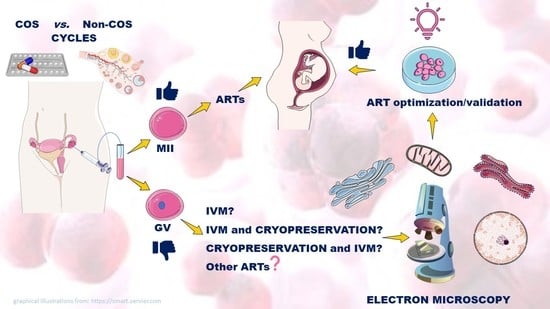
Ultrastructural Evaluation of the Human Oocyte at the Germinal Vesicle Stage during the Application of Assisted Reproductive Technologies
Abstract
:1. Introduction
1.1. From the Ovum to the Cicatricula
1.2. The Identification of the Germinal Vesicle
1.3. GV-Stage Oocytes as a Potential Resource in ARTs
2. Materials and Methods
Inclusion and Exclusion Criteria for the Research in MEDLINE–PubMed, Scopus and ISI Web of Science Databases
3. Ultrastructure of GV-Stage Oocytes
3.1. Nucleus
3.2. Ooplasm
3.2.1. Mitochondria
3.2.2. Mitochondria-Smooth Endoplasmic Reticulum (M-SER) Aggregates
3.2.3. Golgi Apparatus and Smooth Endoplasmic Reticulum (ER)
3.2.4. Cortical Granules (CGs)
3.2.5. Vacuoles
3.2.6. Lysosomes
3.3. Oolemma
Microvilli, Perivitelline Space (PVS) and Zona Pellucida (ZP) Texture
4. Oocyte Quality and Early Embryo Development from ART Cycles
5. Unstimulated Vs. Stimulated GV-Stage Oocytes
6. In Vitro Maturation (IVM)
6.1. IVM of Unstimulated GV-Stage Oocytes
6.2. Rescue IVM from Stimulated Oocytes
7. Cryopreservation
7.1. Slow Freezing
7.2. Vitrification
8. Mitochondrial Replacement
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Aristotle. Generation of Animals, with an English Translation by A.L. Peck; William Heinemann LTD: London, UK; Harvard University Press: Cambridge, MA, USA, 1943. [Google Scholar]
- Harvey, W. Exercises of Animal Generation. In Exercitationes de Generatione Animalium; Pulleyn, I.O., Ed.; Typis Du-Gardianis: London, UK, 1651. [Google Scholar]
- Cobb, M. An amazing 10 years: The discovery of egg and sperm in the 17th century. Reprod. Domest. Anim. 2012, 47, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Turriziani Colonna, F. De Ovi Mammalium et Hominis Genesi (1827), by Karl Ernst Von Baer; Embryo Project Encyclopedia; Center for Biology and Society, School of Life Sciences, Arizona State University: Tempe, AZ, USA, 2017; Available online: http://embryo.asu.edu/handle/10776/11405 (accessed on 25 March 2022).
- De Graaf, R. The Female Organs in the Service of Generation. In De Mulierum Organis Generationi Inservientibus; Ex Officina Hackiana: Leiden, The Netherlands, 1672. [Google Scholar]
- Spallanzani, L. Saggio di Osservazioni Microscopiche Concernenti il Sistema Della Generazione Dei Signori Needham e Buffon; Società Tipografica Editrice Barese: Bari, Italy, 1914. [Google Scholar]
- Prévost, J.L.; Dumas, J.B.A. Nouvelle Théorie de la Generation. In Physiologie et Anatomie Animale; Annales des Sciences Naturelles: Paris, France, 1984. [Google Scholar]
- Buess, H. The contribution of Geneva physicians to the physiology of development in the 19th century. Bull. Hist. Med. 1947, 21, 871–897. [Google Scholar] [PubMed]
- Purkinje, J.E. De evolutione vesiculae germinativae. In Symbolae ad Ovi Avium Historiam ante Incubationem; Leopold Vossi: Leipzig, Germany, 1830; pp. 1–24. [Google Scholar]
- Von Baer, K.E. De Ovi Mammalium et Hominis Genesi. In On the Genesis of the Ovum of Mammals and of Man; Leopold Voss: Leipzig, Germany, 1827. [Google Scholar]
- Altmäe, S.; Acharya, G.; Salumets, A. Celebrating Baer—A Nordic scientist who discovered the mammalian oocyte. Acta Obstet. Gynecol. Scand. 2017, 96, 1281–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller-Wille, S. Cell theory, specificity, and reproduction, 1837–1870. Stud. Hist. Philos. Biol. Biomed. Sci. 2010, 41, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Kruta, V. The letters of Purkinje. Lancet 1956, 268, 360. [Google Scholar] [CrossRef]
- Harris, H. The Discovery of the Cell Nucleus. In The Birth of the Cell; Harris, H., Ed.; Yale University Press: New Haven, CT, USA, 1999; pp. 76–93. [Google Scholar]
- Alexandre, H. A history of mammalian embryological research. Int. J. Dev. Biol. 2001, 45, 457–467. [Google Scholar]
- Makabe, S.; Naguro, T.; Nottola, S.A.; Van Blerkom, J. Atlas of Human Female Reproductive Function: Ovarian Development to Early Embryogenesis after In Vitro Fertilization; Taylor & Francis: London, UK; New York, NY, USA, 2006; p. 89. [Google Scholar]
- Coticchio, G.; Canto, M.D.; Guglielmo, M.C.; Albertini, D.F.; Renzini, M.M.; Merola, M.; Lain, M.; Sottocornola, M.; De Ponti, E.; Fadini, R. Double-strand DNA breaks and repair response in human immature oocytes and their relevance to meiotic resumption. J. Assist. Reprod. Genet. 2015, 32, 1509–1516. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.-H.; Zhou, W.-H.; Chu, D.-P.; Fu, L.; Sha, W.; Li, Y. Ultrastructural Changes and Methylation of Human Oocytes Vitrified at the Germinal Vesicle Stage and Matured in vitro after Thawing. Gynecol. Obstet. Investig. 2017, 82, 252–261. [Google Scholar] [CrossRef]
- Peinado, I.; Moya, I.; Sáez-Espinosa, P.; Barrera, M.; García-Valverde, L.; Francés, R.; Torres, P.; Gómez-Torres, M. Impact of Maturation and Vitrification Time of Human GV Oocytes on the Metaphase Plate Configuration. Int. J. Mol. Sci. 2021, 22, 1125. [Google Scholar] [CrossRef]
- Nilsson, B.O.; Liedholm, P.; Larsson, E. Ultrastructural characteristics of human oocytes fixed at follicular puncture or after culture. J. Assist. Reprod. Genet. 1985, 2, 195–206. [Google Scholar] [CrossRef]
- Pires-Luís, A.S.; Rocha, E.; Bartosch, C.; Oliveira, E.; Silva, J.; Barros, A.; Sá, R.; Sousa, M. A stereological study on organelle distribution in human oocytes at prophase I. Zygote 2015, 24, 346–354. [Google Scholar] [CrossRef] [PubMed]
- Palmerini, M.G.; Antinori, M.; Maione, M.; Cerusico, F.; Versaci, C.; Nottola, S.A.; Macchiarelli, G.; Khalili, M.A.; Antinori, S. Ultrastructure of immature and mature human oocytes after cryotop vitrification. J. Reprod. Dev. 2014, 60, 411–420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suzuki, S.; Kitai, H.; Tojo, R.; Seki, K.; Oba, M.; Fujiwara, T.; Iizuka, R. Ultrastructure and some biologic properties of human oocytes and granulosa cells cultured in vitro. Fertil. Steril. 1981, 35, 142–148. [Google Scholar] [CrossRef]
- Sathananthan, A.H.; Selvaraj, K.; Girijashankar, M.L.; Ganesh, V.; Selvaraj, P.; Trounson, A.O. From oogonia to mature oocytes: Inactivation of the maternal centrosome in humans. Microsc. Res. Tech. 2006, 69, 396–407. [Google Scholar] [CrossRef]
- Sathananthan, A.H.; Trounson, A.; Wood, C. Atlas of Fine Structure of Human Sperm Penetration, Eggs, and Embryos Cultured In Vitro; Praeger: New York, NY, USA, 1985. [Google Scholar]
- Trebichalská, Z.; Kyjovská, D.; Kloudová, S.; Otevřel, P.; Hampl, A.; Holubcová, Z. Cytoplasmic maturation in human oocytes: An ultrastructural study. Biol. Reprod. 2020, 104, 106–116. [Google Scholar] [CrossRef]
- Szollosi, D.; Szöllösi, M.S.; Czolowska, R.; Tarkowski, A.K. Sperm penetration into immature mouse oocytes and nuclear changes during maturation: An EM study. Biol. Cell 1990, 69, 53–64. [Google Scholar] [CrossRef]
- Yang, Y.-J.; Zhang, Y.-J.; Li, Y. Ultrastructure of human oocytes of different maturity stages and the alteration during in vitro maturation. Fertil. Steril. 2009, 92, 396.e1–396.e6. [Google Scholar] [CrossRef]
- Khalili, M.A.; Shahedi, A.; Ashourzadeh, S.; Nottola, S.A.; Macchiarelli, G.; Palmerini, M.G. Vitrification of human immature oocytes before and after in vitro maturation: A review. J. Assist. Reprod. Genet. 2017, 34, 1413–1426. [Google Scholar] [CrossRef]
- Coticchio, G.; Canto, M.D.; Fadini, R.; Renzini, M.M.; Guglielmo, M.C.; Miglietta, S.; Palmerini, M.G.; Macchiarelli, G.; Nottola, S.A. Ultrastructure of human oocytes after in vitro maturation. Mol. Hum. Reprod. 2016, 22, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, Y. Ultrastructure of the Human Oocytes during in Vitro Maturation. J. Mamm. Ova Res. 2009, 26, 10–17. [Google Scholar] [CrossRef]
- Takahashi, Y.; Hashimoto, S.; Yamochi, T.; Goto, H.; Yamanaka, M.; Amo, A.; Matsumoto, H.; Inoue, M.; Ito, K.; Nakaoka, Y.; et al. Dynamic changes in mitochondrial distribution in human oocytes during meiotic maturation. J. Assist. Reprod. Genet. 2016, 33, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Blerkom, J. Developmental Failure in Human Reproduction Associated with Preovulatory Oogenesis and Preimplantation Embryogenesis. In Ultrastructure of Human Gametogenesis and Early Embryogenesis; Van Blerkom, J., Motta, P.M., Eds.; Springer: New York, NY, USA, 1989. [Google Scholar] [CrossRef]
- Ghetler, Y.; Skutelsky, E.; Ben Nun, I.; Ben Dor, L.; Amihai, D.; Shalgi, R. Human oocyte cryopreservation and the fate of cortical granules. Fertil. Steril. 2006, 86, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Monti, M.; Calligaro, A.; Behr, B.; Pera, R.R.; Redi, C.A.; Wossidlo, M. Functional topography of the fully grown human oocyte. Eur. J. Histochem. 2017, 61, 2769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Familiari, G.; Nottola, S.A.; Micara, G.; Aragona, C.; Motta, P.M. Is the sperm-binding capability of the zona pellucida linked to its surface structure? A scanning electron microscopic study of human in vitro fertilization. J. Assist. Reprod. Genet. 1988, 5, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-K.; Lee, S.-C.; Kim, K.-J.; Han, C.-H.; Kim, J.-H. In vitro maturation, fertilization, and development of human germinal vesicle oocytes collected from stimulated cycles. Fertil. Steril. 2000, 74, 1153–1158. [Google Scholar] [CrossRef]
- Halvaei, I.; Khalili, M.A.; Razi, M.H.; Nottola, S.A. The effect of immature oocytes quantity on the rates of oocytes maturity and morphology, fertilization, and embryo development in ICSI cycles. J. Assist. Reprod. Genet. 2012, 29, 803–810. [Google Scholar] [CrossRef] [Green Version]
- Alcoba, D.D.; Pimentel, A.M.; Brum, I.S.; Corleta, H.V.E. Developmental potential of in vitro or in vivo matured oocytes. Zygote 2013, 23, 93–98. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.E.; Kim, S.D.; Jee, B.C.; Suh, C.S.; Kim, S.H. Oocyte maturity in repeated ovarian stimulation. Clin. Exp. Reprod. Med. 2011, 38, 234–237. [Google Scholar] [CrossRef]
- Astbury, P.; Subramanian, G.N.; Greaney, J.; Roling, C.; Irving, J.; Homer, H.A. The Presence of Immature GV–Stage Oocytes during IVF/ICSI Is a Marker of Poor Oocyte Quality: A Pilot Study. Med. Sci. 2020, 8, 4. [Google Scholar] [CrossRef] [Green Version]
- Siristatidis, C.S.; Maheshwari, A.; Bhattacharya, S. In vitro maturation in sub fertile women with polycystic ovarian syndrome undergoing assisted reproduction. Cochrane Database Syst. Rev. 2009, 1, CD006606. [Google Scholar] [CrossRef]
- Nikbakht, R.; Mohammadjafari, R.; Rajabalipour, M.; Moghadam, M.T. Evaluation of oocyte quality in Polycystic ovary syndrome patients undergoing ART cycles. Fertil. Res. Pract. 2021, 7, 2. [Google Scholar] [CrossRef] [PubMed]
- Rienzi, L.; Balaban, B.; Ebner, T.; Mandelbaum, J. The oocyte. Hum. Reprod. 2012, 27, i2–i21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hatırnaz, Ş.; Ata, B.; Hatırnaz, E.S.; Dahan, M.; Tannus, S.; Tan, J.; Tan, S.L. Oocyte in vitro maturation: A systematic review. J. Turk. Soc. Obstet. Gynecol. 2018, 15, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Escrich, L.; Grau, N.; Santos, M.J.D.L.; Romero, J.-L.; Pellicer, A.; Escribá, M.-J. The dynamics of in vitro maturation of germinal vesicle oocytes. Fertil. Steril. 2012, 98, 1147–1151. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, L.; Wang, M.; Huang, B.; Li, Z.; Hu, J.; Xi, Q.; Liu, J.; Jin, L. Analysis of maturation dynamics and developmental competence of in vitro matured oocytes under time-lapse monitoring. Reprod. Biol. Endocrinol. 2021, 19, 183. [Google Scholar] [CrossRef]
- Lee, H.-J.; Barad, D.H.; Kushnir, V.A.; Shohat-Tal, A.; Lazzaroni-Tealdi, E.; Wu, Y.-G.; Gleicher, N. Rescue in vitro maturation (IVM) of immature oocytes in stimulated cycles in women with low functional ovarian reserve (LFOR). Endocrine 2015, 52, 165–171. [Google Scholar] [CrossRef]
- Liu, Y.; Jiang, H.; Du, X.; Huang, J.; Wang, X.; Hu, Y.; Ni, F.; Liu, C. Contribution of rescue in-vitro maturation versus double ovarian stimulation in ovarian stimulation cycles of poor-prognosis women. Reprod. Biomed. Online 2020, 40, 511–517. [Google Scholar] [CrossRef]
- Steptoe, P.; Edwards, R. Birth after the reimplantation of a human embryo. Lancet 1978, 312, 366. [Google Scholar] [CrossRef]
- Edwards, R.G.; Steptoe, P.C.; Purdy, J.M. Establishing full-term human pregnancies using cleaving embryos grown in vitro. BJOG Int. J. Obstet. Gynaecol. 1980, 87, 737–756. [Google Scholar] [CrossRef]
- Pelinck, M.; Vogel, N.; Arts, E.; Simons, A.; Heineman, M.; Hoek, A. Cumulative pregnancy rates after a maximum of nine cycles of modified natural cycle IVF and analysis of patient drop-out: A cohort study. Hum. Reprod. 2007, 22, 2463–2470. [Google Scholar] [CrossRef] [Green Version]
- Mak, W.; Kondapalli, L.A.; Celia, G.; Gordon, J.; DiMattina, M.; Payson, M. Natural cycle IVF reduces the risk of low birthweight infants compared with conventional stimulated IVF. Hum. Reprod. 2016, 31, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Von Wolff, M. The role of Natural Cycle IVF in assisted reproduction. Best Pract. Res. Clin. Endocrinol. Metab. 2018, 33, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Ingerslev, H.J.; Højgaard, A.; Hindkjær, J.; Kesmodel, U. A randomized study comparing IVF in the unstimulated cycle with IVF following clomiphene citrate. Hum. Reprod. 2001, 16, 696–702. [Google Scholar] [CrossRef] [PubMed]
- Macklon, N.S.; Stouffer, R.L.; Giudice, L.C.; Fauser, B.C.J.M. The Science behind 25 Years of Ovarian Stimulation for in Vitro Fertilization. Endocr. Rev. 2006, 27, 170–207. [Google Scholar] [CrossRef]
- Ubaldi, F.; Rienzi, L.; Baroni, E.; Ferrero, S.; Iacobelli, M.; Minasi, M.; Sapienza, F.; Romano, S.; Colasante, A.; Litwicka, K.; et al. Hopes and facts about mild ovarian stimulation. Reprod. Biomed. Online 2007, 14, 675–681. [Google Scholar] [CrossRef]
- Escrich, L.; Galiana, Y.; Grau, N.; Insua, F.; Soler, N.; Pellicer, A.; Escribá, M. Do immature and mature sibling oocytes recovered from stimulated cycles have the same reproductive potential? Reprod. Biomed. Online 2018, 37, 667–676. [Google Scholar] [CrossRef]
- Greenblatt, E.M.; Meriano, J.S.; Casper, R.F. Type of stimulation protocol affects oocyte maturity, fertilization rate, and cleavage rate after intracytoplasmic sperm injection. Fertil. Steril. 1995, 64, 557–563. [Google Scholar] [CrossRef]
- Nogueira, D.; Friedler, S.; Schachter, M.; Raziel, A.; Ron-El, R.; Smitz, J. Oocyte maturity and preimplantation development in relation to follicle diameter in gonadotropin-releasing hormone agonist or antagonist treatments. Fertil. Steril. 2006, 85, 578–583. [Google Scholar] [CrossRef]
- Huddleston, H.G.; Jackson, K.V.; Doyle, J.O.; Racowsky, C. hMG increases the yield of mature oocytes and excellent-quality embryos in patients with a previous cycle having a high incidence of oocyte immaturity. Fertil. Steril. 2009, 92, 946–949. [Google Scholar] [CrossRef]
- Ming, T.X.; Nielsen, H.I.; Chen, Z.Q. Maturation arrest of human oocytes at germinal vesicle stage. J. Hum. Reprod. Sci. 2010, 3, 153–157. [Google Scholar] [CrossRef]
- Cha, K.Y.; Koo, J.J.; Ko, J.J.; Choi, D.H.; Han, S.Y.; Yoon, T.K. Pregnancy after in vitro fertilization of human follicular oocytes collected from nonstimulated cycles, their culture in vitro and their transfer in a donor oocyte program. Fertil. Steril. 1991, 55, 109–113. [Google Scholar] [CrossRef]
- Fatum, M.; Bergeron, M.-E.; Ross, C.; Bhevan, A.; Turner, K.; Child, T. Rescue In Vitro Maturation in Polycystic Ovarian Syndrome Patients Undergoing In Vitro Fertilization Treatment who Overrespond or Underrespond to Ovarian Stimulation: Is It a Viable Option? A Case Series Study. Int. J. Fertil. Steril. 2020, 14, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.M.; Cram, D.S.; Song, B.; Magli, M.C.; Gianaroli, L.; Lacham-Kaplan, O.; Findlay, J.K.; Jenkin, G.; Trounson, A.O. Gene expression profiling of human oocytes following in vivo or in vitro maturation. Hum. Reprod. 2008, 23, 1138–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilchrist, R.B.; Smitz, J.E.J.; Thompson, J.G. Current Status and Future Trends of the Clinical Practice of Human Oocyte In Vitro Maturation. In Human Assisted Reproductive Technology Future Trends in Laboratory and Clinical Practice; Gardner, D.K., Rizk, B., Falcone, T., Eds.; Cambridge University Press: Cambridge, UK, 2011; pp. 186–198. [Google Scholar]
- Khalili, M.A.; A Nottola, S.; Shahedi, A.; Macchiarelli, G. Contribution of human oocyte architecture to success of in vitro maturation technology. Iran. J. Reprod. Med. 2013, 11, 1–10. [Google Scholar]
- Trounson, A.; Wood, C.; Kausche, A. In vitro maturation and the fertilization and developmental competence of oocytes recovered from untreated polycystic ovarian patients. Fertil. Steril. 1994, 62, 353–362. [Google Scholar] [CrossRef]
- Barnes, F.L.; Kausche, A.; Tiglias, J.; Wood, C.; Wilton, L.; Trounson, A. Production of embryos from in vitro-matured primary human oocytes. Fertil. Steril. 1996, 65, 1151–1156. [Google Scholar] [CrossRef]
- Russell, J.B.; Knezevich, K.M.; Fabian, K.F.; Dickson, J.A. Unstimulated immature oocyte retrieval: Early versus midfollicular endometrial priming. Fertil. Steril. 1997, 67, 616–620. [Google Scholar] [CrossRef]
- Chian, R.C.; Park, S.E.; Park, E.H.; Son, W.Y.; Chung, H.M.; Lim, J.G.; Ko, J.J.; Cha, K.Y. Molecular and structural characteristics between immature human oocytes retrieved from stimulated and unstimulated ovaries. In In Vitro Fertilization and Assisted Reproduction; Gromel, V., Leung, P.C.K., Eds.; Monduzzi Editore: Bologna, Italy, 1997; pp. 315–319. [Google Scholar]
- Chian, R.-C.; Buckett, W.M.; Tan, S.-L. In-vitro maturation of human oocytes. Reprod. Biomed. Online 2004, 8, 148–166. [Google Scholar] [CrossRef]
- Choi, J.; Kim, D.; Cha, J.; Lee, S.; Yoon, S.; Lim, J. P-705: The relationship between oocyte size and developmental competence in unstimulated cycles. Fertil. Steril. 2006, 86, S395. [Google Scholar] [CrossRef]
- Cavilla, J.; Byskov, A.; Hartshorne, G.; Kennedy, C. Human immature oocytes grow during culture for IVM. Hum. Reprod. 2007, 23, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Child, T.J.; Abdul-Jalil, A.K.; Gulekli, B.; Tan, S.L. In vitro maturation and fertilization of oocytes from unstimulated normal ovaries, polycystic ovaries, and women with polycystic ovary syndrome. Fertil. Steril. 2001, 76, 936–942. [Google Scholar] [CrossRef]
- Mikkelsen, A.L. Strategies in human in-vitro maturation and their clinical outcome. Reprod. Biomed. Online 2005, 10, 593–599. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, J.-J.; Feng, H.-L.; Ma, S.-Y.; Li, M.; Li, Y. Evaluation of the immature human oocytes from unstimulated cycles in polycystic ovary syndrome patients using a novel scoring system. Fertil. Steril. 2010, 93, 2202–2209. [Google Scholar] [CrossRef] [PubMed]
- Gleicher, N.; Weghofer, A.; Barad, D.H. Defining ovarian reserve to better understand ovarian aging. Reprod. Biol. Endocrinol. 2011, 9, 23. [Google Scholar] [CrossRef] [Green Version]
- Álvarez, C.; García-Garrido, C.; Taronger, R.; González de Merlo, G. In vitro maturation, fertilization, embryo development & clinical outcome of human metaphase-I oocytes retrieved from stimulated intracytoplasmic sperm injection cycles. Indian J. Med. Res. 2013, 137, 331–338. [Google Scholar]
- Son, W.-Y.; Lee, S.-Y.; Lim, J.-H. Fertilization, cleavage and blastocyst development according to the maturation timing of oocytes in in vitro maturation cycles. Hum. Reprod. 2005, 20, 3204–3207. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, I.; Komsky, A.; Bern, O.; Kasterstein, E.; Komarovsky, D.; Ron-El, R. In vitro maturation of human germinal vesicle-stage oocytes: Role of epidermal growth factor-like growth factors in the culture medium. Hum. Reprod. 2010, 26, 76–81. [Google Scholar] [CrossRef] [Green Version]
- Levi, M.; Ghetler, Y.; Shulman, A.; Shalgi, R. Morphological and molecular markers are correlated with maturation-competence of human oocytes. Hum. Reprod. 2013, 28, 2482–2489. [Google Scholar] [CrossRef] [Green Version]
- Faramarzi, A.; Khalili, M.A.; Ashourzadeh, S.; Palmerini, M.G. Does rescue in vitro maturation of germinal vesicle stage oocytes impair embryo morphokinetics development? Zygote 2018, 26, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Tucker, M.J.; Morton, P.C.; Wright, G.; Sweitzer, C.L.; Massey, J.B. Clinical application of human egg cryopreservation. Hum. Reprod. 1998, 13, 3156–3159. [Google Scholar] [CrossRef] [Green Version]
- Mandelbaum, J.; Junca, A.M.; Tibi, C.; Plachot, M.; Alnot, M.O.; Rim, H.; Salat-Baroux, J.; Cohen, J. Cryopreservation of Immature and Mature Hamster and Human Oocytes. Ann. N. Y. Acad. Sci. 1988, 541, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R. Cryopreservation of Human Oocytes and Ovarian Tissue. Cell Tissue Bank. 2006, 7, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Minasi, M.G.; Fabozzi, G.; Casciani, V.; Ferrero, S.; Litwicka, K.; Greco, E. Efficiency of slush nitrogen vitrification of human oocytes vitrified with or without cumulus cells in relation to survival rate and meiotic spindle competence. Fertil. Steril. 2012, 97, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Fabbri, R.; Porcu, E.; Marsella, T.; Rocchetta, G.; Venturoli, S.; Flamigni, C. Human oocyte cryopreservation: New perspectives regarding oocyte survival. Hum. Reprod. 2001, 16, 411–416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albertini, D.F.; Olsen, R. Effects of Fertility Preservation on Oocyte Genomic Integrity. In Oocyte Biology in Fertility Preservation; Springer: New York, NY, USA, 2013; Volume 761, pp. 19–27. [Google Scholar] [CrossRef]
- Chian, R.C.; Xu, Y.; Keilty, D. Chapter 3 Current Challenges in Immature Oocyte Cryopreservation. Methods Mol. Biol. 2017, 1568, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Youm, H.S.; Choi, J.R.; Oh, D.; Rho, Y.H. Vitrfication and Slow Freezing for Cryopreservation of Germinal Vesicle-Stage Human Oocytes: A Bayesian Meta-Analysis. Cryoletters 2017, 38, 455–462. [Google Scholar]
- Jang, T.H.; Park, S.C.; Yang, J.H.; Kim, J.Y.; Seok, J.H.; Park, U.S.; Choi, C.W.; Lee, S.R.; Han, J. Cryopreservation and its clinical applications. Integr. Med. Res. 2017, 6, 12–18. [Google Scholar] [CrossRef]
- Parmegiani, L.; Tatone, C.; Cognigni, G.E.; Bernardi, S.; Troilo, E.; Arnone, A.; Maccarini, A.M.; Di Emidio, G.; Vitti, M.; Filicori, M. Rapid warming increases survival of slow-frozen sibling oocytes: A step towards a single warming procedure irrespective of the freezing protocol? Reprod. Biomed. Online 2014, 28, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Stimpfel, M.; Vrtacnik-Bokal, E.; Virant-Klun, I. No difference in mitochondrial distribution is observed in human oocytes after cryopreservation. Arch. Gynecol. Obstet. 2017, 296, 373–381. [Google Scholar] [CrossRef]
- Bosch, E.; De Vos, M.; Humaidan, P. The Future of Cryopreservation in Assisted Reproductive Technologies. Front. Endocrinol. 2020, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Vajta, G.; Rienzi, L.; Ubaldi, F.M. Open versus closed systems for vitrification of human oocytes and embryos. Reprod. Biomed. Online 2015, 30, 325–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gullo, G.; Petousis, S.; Papatheodorou, A.; Panagiotidis, Y.; Margioula-Siarkou, C.; Prapas, N.; D’Anna, R.; Perino, A.; Cucinella, G.; Prapas, Y. Closed vs. Open Oocyte Vitrification Methods Are Equally Effective for Blastocyst Embryo Transfers: Prospective Study from a Sibling Oocyte Donation Program. Gynecol. Obstet. Investig. 2020, 85, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Gosden, R.G. General principles of cryopreservation. Methods Mol. Biol. 2014, 1154, 261–268. [Google Scholar] [CrossRef]
- Levi-Setti, P.E.; Patrizio, P.; Scaravelli, G. Evolution of human oocyte cryopreservation. Curr. Opin. Endocrinol. Diabetes Obes. 2016, 23, 445–450. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Xing, Q.; Zhang, Z.-G.; Wei, Z.-L.; Zhou, P.; Cong, L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod. Biomed. Online 2009, 19, 369–373. [Google Scholar] [CrossRef]
- Fahy, G.M. Theoretical considerations for oocyte cryopreservation by freezing. Reprod. Biomed. Online 2007, 14, 709–714. [Google Scholar] [CrossRef]
- Cobo, A.; Diaz, C. Clinical application of oocyte vitrification: A systematic review and meta-analysis of randomized controlled trials. Fertil. Steril. 2011, 96, 277–285. [Google Scholar] [CrossRef]
- Taylor, R.W.; Taylor, G.A.; Durham, S.E.; Turnbull, D.M. The determination of complete human mitochondrial DNA sequences in single cells: Implications for the study of somatic mitochondrial DNA point mutations. Nucleic Acids Res. 2001, 29, e74. [Google Scholar] [CrossRef]
- Schon, E.A.; DiMauro, S.; Hirano, M. Human mitochondrial DNA: Roles of inherited and somatic mutations. Nat. Rev. Genet. 2012, 13, 878–890. [Google Scholar] [CrossRef]
- Ye, M.; Shi, W.; Hao, Y.; Zhang, L.; Chen, S.; Wang, L.; He, X.; Li, S.; Xu, C. Associations of mitochondrial DNA copy number and deletion rate with early pregnancy loss. Mitochondrion 2020, 55, 48–53. [Google Scholar] [CrossRef]
- Bianco, B.; Montagna, E. The advances and new technologies for the study of mitochondrial diseases. Einstein 2016, 14, 291–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farnezi, H.C.M.; Goulart, A.C.X.; Dos Santos, A.; Ramos, M.G.; Penna, M.L.F. Three-parent babies: Mitochondrial replacement therapies. JBRA Assist. Reprod. 2020, 24, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Richardson, J.; Irving, L.; Hyslop, L.A.; Choudhary, M.; Murdoch, A.; Turnbull, D.M.; Herbert, M. Concise Reviews: Assisted Reproductive Technologies to Prevent Transmission of Mitochondrial DNA Disease. Stem. Cells 2015, 33, 639–645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craven, L.; Tang, M.-X.; Gorman, G.; De Sutter, P.; Heindryckx, B. Novel reproductive technologies to prevent mitochondrial disease. Hum. Reprod. Updat. 2017, 23, 501–519. [Google Scholar] [CrossRef] [Green Version]
- Sendra, L.; García-Mares, A.; Herrero, M.J.; Aliño, S.F. Mitochondrial DNA Replacement Techniques to Prevent Human Mitochondrial Diseases. Int. J. Mol. Sci. 2021, 22, 551. [Google Scholar] [CrossRef]
- Darbandi, S.; Darbandi, M.; Khorshid, H.R.K.; Sadeghi, M.R.; Agarwal, A.; Sengupta, P.; Al-Hasani, S.; Akhondi, M.M. Ooplasmic transfer in human oocytes: Efficacy and concerns in assisted reproduction. Reprod. Biol. Endocrinol. 2017, 15, 77. [Google Scholar] [CrossRef] [Green Version]
- Cree, L.; Loi, P. Mitochondrial replacement: From basic research to assisted reproductive technology portfolio tool—technicalities and possible risks. Mol. Hum. Reprod. 2014, 21, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Tachibana, M.; Amato, P.; Sparman, M.; Woodward, J.; Sanchis, D.M.; Ma, H.; Gutierrez, N.M.; Tippner-Hedges, R.; Kang, E.; Lee, H.-S.; et al. Towards germline gene therapy of inherited mitochondrial diseases. Nature 2012, 493, 627–631. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, H.; Luo, S.; Lu, Z.; Chávez-Badiola, A.; Liu, Z.; Yang, M.; Merhi, Z.; Silber, S.J.; Munné, S.; et al. Live birth derived from oocyte spindle transfer to prevent mitochondrial disease. Reprod. Biomed. Online 2017, 34, 361–368. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Wang, K.; Kellam, L.D.; Lee, Y.S.; Liang, C.-G.; Han, Z.; Mtango, N.R.; Latham, K.E. Effects of Ooplasm Manipulation on DNA Methylation and Growth of Progeny in Mice1. Biol. Reprod. 2009, 80, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Neupane, J.; Vandewoestyne, M.; Ghimire, S.; Lu, Y.; Qian, C.; Van Coster, R.; Gerris, J.; Deroo, T.; Deforce, D.; De Sutter, P.; et al. Assessment of nuclear transfer techniques to prevent the transmission of heritable mitochondrial disorders without compromising embryonic development competence in mice. Mitochondrion 2014, 18, 27–33. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Palmerini, M.G.; Antonouli, S.; Macchiarelli, G.; Cecconi, S.; Bianchi, S.; Khalili, M.A.; Nottola, S.A. Ultrastructural Evaluation of the Human Oocyte at the Germinal Vesicle Stage during the Application of Assisted Reproductive Technologies. Cells 2022, 11, 1636. https://doi.org/10.3390/cells11101636
Palmerini MG, Antonouli S, Macchiarelli G, Cecconi S, Bianchi S, Khalili MA, Nottola SA. Ultrastructural Evaluation of the Human Oocyte at the Germinal Vesicle Stage during the Application of Assisted Reproductive Technologies. Cells. 2022; 11(10):1636. https://doi.org/10.3390/cells11101636
Chicago/Turabian StylePalmerini, Maria Grazia, Sevastiani Antonouli, Guido Macchiarelli, Sandra Cecconi, Serena Bianchi, Mohammad Ali Khalili, and Stefania Annarita Nottola. 2022. "Ultrastructural Evaluation of the Human Oocyte at the Germinal Vesicle Stage during the Application of Assisted Reproductive Technologies" Cells 11, no. 10: 1636. https://doi.org/10.3390/cells11101636
APA StylePalmerini, M. G., Antonouli, S., Macchiarelli, G., Cecconi, S., Bianchi, S., Khalili, M. A., & Nottola, S. A. (2022). Ultrastructural Evaluation of the Human Oocyte at the Germinal Vesicle Stage during the Application of Assisted Reproductive Technologies. Cells, 11(10), 1636. https://doi.org/10.3390/cells11101636