci, L.; Huisman, M.; Skoog, I.; Piccinin, A.M.; Hofer, S.M.; et al. Gait speed as predictor of transition into cognitive impairment: Findings from three longitudinal studies on aging. Exp. Gerontol. 2020, 129, 110783. [Google Scholar] [CrossRef] [PubMed]
- Shirakabe, A.; Ikeda, Y.; Sciarretta, S.; Zablocki, D.K.; Sadoshima, J. Aging and Autophagy in the Heart. Circ. Res. 2016, 118, 1563–1576. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, Y.; Sciarretta, S.; Nagarajan, N.; Rubattu, S.; Volpe, M.; Frati, G.; Sadoshima, J. New insights into the role of mitochondrial dynamics and autophagy during oxidative stress and aging in the heart. Oxid. Med. Cell. Longev. 2014, 2014, 210934. [Google Scholar] [CrossRef]
- Wohlgemuth, S.E.; Julian, D.; Akin, D.E.; Fried, J.; Toscano, K.; Leeuwenburgh, C.; Dunn, W.A., Jr. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007, 10, 281–292. [Google Scholar] [CrossRef]
- Hay, N.; Sonenberg, N. Upstream and downstream of mTOR. Genes Dev. 2004, 18, 1926–1945. [Google Scholar] [CrossRef] [Green Version]
- Wullschleger, S.; Loewith, R.; Hall, M.N. TOR signaling in growth and metabolism. Cell 2006, 124, 471–484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laplante, M.; Sabatini, D.M. An emerging role of mTOR in lipid biosynthesis. Curr. Biol. 2009, 19, R1046–R1052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dann, S.G.; Selvaraj, A.; Thomas, G. mTOR Complex1-S6K1 signaling: At the crossroads of obesity, diabetes and cancer. Trends Mol. Med. 2007, 13, 252–259. [Google Scholar] [CrossRef]
- Bentzinger, C.F.; Romanino, K.; Cloetta, D.; Lin, S.; Mascarenhas, J.B.; Oliveri, F.; Xia, J.; Casanova, E.; Costa, C.F.; Brink, M.; et al. Skeletal muscle-specific ablation of raptor, but not of rictor, causes metabolic changes and results in muscle dystrophy. Cell Metab. 2008, 8, 411–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masiero, E.; Agatea, L.; Mammucari, C.; Blaauw, B.; Loro, E.; Komatsu, M.; Metzger, D.; Reggiani, C.; Schiaffino, S.; Sandri, M. Autophagy is required to maintain muscle mass. Cell Metab. 2009, 10, 507–515. [Google Scholar] [CrossRef]
- Gottlieb, R.A.; Mentzer, R.M. Autophagy during cardiac stress: Joys and frustrations of autophagy. Annu. Rev. Physiol. 2010, 72, 45–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakai, A.; Yamaguchi, O.; Takeda, T.; Higuchi, Y.; Hikoso, S.; Taniike, M.; Omiya, S.; Mizote, I.; Matsumura, Y.; Asahi, M.; et al. The role of autophagy in cardiomyocytes in the basal state and in response to hemodynamic stress. Nat. Med. 2007, 13, 619–624. [Google Scholar] [CrossRef] [PubMed]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sujkowski, A.; Wessells, R. Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms. Cells 2021, 10, 2479. https://doi.org/10.3390/cells10092479
Sujkowski A, Wessells R. Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms. Cells. 2021; 10(9):2479. https://doi.org/10.3390/cells10092479
Chicago/Turabian StyleSujkowski, Alyson, and Robert Wessells. 2021. "Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms" Cells 10, no. 9: 2479. https://doi.org/10.3390/cells10092479
APA StyleSujkowski, A., & Wessells, R. (2021). Exercise and Sestrin Mediate Speed and Lysosomal Activity in Drosophila by Partially Overlapping Mechanisms. Cells, 10(9), 2479. https://doi.org/10.3390/cells10092479