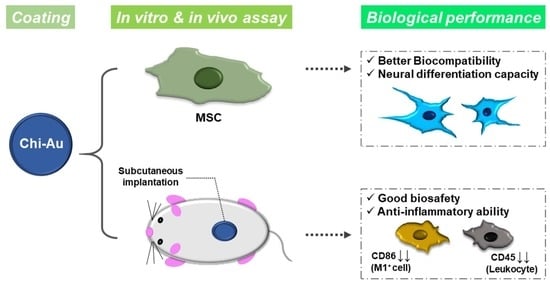
Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material Preparation
2.1.1. Chitosan Solution (Chi) Preparation
2.1.2. Chitosan–Nanogold Composites Preparation
2.2. Characterization of Materials
2.2.1. Ultraviolet-Visible Spectroscopy (UV-Vis)
2.2.2. Fourier-Transform Infrared Spectroscopy (FTIR)
2.2.3. Atomic Force Microscopy (AFM)
2.2.4. Dynamic Light Scattering (DLS)
2.2.5. Scanning Electron Microscopy (SEM)
2.3. Assessments of Biocompatibility
2.3.1. Culture of Mesenchymal Stem Cells (MSCs)
2.3.2. Cell Colony Formation Assay
2.3.3. Platelet and Monocyte Activation Assessments
2.3.4. Examination of Reactive Oxygen Species (ROS)
2.4. Examination of Matrix Metalloproteinase Expression
2.5. Real-Time Polymerase Chain Reaction (PCR)
2.6. Immunofluorescence (IF) and Fluorescence Activated Cell Sorting (FACS) Analysis
2.7. In Vivo Assessments
2.8. Statistical Analysis
3. Results
3.1. Materials Characterization
3.2. Phenotype Characterization of MSCs
3.3. Colonization Capacity of MSCs
3.4. Biocompatibility Assessments
3.5. Assessments of Matrix Metalloproteinase (MMP) Activity and Vinculin Expression
3.6. Real-Time PCR Assay for mRNA Expression Induced by Chi–Au
3.7. FACS and IF Measurement for Protein Expression Induced by Chi–Au
3.8. Subcutaneous Implantation in an Animal Model for Biocompatibility Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanza, R.; Langer, R.; Vacanti, J.P.; Atala, A. Principle of Tissue Engineering; Elsevier: Amsterdam, The Netherland, 2020. [Google Scholar]
- Pashneh-Tala, S.; MacNeil, S.; Claeyssens, F. The tissue-engineered vascular graft—Past, present, and future. Tissue Eng. Part. B Rev. 2016, 22, 68–100. [Google Scholar] [CrossRef]
- Goodarzi, P.; Falahzadeh, K.; Nematizadeh, M.; Farazandeh, P.; Payab, M.; Larijani, B.; Tayanloo Beik, A.; Arjmand, B. Tissue engineered skin substitutes. Cell Biol. Transl. Med. 2018, 3, 143–188. [Google Scholar]
- Kawecki, F.; Clafshenkel, W.P.; Fortin, M.; Auger, F.A.; Fradette, J. Biomimetic tissue-engineered bone substitutes for maxillofacial and craniofacial repair: The potential of cell sheet technologies. Adv. Healthc. Mater. 2018, 7, 1700919. [Google Scholar] [CrossRef]
- Jiang, S.; Guo, W.; Tian, G.; Luo, X.; Peng, L.; Liu, S.; Sui, X.; Guo, Q.; Li, X. Clinical application status of articular cartilage regeneration techniques: Tissue-engineered cartilage brings new hope. Stem Cells Int. 2020, 2020, 5690252. [Google Scholar] [CrossRef]
- Nasr, S.M.; Rabiee, N.; Hajebi, S.; Ahmadi, S.; Fatahi, Y.; Hosseini, M.; Bagherzadeh, M.; Ghadiri, A.M.; Rabiee, M.; Jajarmi, V. Biodegradable nanopolymers in cardiac tissue engineering: From concept towards nanomedicine. Int. J. Nanomed. 2020, 15, 4205. [Google Scholar] [CrossRef]
- Künzle, M.; Lach, M.; Budiarta, M.; Beck, T. Higher-Order Structures Based on Molecular Interactions for the Formation of Natural and Artificial Biomaterials. Chem. Biol. Chem. 2019, 20, 1637–1641. [Google Scholar] [CrossRef]
- Asghari, F.; Samiei, M.; Adibkia, K.; Akbarzadeh, A.; Davaran, S. Biodegradable and biocompatible polymers for tissue engineering application: A review. Artif. Cells Nanomed. Biotechnol. 2017, 45, 185–192. [Google Scholar] [CrossRef]
- Augustine, R. Skin bioprinting: A novel approach for creating artificial skin from synthetic and natural building blocks. Prog. Biomater. 2018, 7, 77–92. [Google Scholar] [CrossRef] [Green Version]
- Caldorera-Moore, M.; Peppas, N.A. Micro-and nanotechnologies for intelligent and responsive biomaterial-based medical systems. Adv. Drug Deliv. Rev. 2009, 61, 1391–1401. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal stem cell–derived exosomes: A promising biological tool in nanomedicine. Front. Pharmacol. 2021, 11, 590470. [Google Scholar] [CrossRef]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947–966. [Google Scholar] [CrossRef]
- Daum, R.; Visser, D.; Wild, C.; Kutuzova, L.; Schneider, M.; Lorenz, G.; Weiss, M.; Hinderer, S.; Stock, U.A.; Seifert, M. Fibronectin adsorption on electrospun synthetic vascular grafts attracts endothelial progenitor cells and promotes endothelialization in dynamic in vitro culture. Cells 2020, 9, 778. [Google Scholar] [CrossRef] [Green Version]
- Hung, H.-S.; Chang, C.-H.; Chang, C.-J.; Tang, C.-M.; Kao, W.-C.; Lin, S.-Z.; Hsieh, H.-H.; Chu, M.-Y.; Sun, W.-S.; Hsu, S.-H. In vitro study of a novel nanogold-collagen composite to enhance the mesenchymal stem cell behavior for vascular regeneration. PLoS ONE 2014, 9, e104019. [Google Scholar] [CrossRef]
- Bujoli, B.; Scimeca, J.-C.; Verron, E. Fibrin as a multipurpose physiological platform for bone tissue engineering and targeted delivery of bioactive compounds. Pharmaceutics 2019, 11, 556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.-W.; Hsieh, S.-C.; Yang, Y.-C.; Hsu, S.-H.; Kung, M.-L.; Lin, P.-Y.; Hsieh, H.-H.; Lin, C.-H.; Tang, C.-M.; Hung, H.-S. Functional engineered mesenchymal stem cells with fibronectin-gold composite coated catheters for vascular tissue regeneration. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Croisier, F.; Jérôme, C. Chitosan-based biomaterials for tissue engineering. Eur. Polym. J. 2013, 49, 780–792. [Google Scholar] [CrossRef] [Green Version]
- Kumar, M.R.; Muzzarelli, R.A.; Muzzarelli, C.; Sashiwa, H.; Domb, A. Chitosan chemistry and pharmaceutical perspectives. Chem. Rev. 2004, 104, 6017–6084. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Bhuiyan, M.; Islam, M. Chitin and chitosan: Structure, properties and applications in biomedical engineering. J. Polym. Environ. 2017, 25, 854–866. [Google Scholar] [CrossRef]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Prado-Gotor, R.; López-Pérez, G.; Martín, M.; Cabrera-Escribano, F.; Franconetti, A. Use of gold nanoparticles as crosslink agent to form chitosan nanocapsules: Study of the direct interaction in aqueous solutions. J. Inorg. Biochem. 2014, 135, 77–85. [Google Scholar] [CrossRef]
- Shirvan, A.R.; Shakeri, M.; Bashari, A. Recent advances in application of chitosan and its derivatives in functional finishing of textiles. In The Impact and Prospects of Green Chemistry for Textile Technology; Elsevier: Amsterdam, The Netherland, 2019; pp. 107–133. [Google Scholar]
- Luna, S.M.; Silva, S.S.; Gomes, M.E.; Mano, J.F.; Reis, R.L. Cell adhesion and proliferation onto chitosan-based membranes treated by plasma surface modification. J. Biomater. Appl. 2011, 26, 101–116. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Xu, J.; Wang, K.; Cheng, L.; Wang, J.; Liu, B. Preparation and characterization of chitosan nanocomposites with vermiculite of different modification. Polym. Degrad. Stab. 2009, 94, 2121–2127. [Google Scholar] [CrossRef]
- Elahi, N.; Kamali, M.; Baghersad, M.H. Recent biomedical applications of gold nanoparticles: A review. Talanta 2018, 184, 537–556. [Google Scholar] [CrossRef]
- Torres-Ferrer, L.R.; López-Romero, J.M.; Mendez-Nonell, J.; Rivas-Arreola, M.J.; Moreno-Ríos, M.; Ávila-Dávila, E.O.; Prokhorov, E.; Kovalenko, Y.; Zárate-Triviño, D.G.; Revilla-Vazquez, J.R. Tuning HAuCl4/Sodium Citrate Stoichiometry to Fabricate Chitosan-Au Nanocomposites. Polymers 2022, 14, 788. [Google Scholar] [CrossRef]
- Huang, N.; Martínez, L.J.; Jaquay, E.; Nakano, A.; Povinelli, M.L. Optical epitaxial growth of gold nanoparticle arrays. Nano Lett. 2015, 15, 5841–5845. [Google Scholar] [CrossRef]
- Cross, C.; Hemminger, J.; Penner, R. Physical vapor deposition of one-dimensional nanoparticle arrays on graphite: Seeding the electrodeposition of gold nanowires. Langmuir 2007, 23, 10372–10379. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.-H.; Lin, Z.-C. Biocompatibility and biostability of a series of poly (carbonate) urethanes. Colloids Surf. B Biointerfaces 2004, 36, 1–12. [Google Scholar] [CrossRef]
- Sani, A.; Cao, C.; Cui, D. Toxicity of gold nanoparticles (AuNPs): A review. Biochem. Biophys. Rep. 2021, 26, 100991. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Tang, Z.; Park, I.-H.; Zhu, Y.; Patel, S.; Daley, G.Q.; Li, S. Induced pluripotent stem cells for neural tissue engineering. Biomaterials 2011, 32, 5023–5032. [Google Scholar] [CrossRef] [Green Version]
- Sakthiswary, R.; Raymond, A.A. Stem cell therapy in neurodegenerative diseases: From principles to practice. Neural Regen. Res. 2012, 7, 1822. [Google Scholar]
- Samsonraj, R.M.; Raghunath, M.; Nurcombe, V.; Hui, J.H.; van Wijnen, A.J.; Cool, S.M. Concise review: Multifaceted characterization of human mesenchymal stem cells for use in regenerative medicine. Stem Cells Transl. Med. 2017, 6, 2173–2185. [Google Scholar] [CrossRef] [Green Version]
- Aust, L.; Devlin, B.; Foster, S.; Halvorsen, Y.; Hicok, K.d.; Du Laney, T.; Sen, A.; Willingmyre, G.; Gimble, J. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy 2004, 6, 7–14. [Google Scholar] [CrossRef]
- Jackson, W.M.; Nesti, L.J.; Tuan, R.S. Potential therapeutic applications of muscle-derived mesenchymal stem and progenitor cells. Expert Opin. Biol. Ther. 2010, 10, 505–517. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Gronthos, S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J. Bone Miner. Res. 2003, 18, 696–704. [Google Scholar] [CrossRef] [Green Version]
- Corrao, S.; La Rocca, G.; Lo Iacono, M.; Corsello, T.; Farina, F.; Anzalone, R. Umbilical cord revisited: From Wharton’s jelly myofibroblasts to mesenchymal stem cells. Histol. Histopathol. 2013, 28, 1235–1244. [Google Scholar] [CrossRef]
- Han, Y.; Li, X.; Zhang, Y.; Han, Y.; Chang, F.; Ding, J. Mesenchymal stem cells for regenerative medicine. Cells 2019, 8, 886. [Google Scholar] [CrossRef] [Green Version]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal stem/stromal cell-derived extracellular vesicles and their potential as novel immunomodulatory therapeutic agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef] [Green Version]
- Przyborski, S.A.; Hardy, S.A.; Maltman, D.J. Mesenchymal stem cells as mediators of neural differentiation. Curr. Stem Cell Res. Ther. 2008, 3, 43–52. [Google Scholar] [CrossRef]
- Boomsma, R.A.; Geenen, D.L. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS ONE 2012, 7, e35685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanabdali, R.; Saadat, A.; Fazilah, M.; Bazli, K.F.K.; Qazi, R.-e.-M.; Khalid, R.S.; Adli, D.S.H.; Moghadamtousi, S.Z.; Naeem, N.; Khan, I. Promoting effect of small molecules in cardiomyogenic and neurogenic differentiation of rat bone marrow-derived mesenchymal stem cells. Drug Des. Dev. Ther. 2016, 10, 81. [Google Scholar]
- Hsieh, S.-C.; Chen, H.-J.; Hsu, S.-h.; Yang, Y.-C.; Tang, C.-M.; Chu, M.-Y.; Lin, P.-Y.; Fu, R.-H.; Kung, M.-L.; Chen, Y.-W. Prominent vascularization capacity of mesenchymal stem cells in collagen–gold nanocomposites. ACS Appl. Mater. Interfaces 2016, 8, 28982–29000. [Google Scholar] [CrossRef]
- Hung, H.-S.; Kao, W.-C.; Shen, C.-C.; Chang, K.-B.; Tang, C.-M.; Yang, M.-Y.; Yang, Y.-C.; Yeh, C.-A.; Li, J.-J.; Hsieh, H.-H. Inflammatory Modulation of Polyethylene Glycol-AuNP for Regulation of the Neural Differentiation Capacity of Mesenchymal Stem Cells. Cells 2021, 10, 2854. [Google Scholar] [CrossRef]
- Schmidt, C.E.; Leach, J.B. Neural tissue engineering: Strategies for repair and regeneration. Annu. Rev. Biomed. Eng. 2003, 5, 293–347. [Google Scholar] [CrossRef] [Green Version]
- Sedaghati, T.; Yang, S.Y.; Mosahebi, A.; Alavijeh, M.S.; Seifalian, A.M. Nerve regeneration with aid of nanotechnology and cellular engineering. Biotechnol. Appl. Biochem. 2011, 58, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Cunha, C.; Panseri, S.; Antonini, S. Emerging nanotechnology approaches in tissue engineering for peripheral nerve regeneration. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 50–59. [Google Scholar] [CrossRef]
- Chamberlain, L.; Yannas, I.; Arrizabalaga, A.; Hsu, H.-P.; Norregaard, T.; Spector, M. Early peripheral nerve healing in collagen and silicone tube implants: Myofibroblasts and the cellular response. Biomaterials 1998, 19, 1393–1403. [Google Scholar] [CrossRef]
- Wang, W.; Itoh, S.; Matsuda, A.; Ichinose, S.; Shinomiya, K.; Hata, Y.; Tanaka, J. Influences of mechanical properties and permeability on chitosan nano/microfiber mesh tubes as a scaffold for nerve regeneration. J. Biomed. Mater. Res. Part. A 2008, 84, 557–566. [Google Scholar]
- Vial, S.; Reis, R.L.; Oliveira, J.M. Recent advances using gold nanoparticles as a promising multimodal tool for tissue engineering and regenerative medicine. Curr. Opin. Solid State Mater. Sci. 2017, 21, 92–112. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Wang, Y.; Song, Z.; Feng, Y.; Chen, Y.; Zhang, D.; Feng, L. The basic properties of gold nanoparticles and their applications in tumor diagnosis and treatment. Int. J. Mol. Sci. 2020, 21, 2480. [Google Scholar] [CrossRef] [Green Version]
- Jiang, C.; Zhu, J.; Li, Z.; Luo, J.; Wang, J.; Sun, Y. Chitosan–gold nanoparticles as peroxidase mimic and their application in glucose detection in serum. Rsc Adv. 2017, 7, 44463–44469. [Google Scholar] [CrossRef] [Green Version]
- Silva, I.O.; Ladchumananandasivam, R.; Nascimento, J.H.O.; Silva, K.K.O.; Oliveira, F.R.; Souto, A.P.; Felgueiras, H.P.; Zille, A. Multifunctional chitosan/gold nanoparticles coatings for biomedical textiles. Nanomaterials 2019, 9, 1064. [Google Scholar] [CrossRef] [Green Version]
- Komalam, A.; Muraleegharan, L.G.; Subburaj, S.; Suseela, S.; Babu, A.; George, S. Designed plasmonic nanocatalysts for the reduction of eosin Y: Absorption and fluorescence study. Int. Nano Lett. 2012, 2, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Primo, A.; Quignard, F. Chitosan as efficient porous support for dispersion of highly active gold nanoparticles: Design of hybrid catalyst for carbon–carbon bond formation. Chem. Commun. 2010, 46, 5593–5595. [Google Scholar] [CrossRef] [PubMed]
- Regiel-Futyra, A.; Kus-Liśkiewicz, M.; Sebastian, V.; Irusta, S.; Arruebo, M.; Stochel, G.y.; Kyzioł, A. Development of noncytotoxic chitosan–gold nanocomposites as efficient antibacterial materials. Acs Appl. Mater. Interfaces 2015, 7, 1087–1099. [Google Scholar] [CrossRef]
- Hung, H.-S.; Tang, C.-M.; Lin, C.-H.; Lin, S.-Z.; Chu, M.-Y.; Sun, W.-S.; Kao, W.-C.; Hsien-Hsu, H.; Huang, C.-Y.; Hsu, S.-H. Biocompatibility and favorable response of mesenchymal stem cells on fibronectin-gold nanocomposites. PLoS ONE 2013, 8, e65738. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.-W.; Liu, D.-Z.; Wang, S.-Y.; Wang, S.-S. Enhancement of the growth of human endothelial cells by surface roughness at nanometer scale. Biomaterials 2003, 24, 4655–4661. [Google Scholar] [CrossRef]
- Qi, B.; Feng, H.; Qiu, X.; Beaune, G.G.; Guo, X.; Brochard-Wyart, F.O.; Winnik, F.O.M. Spreading of Cell Aggregates on Zwitterion-Modified Chitosan Films. Langmuir 2018, 35, 1902–1908. [Google Scholar] [CrossRef]
- Kumar, P.P.P.; Lim, D.-K. Gold-polymer nanocomposites for future therapeutic and tissue engineering applications. Pharmaceutics 2021, 14, 70. [Google Scholar] [CrossRef] [PubMed]
- Yadid, M.; Feiner, R.; Dvir, T. Gold nanoparticle-integrated scaffolds for tissue engineering and regenerative medicine. Nano Lett. 2019, 19, 2198–2206. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Doroudian, M.; Ahadpour, A.; Azari, S. Injectable chitosan/κ-carrageenan hydrogel designed with au nanoparticles: A conductive scaffold for tissue engineering demands. Int. J. Biol. Macromol. 2019, 126, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Mao, J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006, 420, 339–361. [Google Scholar]
- Wei, M.; Li, S.; Yang, Z.; Zheng, W.; Le, W. Gold nanoparticles enhance the differentiation of embryonic stem cells into dopaminergic neurons via mTOR/p70S6K pathway. Nanomedicine 2017, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Hang, Y.; Wu, J.; Tang, Z.; Li, X.; Zhang, S.; Wang, L.; Brash, J.L.; Chen, H. Dual pathway for promotion of stem cell neural differentiation mediated by gold nanocomposites. ACS Appl. Mater. Interfaces 2020, 12, 22066–22073. [Google Scholar] [CrossRef]
- Lin, Y.-L.; Jen, J.-C.; Hsu, S.-H.; Chiu, M. Sciatic nerve repair by microgrooved nerve conduits made of chitosan-gold nanocomposites. Surg. Neurol. 2008, 70, S9–S18. [Google Scholar] [CrossRef] [PubMed]
- Amariglio, N.; Hirshberg, A.; Scheithauer, B.W.; Cohen, Y.; Loewenthal, R.; Trakhtenbrot, L.; Paz, N.; Koren-Michowitz, M.; Waldman, D.; Leider-Trejo, L. Donor-derived brain tumor following neural stem cell transplantation in an ataxia telangiectasia patient. PLoS Med. 2009, 6, e1000029. [Google Scholar] [CrossRef]
- Razavi, S.; Seyedebrahimi, R.; Jahromi, M. Biodelivery of nerve growth factor and gold nanoparticles encapsulated in chitosan nanoparticles for schwann-like cells differentiation of human adipose-derived stem cells. Biochem. Biophys. Res. Commun. 2019, 513, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Hassanshahi, A.; Hassanshahi, M.; Khabbazi, S.; Hosseini-Khah, Z.; Peymanfar, Y.; Ghalamkari, S.; Su, Y.W.; Xian, C.J. Adipose-derived stem cells for wound healing. J. Cell. Physiol. 2019, 234, 7903–7914. [Google Scholar] [CrossRef]
- Lendahl, U.; Zimmerman, L.B.; McKay, R.D. CNS stem cells express a new class of intermediate filament protein. Cell 1990, 60, 585–595. [Google Scholar] [CrossRef]
- Filippov, V.; Kronenberg, G.; Pivneva, T.; Reuter, K.; Steiner, B.; Wang, L.-P.; Yamaguchi, M.; Kettenmann, H.; Kempermann, G. Subpopulation of nestin-expressing progenitor cells in the adult murine hippocampus shows electrophysiological and morphological characteristics of astrocytes. Mol. Cell. Neurosci. 2003, 23, 373–382. [Google Scholar] [CrossRef]
- Chen, H.; Wu, H.; Yin, H.; Wang, J.; Dong, H.; Chen, Q.; Li, Y. Effect of photobiomodulation on neural differentiation of human umbilical cord mesenchymal stem cells. Lasers Med. Sci. 2019, 34, 667–675. [Google Scholar] [CrossRef]
- Sheikh, A.M.; Yano, S.; Tabassum, S.; Omura, K.; Araki, A.; Mitaki, S.; Ito, Y.; Huang, S.; Nagai, A. Alteration of neural stem cell functions in ataxia and male sterility mice: A possible role of β-tubulin glutamylation in neurodegeneration. Cells 2021, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Svirshchevskaya, E.V.; Sharonova, N.V.; Poltavtseva, R.A.; Konovalova, M.V.; Efimov, A.E.; Popov, A.A.; Sizova, S.V.; Solovyeva, D.O.; Bogdanov, I.V.; Oleinikov, V.A. Silicon–Gold Nanoparticles Affect Wharton’s Jelly Phenotype and Secretome during Tri-Lineage Differentiation. Int. J. Mol. Sci. 2022, 23, 2134. [Google Scholar] [CrossRef]
- Barkho, B.Z.; Munoz, A.E.; Li, X.; Li, L.; Cunningham, L.A.; Zhao, X. Endogenous matrix metalloproteinase (MMP)-3 and MMP-9 promote the differentiation and migration of adult neural progenitor cells in response to chemokines. Stem Cells 2008, 26, 3139–3149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, H.; Rao, R.; Ma, D.D. Cytokine-induced stable neuronal differentiation of human bone marrow mesenchymal stem cells in a serum/feeder cell-free condition. Dev. Growth Differ. 2005, 47, 423–433. [Google Scholar] [CrossRef] [PubMed]









| GFAP mRNA Expression (Relative Fold) | Day 3 | Day 5 | Day 7 |
|---|---|---|---|
| Control | 1 | 1 | 1 |
| Chi | ~1.44 | ~1.74 | ~4.16 |
| Chi–Au 25 ppm | ~2.5 | ~1.71 | ~3.92 |
| Chi–Au 50 ppm | ~3.37 * | ~3.61 ** | ~7.96 *** |
| Chi–Au 100 ppm | ~1.51 | ~1.81 | ~4.92 |
| β-Tubulin mRNA Expression (Relative Fold) | Day 3 | Day 5 | Day 7 |
|---|---|---|---|
| Control | 1 | 1 | 1 |
| Chi | ~1.17 | ~2.48 ** | ~5.1 * |
| Chi–Au 25 ppm | ~2.02 | ~2.7 ** | ~4.49 * |
| Chi–Au 50 ppm | ~3.2 * | ~3.07 * | ~9.76 *** |
| Chi–Au 100 ppm | ~1.3 | ~2.59 ** | ~6.77 ** |
| Nestin mRNA Expression (Relative Fold) | Day 3 | Day 5 | Day 7 |
|---|---|---|---|
| Control | 1 | 1 | 1 |
| Chi | ~1.72 | ~2.16 | ~3.98 |
| Chi–Au 25 ppm | ~1.23 | ~2.56 | ~3.89 |
| Chi–Au 50 ppm | ~1.84 ** | ~3.89 *** | ~8.46 *** |
| Chi–Au 100 ppm | ~1.82 * | ~2.34 | ~6.29 |
| CD31 mRNA Expression (Relative Fold) | Day 3 | Day 5 | Day 7 |
|---|---|---|---|
| Control | 1 | 1 | 1 |
| Chi | ~1.19 | ~0.93 | ~0.81 |
| Chi–Au 25 ppm | ~2.84 ** | ~3.13 *** | ~4.23 *** |
| Chi–Au 50 ppm | ~2.18 | ~2.19 | ~2.47 |
| Chi–Au 100 ppm | ~1.11 | ~1.33 | ~1.86 |
| vWF mRNA Expression (Relative Fold) | Day 3 | Day 5 | Day 7 |
|---|---|---|---|
| Control | 1 | 1 | 1 |
| Chi | ~1.18 | ~1.44 | ~1.54 |
| Chi–Au 25 ppm | ~2.28 ** | ~1.95 * | ~5.14 *** |
| Chi–Au 50 ppm | ~1.63 | ~1.97 * | ~4.61 |
| Chi–Au 100 ppm | ~1.58 | ~1.62 | ~3.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hung, H.-S.; Yang, Y.-C.; Chang, C.-H.; Chang, K.-B.; Shen, C.-C.; Tang, C.-L.; Liu, S.-Y.; Lee, C.-H.; Yen, C.-M.; Yang, M.-Y. Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites. Cells 2022, 11, 1861. https://doi.org/10.3390/cells11121861
Hung H-S, Yang Y-C, Chang C-H, Chang K-B, Shen C-C, Tang C-L, Liu S-Y, Lee C-H, Yen C-M, Yang M-Y. Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites. Cells. 2022; 11(12):1861. https://doi.org/10.3390/cells11121861
Chicago/Turabian StyleHung, Huey-Shan, Yi-Chin Yang, Chih-Hsuan Chang, Kai-Bo Chang, Chiung-Chyi Shen, Chien-Lun Tang, Szu-Yuan Liu, Chung-Hsin Lee, Chun-Ming Yen, and Meng-Yin Yang. 2022. "Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites" Cells 11, no. 12: 1861. https://doi.org/10.3390/cells11121861
APA StyleHung, H. -S., Yang, Y. -C., Chang, C. -H., Chang, K. -B., Shen, C. -C., Tang, C. -L., Liu, S. -Y., Lee, C. -H., Yen, C. -M., & Yang, M. -Y. (2022). Neural Differentiation Potential of Mesenchymal Stem Cells Enhanced by Biocompatible Chitosan-Gold Nanocomposites. Cells, 11(12), 1861. https://doi.org/10.3390/cells11121861