1. Introduction
Protein phosphorylation is an essential post-translational modification that can regulate almost all aspects of cellular processes, including metabolism, growth, division, differentiation, apoptosis, and signal transduction pathways [
1]. Abnormal phosphorylation is the cause or consequence of many diseases [
2]. Approximately one-third of all proteins in eukaryotic cells can be phosphorylated at any given time [
3]. Phosphorylation primarily occurs on the side-chain hydroxyl groups of serine (Ser, S), threonine (Thr, T), and tyrosine (Tyr, Y), which is called O-phosphorylation. Though phosphorylation can also occur on other amino acid residues, including histidine (His, H), lysine (Lys, K), arginine (Arg, R), aspartic acid (Asp, D), glutamic acid (Glu, E), and cysteine (Cys, C), O-phosphorylation is the primary type of phosphorylation in eukaryotes [
4]. Due to their chemical stability in acidic and neutral milieu, analytical methods have primarily been developed for O-phosphorylation, making it the best-studied type of phosphorylation in cell biology and phosphoproteomics [
5].
Despite advances in mass spectrometry (MS) technology, many challenges exist in phosphoproteomic analysis. First, phosphoproteins (or phosphopeptides) have very low stoichiometry compared to non-phosphorylated proteins (or peptides), which makes them difficult to identify. Second, phosphopeptides ionize with lower efficiency than non-phosphorylated peptides; therefore, removing non-phosphorylated peptides before MS analysis can boost the MS ion signal of phosphopeptides and increase detection sensitivity [
6]. Therefore, the development of effective and specific phosphopeptide enrichment methods is essential for performing comprehensive phosphoproteomic analyses.
In the past two decades, many phosphopeptide enrichment approaches have been described, including antibody-based immunoprecipitation [
7], immobilized metal affinity chromatography (IMAC) [
8], metal oxide affinity chromatography (MOAC) [
9], and sequential elution from IMAC (SIMAC) [
10], among others [
11,
12]. IMAC and MOAC are the most popular techniques for phosphopeptide enrichment since they are simple and effective. However, many molecules, including salts, detergents, and other low-molecular compounds in biological samples, can influence the performance of IMAC [
13]. MOAC uses metal oxides to electrostatically interact with phosphopeptides for adsorption and purification, while titanium dioxide (TiO
2) is the most frequently used chemical species for MOAC [
14]. Additionally, TiO
2-MOAC has higher specificity than IMAC [
15].
The enrichment specificity of TiO
2 is affected by various factors, such as the ratio of peptide-to-TiO
2 beads [
16], the composition of the sample loading buffer, and the washing buffer [
13]. The type of non-phosphopeptide excluders in the sample loading buffer significantly affects phosphopeptide enrichment specificity [
13]. It has been reported that the inclusion of non-phosphopeptide excluders in the sample loading buffer, such as glycolic acid [
13], glutamic acid [
17], lactic acid [
18], citric acid [
19], and 2,5-dihydroxybenzoic acid (DHB) [
9,
20], can effectively decrease the binding of non-phosphorylated peptides to TiO
2 without affecting the binding of phosphopeptides. However, conflicting results have been reported for the functions of non-phosphopeptide excluders during phosphopeptide enrichment. Larsen et al. demonstrated that adding DHB to a sample loading buffer can effectively remove acidic non-phosphorylated peptides (non-phosphorylated peptides containing acidic amino acids (D and E)) during TiO
2 phosphopeptide enrichment for Matrix-assisted Laser Desorption/Ionization (MALDI)-MS analysis [
21]. However, this method is not appropriate for liquid chromatography-tandem mass spectrometry (LC-MS/MS) analysis, as DHB is assumed to cause the contamination of both the LC system and the inlet of the mass spectrometer [
13,
18]. Jensen et al. reported that glycolic acid was an efficient non-phosphopeptide excluder in the TiO
2 micro-column [
13]. However, adding glycolic acid to the sample loading buffer increased the non-specific binding of peptides to TiO
2 [
15,
18], and non-specific binding increased as the concentration of glycolic acid in the sample loading buffer increased from 0.25 M to 1 M [
15]. These conflicting results make it difficult to identify the best method of enriching phosphopeptides suitable for different biological studies. The aforementioned studies [
13,
15,
18] used simple standard protein or peptide mixtures to compare the effects of non-phosphopeptide excluders on phosphopeptide enrichment with TiO
2. However, very few studies have thoroughly compared TiO
2 phosphopeptide enrichment methods with different non-phosphopeptide excluders using complex protein samples. In this study, we comprehensively analyzed the effects of four commonly used non-phosphopeptide excluders in the sample loading buffer, including glutamic acid, lactic acid, glycolic acid, and DHB, on phosphopeptide enrichment with TiO
2 using 293T cell lysate.
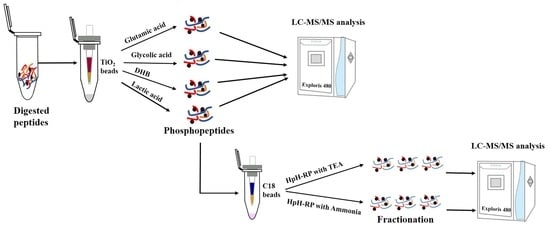
Conventional large-scale phosphoproteomic analysis requires laborious offline peptide fractionation before phosphopeptide enrichment to reduce sample complexity and perform in-depth phosphoproteomic analyses. However, this “fractionation before enrichment” strategy requires a large number of samples (usually 2–3 mg of protein/peptide mixture), which is unsuitable when samples are limited. Additionally, this workflow is time- and labor-intensive. Here, we described a “fractionation after enrichment” strategy, which fractionates purified phosphopeptides with StageTip-based HpH-RP chromatography using Triethylamine (TEA) to achieve in-depth phosphoproteome coverage. This strategy can identify more than 30,000 phosphopeptides corresponding to approximately 20,000 localized phosphosites from a 1 mg 293T peptide mixture. This study will provide a basis for selecting suitable phosphopeptide enrichment methods and provide a more effective fractionation strategy for in-depth phosphoproteomics.
2. Experimental Procedures
2.1. Cell Culture and Lysis
Human kidney epithelial cell lines 293T were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37 °C and 5% CO2. Once grown to 90% confluence, the cell culture media were removed, and the cells were washed three times with ice-cold PBS. The 293T cells were then harvested in the lysis buffer containing 8 M urea and 100 mM Tris-HCl (pH 8.5) supplemented with an EDTA-free complete protease inhibitor cocktail (Roche, Basel, Switzerland) and phosphatase inhibitor cocktail (PhosSTOP, Roche). The cells were then lysed with Precellys Evolution homogenizer (Bertin Technologies, Paris, France). After centrifugation at 20,000× g for 20 min at 4 °C, the supernatant was collected, and the protein concentration was determined using a BCA (bicinchoninic acid) protein assay kit (Thermo Fisher Scientific, Waltham, MA, USA).
2.2. In-Solution Digestion of Proteins
Proteins were reduced with DTT at a final concentration of 10 mM at 30 °C for 1 h. The resulting free thiols were alkylated with IAM at a final concentration of 40 mM for 45 min at room temperature in the dark. The same amount of DTT was subsequently added to remove excess IAM at 30 °C for 30 min. Proteins were then digested with Lys-C (Wako Pure Chemical Industries, Osaka, Japan) at an enzyme/protein ratio of 1:100 (w/w) at 37 °C for 3 h. After dilution with 50 mM Tris-HCl (pH 8.0), samples were digested with sequencing grade modified trypsin (Promega, Madison, WI, USA) at an enzyme/protein ratio of 1:50 (w/w) at 37 °C overnight. The enzymatic digestion was stopped with formic acid (FA), and the supernatant was collected after centrifugation at 20,000× g for 20 min. Peptides were desalted on HLB cartridges (Waters, Milford, MA, USA) and dried in SpeedVac (LABCONCO, Kansas City, MO, USA). After dissolving the desalted peptides with 0.1% FA, the peptide concentration was determined using a BCA peptide assay kit (Thermo Fisher Scientific). The peptides were then split into different amounts according to the different experiments, dried, and stored at −80 °C for phosphopeptide enrichment. An aliquot of peptide digests from each digestion batch was analyzed with LC-MS/MS to obtain peptide identification data from cell lysate.
2.3. Phosphopeptide Enrichment with Different Methods
2.3.1. Enrichment of Phosphopeptides Using TiO2 with Glutamic Acid
The phosphopeptides were enriched using TiO
2 with glutamic acid according to the method previously described [
16,
17], with some modifications. The peptides were resolubilized in a freshly prepared sample loading buffer containing 65% ACN, 2% trifluoroacetic acid (TFA), and saturated glutamic acid (cat. no. G1251, Sigma Aldrich, Burlington, MA, USA) to a final concentration of 2 μg/μL. TiO
2 beads (5 μm Titansphere, GL Sciences, Tokyo, Japan) were preconditioned with sample loading buffer for 5 min, and the process was repeated three times. The peptides were then incubated with TiO
2 beads at a peptides/TiO
2 ratio of 1:6 (
w/w) for 15 min at room temperature. After pelleted TiO
2 beads, the supernatant was transferred to another tube and incubated with half the amount of TiO
2 beads used in the first incubation. The third incubation was performed with 1/4 the amount of TiO
2 beads used in the first incubation. TiO
2 beads from three incubations were pooled with loading buffer and transferred to preconditioned C8 StageTips. The TiO
2 beads were sequentially washed with sample loading buffer, washing buffer 1 (65% ACN and 0.5% TFA), and washing buffer 2 (65% ACN and 0.1% TFA). The times and/or volume of the loading and washing buffers were the same for all four methods. The phosphopeptides were then eluted with elution buffer 1 (4% ammonia (NH
3·H
2O) (Sigma Aldrich)) and elution buffer 2 (4% NH
3·H
2O and 50% ACN). The eluted phosphopeptides were immediately acidified with 10% FA and dried in SpeedVac. The phosphopeptides were then desalted with homemade OLIGO
TM R3 (Thermo Fisher Scientific) C18 StageTips before LC-MS/MS analysis.
2.3.2. Enrichment of Phosphopeptides Using TiO2 with Lactic Acid
The enrichment of phosphopeptides using TiO
2 with lactic acid was performed according to the method previously described [
22], with some modifications. The phosphopeptide enrichment procedure using lactic acid was consistent with the protocol for glutamic acid described above, except that different sample loading and washing buffers were used. The sample loading buffer was 70% ACN, 5% TFA, and 20% lactic acid (cat. no. L6661, Sigma Aldrich); washing buffer 1 was 30% ACN and 0.5% TFA; and washing buffer 2 was 80% ACN and 0.4% TFA.
2.3.3. Enrichment of Phosphopeptides Using TiO2 with Glycolic Acid
The enrichment of phosphopeptides using TiO
2 with glycolic acid was performed according to the method previously described [
23], with some modifications. The phosphopeptide enrichment procedure using glycolic acid was consistent with the protocol for glutamic acid described above, except that different loading and washing buffers were used. The sample loading buffer was 80% ACN, 5% TFA, and 1 M glycolic acid (cat. no. 12473-7, Sigma Aldrich); washing buffer 1 was 80% ACN and 1% TFA; and washing buffer 2 was 20% ACN and 0.1% TFA.
In addition to the normal glycolic acid phosphopeptide enrichment protocol, there were other four modified phosphopeptide enrichment protocols that used glycolic acid: (1) PNGase F: the phosphopeptide enrichment procedure was consistent with the normal glycolic acid enrichment protocol, except that peptides were digested with PNGase F (New England BioLabs, Ipswich, MA, USA) at 37 °C overnight to remove glycans from peptides before phosphopeptide enrichment with TiO
2. (2) 2X washings: the volume of the loading and washing buffers was double that of the normal glycolic acid enrichment protocol. (3) Changed washing buffer: washing buffer 1 was 50% ACN and 0.5% TFA, washing buffer 2 was 50% ACN and 0.1% TFA, and washing buffer 3 (80% ACN and 0.4% TFA) was used. (4) Double TiO
2: phosphopeptides were enriched with TiO
2 beads using the normal glycolic acid enrichment protocol described above. After they dried, the enriched phosphopeptides were subjected to a second round of TiO
2 enrichment using the method previously described [
24]. Briefly, phosphopeptides enriched from the first round of TiO
2 were resolubilized in 70% ACN and 2% TFA and incubated with TiO
2 beads at a peptide/TiO
2 ratio of 1:6 (
w/w) for 15 min at room temperature. After centrifugation, TiO
2 beads were transferred to preconditioned C8 StageTips, and the supernatant was collected as flow-through (FT). The TiO
2 beads were then washed with 50% ACN and 0.1% TFA, and the supernatant was collected and pooled with the FT fraction. The pooled supernatant (FT and washing) was designed as TiO
2-TiO
2-FT. Next, phosphopeptides were eluted from TiO
2 beads with the same elution buffers described above.
2.3.4. Enrichment of Phosphopeptides Using TiO2 with DHB
The enrichment of phosphopeptides using TiO
2 with DHB was performed according to the method previously described [
25], with some modifications. The phosphopeptide enrichment process using DHB was consistent with the protocol described above, except that different sample loading and washing buffers were used. The sample loading buffer was 80% ACN, 5% TFA, and 20 mg/mL DHB (cat. no. 149357, Sigma Aldrich); washing buffer 1 was 30% ACN and 1% TFA ; washing buffer 2 was 50% ACN and 1% TFA; and washing buffer 3 was 80% ACN and 1% TFA.
2.4. StageTip-Based HpH-RP Fractionation of Phosphopeptides
Phosphopeptides were enriched from 1 mg peptides using both the glutamic acid and lactic acid methods. Three biological replicates were performed for each method. After enrichment, 15% of the enriched phosphopeptides were used for single-shot LC-MS/MS analysis, and/or the rest of the samples were used for HpH-RP fractionation.
Two HpH-RP protocols were used to fractionate phosphopeptides using C18 StageTips, and the identification results were compared. C18 StageTip was prepared by plugging a layer of C18 disk (3 M Empore) into a 200 μL pipet tip and methanol-washed C18 beads (cat. no DC930010-L, 3 μm, 150 Å, Agela Technologies, Tianjin, China) were transferred into pipet tips.
For the HpH-RP fractionation of phosphopeptides using ammonia, C18 StageTips were washed with ACN and equilibrated with buffer A (0.1% NH3·H2O, pH 10) before sample loading. Phosphopeptides were then reconstituted in buffer A and loaded onto StageTips, and the FT fraction was collected. StageTips were then washed with buffer A once, and the washing solution was combined with FT fraction. Then, phosphopeptides were fractionated into five fractions by a stepwise gradient of ACN (2%, 5%, 8%, 15%, and 35%) in 0.1% NH3·H2O. After elution, the eluates were immediately acidified with 10% FA. Finally, the six fractions were combined into three fractions for LC-MS/MS analysis: 2% ACN/0.1% NH3·H2O fraction and 15% ACN/0.1% NH3·H2O fraction were combined as Fraction 1, FT/washing fraction and 5% ACN/0.1% NH3·H2O fraction were combined as Fraction 2, and 8% ACN/0.1% NH3·H2O fraction and 35% ACN/0.1% NH3·H2O fraction were combined as Fraction 3.
For the HpH-RP fractionation of phosphopeptides using TEA, C18 StageTips were washed with ACN and equilibrated with buffer A (0.1% TFA) before sample loading. Then, phosphopeptides were reconstituted in buffer A and loaded onto the StageTips. After washing the tips with H2O, phosphopeptides were fractionated into five fractions with a stepwise gradient of ACN (2%, 5%, 8%, 15%, and 35%) in 0.1% TEA (pH 10). After elution, the eluates were immediately acidified with 10% FA. Finally, the five eluates were combined into three fractions: 2% ACN/0.1% TEA fraction and 15% ACN/0.1% TEA fraction were combined as Fraction 1, 5% ACN/0.1% TEA fraction was set as Fraction 2, and 8% ACN/0.1% TEA and 35% ACN/0.1% TEA fraction were combined as Fraction 3.
All samples were dried in SpeedVac and desalted with homemade OLIGOTM R3 C18 StageTips before analyzing with LC-MS/MS.
2.5. LC-MS/MS Analysis
All samples were analyzed on an Easy-nLC 1200 HPLC system (Thermo Fisher Scientific) coupled to an Orbitrap Exploris 480 (Thermo Fisher Scientific) with a high-field asymmetric waveform ion mobility spectrometry (FAIMS) device (Thermo Fisher Scientific). All samples were reconstituted in 0.1% FA and separated on a fused silica trap column (100 μm ID × 2 cm) in-house packed with reversed-phase silica (Reprosil-Pur C18 AQ, 5 μm, Dr. Maisch GmbH, Baden-Wuerttemberg, Germany) coupled to an analytical column (75 μm ID × 20 cm) packed with reversed-phase silica (Reprosil-Pur C18 AQ, 3 μm, Dr. Maisch GmbH). The phosphopeptides were analyzed with 132 min gradient (buffer A: 0.1% FA in H2O, buffer B: 80% ACN, 0.1% FA in H2O) at a flow rate of 300 nL/min (0–5% B, 6 min; 5–20% B, 69 min; 20–30% B, 39 min; 30–99% B, 9 min; 99% B, 9 min). The analysis of 293T peptide digests was performed using 73 min gradient (4–10% B, 3 min; 10–20% B, 22 min; 20–30% B, 20 min; 30–40% B, 15 min; 40–95% B, 3 min; 95% B, 10 min).
MS data were acquired using an Orbitrap mass analyzer in data-dependent acquisition mode. The cycle time was set as 2 s. The spray voltage of the nano-electrospray ion source was 2.0 kV, and with no sheath gas flow, the heated capillary temperature was 320 °C. Full scan MS data were collected at a high resolution of 60,000 (m/z 200) from 350 to 1200 m/z. The automatic gain control target was 3 × 106, dynamic exclusion was 30 s, and the intensity threshold was 5.0 × 104. The precursor ions were selected from each MS full scan with an isolation width of 1.6 m/z for fragmentation with a normalized collision energy of 28%. For phosphopeptide analysis, MS/MS data were acquired at a resolution of 30,000 (m/z 200). The automatic gain control target was 1 × 105, the maximum injection time was 54 ms, dynamic exclusion was 30 s, and the intensity threshold was 5.0 × 104. For peptide analysis, MS/MS data were acquired at a resolution of 15,000 (m/z 200). The automatic gain control target was 7.5 × 104; the maximum injection time was 22 ms. The compensation voltage of FAIMS was set as −45 V and −65 V.
2.6. Data Analysis and Processing
LC-MS/MS raw data were processed with Proteome Discoverer (PD) (version 2.4.1.15) using the Sequest HT search engine for protein identification. To reduce the influence of chimeric spectra, the precursor detector node in PD was added. The database was the UniProt reviewed human protein database (updated April 2021) with 20,386 protein entries and common contaminants. The database searching parameters were set as follows: enzyme specificity for trypsin and up to two mis-cleavages was allowed, the minimum peptide length was 6, and the mass tolerances for precursor and fragment ions were set as 10 ppm and 0.02 Da, respectively. Cysteine carbamidomethylation was set as a fixed modification. Variable modifications were set to methionine oxidation, phosphorylation at serine, threonine, tyrosine, deamidation at asparagine (Asn, N) and glutamine (Gln, Q), and acetylation at the N-terminal of proteins. The false discovery rate (FDR) was calculated using the Percolator algorithm provided by PD. FDR on peptide and protein levels was 1%. PhosphoRS localization probability was set to greater than 0.75 [
26]. Only phosphopeptides with fully localized sites were regarded as localized phosphopeptides. If there were multiple phosphosites within one phosphopeptide and at least one phosphosite was ambiguous, this phosphopeptide was not regarded as a localized phosphopeptide. The LFQ intensity of phosphopeptides was extracted from raw data, and a normalized abundance of phosphopeptides was used for principal component analysis (PCA) and correlation analysis. The number of non-redundant localized phosphopeptides and localized phosphosites identified were extracted with an in-house Python script.
To analyze the motifs of deamidated (phospho)peptides, sequences of amino acids around deamidation sites (deamidated N or Q) were analyzed with the iceLogo resource (
https://iomics.ugent.be/icelogoserver/create, accessed on 16 December 2021) [
28]. First, the sequence windows of ±5 amino acids around localized deamidated sites were created. Then, non-redundant sequence windows were submitted for iceLogo analysis, and the precompiled Swiss-Prot human database was used as the background. To analyze the motifs of phosphopeptides, sequence windows of ±7 amino acids around phosphorylated amino acids were created and analyzed as described above. For all analyses, the
p-value was set as 0.05, and the results were presented as a percentage.
SPSS (version 16.0) was used for statistical calculations, and Origin 8.0 was used to produce figures. The density plots, PCA plots, and correlation plot were prepared with RStudio using in-house scripts.
4. Discussion
Due to the relatively low stoichiometry of phosphoproteins in the whole proteome, the specific enrichment of phosphopeptides is essential for the successful analysis of phosphoproteomes. TiO
2-based MOAC is likely the most common method of enrichment due to its robust protocol, simple procedure, and TiO
2 selectivity. TiO
2 enrichment is based on the interaction between the negatively charged phosphate group and the metal oxide. The key to the specific enrichment of phosphopeptides from complex samples with TiO
2 is to minimize the interference of acidic non-phosphorylated peptides. To improve enrichment specificity, the sample loading buffer for phosphopeptide enrichment should be acidified to pH 2–2.5 with organic acids, such as acetic acid [
39] or TFA [
20]. The pKa value of phosphate groups (pKa
1 of phosphoric acid) is 2.15, while the pKa values of the carboxyl groups of aspartic acid and glutamic acid are 3.65 and 4.25, respectively [
40]. To reduce the binding of acidic non-phosphorylated peptides to TiO
2, the pH of the loading buffer should be between the pKa values of acidic amino acids and phosphoric acid. In this way, the negative charge of the carboxyl groups is covered after the protonation of acidic amino acids and no longer binds to positively charged TiO
2 [
12], while most phosphates are in a non-protonated state and still show negative charges. As such, they can bind to TiO
2.
However, buffer acidification is insufficient for reducing non-specific binding [
12]. Non-phosphopeptide excluders are added to the loading buffer as competitors to prevent the adsorption of non-phosphopeptides to TiO
2 beads. However, the effects of non-phosphopeptide excluders on phosphopeptide enrichment are unclear. This is likely because only simple standard protein or peptide mixtures are used for enrichment, and only MALDI MS, not ESI MS, has been used to acquire MS spectra. In this study, we comprehensively investigated the effects of four commonly-used non-phosphopeptide excluders, including glutamic acid, lactic acid, glycolic acid, and DHB, on phosphopeptide enrichment with TiO
2 using complex proteomic samples. Phosphopeptide enrichment specificity greatly varied among the four enrichment methods using different non-phosphopeptide excluders (
Figure 2B). The selectivity of TiO
2 in the glutamic acid method was high (>85%), which is consistent with previous observations [
17]. The lactic acid method’s high enrichment specificity (about 89%) in this study has also been previously reported [
18,
22]. In this study, high non-specific binding was observed when 1 M glycolic acid was used as a non-phosphopeptide excluder. These results are consistent with those of Sugiyama et al. [
18] but differ from the results from Jensen et al., who found that 1 M glycolic acid could effectively exclude non-phosphopeptides from TiO
2 micro-columns [
13]. An enrichment specificity exceeding 85% can be achieved in the glycolic acid method by washing TiO
2 beads with ammonium acetate (pH~6.8) to remove non-phosphopeptides from the TiO
2 beads [
41]. However, we did not attempt this method and achieved our greatest improvement in enrichment specificity using the glycolic acid method when double TiO
2 enrichment was performed (
Figure 4A). This indicates that two rounds of TiO
2 enrichment are required to obtain a high enrichment specificity for the glycolic acid method. However, the double TiO
2 enrichment method must be approached with caution: some phosphopeptides with a low affinity to TiO
2 beads could be lost during the second TiO
2 enrichment (
Figure 4B), which would decrease phosphoproteome coverage and compromise quantification accuracy.
DHB was the first non-phosphopeptide excluder used to improve phosphopeptide enrichment specificity in TiO
2 [
9], while phosphopeptide enrichment specificity can reach 90% [
25]. However, in this study, the DHB method displayed the lowest enrichment specificity (about 41%). This discrepancy in enrichment specificity could be because the two studies used samples with different complexities. HpH-RP-fractionated or SCX-fractionated peptide mixtures were used in the previous study [
25], while an unfractionated peptide mixture from cell lysate was used in this study. Since DHB was assumed to contaminate both the LC system and the mass spectrometer [
13], we did not perform further investigations using DHB.
A detailed investigation of phosphopeptide characteristics revealed some differences in the phosphopeptides enriched by the four methods. Compared with the other three methods, the lactic acid method purified more multi-phosphorylated peptides (doubly and triply phosphorylated peptides) (
Figure 3A), which led to a lower localization rate of phosphosite(s) within phosphopeptides (
Figure 3B). In addition, the difference in peptide length, hydrophobicity, and pI of phosphopeptides were observed for phosphopeptides identified by the glutamic acid method and lactic acid method. For example, shorter phosphopeptides were purified by the glutamic acid method, while more hydrophilic and more acidic phosphopeptides were purified by the lactic acid method (
Figure 3E–G). These results indicate that the specificity and selectivity of phosphopeptide enrichment with TiO
2 depend on the loading conditions with different non-phosphopeptide excluders. Besides that, the phosphopeptide enrichment specificity of TiO
2 can vary depending on the TiO
2 beads from different vendors [
42]. The extract buffers and enrichment conditions used for phosphopeptide enrichment with different TiO
2 materials should be optimized to obtain high enrichment specificity [
43]. Furthermore, the optimized protocol used here with TiO
2 may not obtain the best performance for phosphopeptide enrichment with other types of IMAC and MOAC, such as Zr-IMAC, Ti-IMAC, Fe-IMAC, Ga-IMAC, or ZrO
2, as specific sample-loading conditions should be used for different enrichment materials to increase their performance in phosphopeptide enrichment [
44].
Aside from these differences, the phosphopeptides identified by the four methods shared some similarities, such as a high percentage of mis-cleavage (
Supplementary Figure S2A), similar peptide sequence patterns (
Figure 3C), and a bias towards S, P, D, and E on amino acid composition (
Figure 3D). A distinct but partially overlapped population of phosphopeptides was purified by the four TiO
2-based phosphopeptide enrichment methods. The same results were obtained when comparing phosphopeptides isolated by the three phosphopeptide isolation methods (phosphoramidate chemistry (PAC), IMAC, and TiO
2) [
31]. Since the overlap of phosphopeptides identified by the four methods was only 35.5% of all phosphopeptides identified (
Figure 2D), no single method can enrich all phosphoproteome parts, and combined phosphopeptides enriched by different methods could increase phosphoproteome coverage.
Deamidation is a chemical reaction in which an amide functional group on the side chain of amino acids Asn or Gln is removed, typically converting Asn to aspartic acid (Asp) or isoaspartic acid (isoAsp) and Gln to glutamic acid (Glu) or isopyroglutamic acid (γ-Glu) [
45]. There are two types of deamidation: enzymatic deamidation, which uses PNGase F to remove N-glycans from N-linked glycoproteins or glycopeptides [
46], and nonenzymatic deamidation (also called chemical deamidation), which occurs spontaneously on proteins and peptides both in vivo and in vitro. The latter is discussed in this study.
Previous mechanistic studies have revealed the process of the deamidation of Asn and Gln [
47]. Under neutral or alkaline conditions, Asn deamidation proceeds via a succinimide intermediate formed by the nucleophilic attack on the side chain carbonyl carbon of Asn by the backbone nitrogen of the ensuing amino acid residue. The cyclic succinimide intermediate is then rapidly hydrolyzed at either the alpha or beta carbonyl group to produce Asp and isoAsp at a ratio of approximately 1:3 [
48]. Under acidic conditions, Asn usually deamidates by direct hydrolysis via acid catalysis [
49]. Deamidation on Gln can occur via a mechanism similar to Asn residue by direct hydrolysis at acidic pH or via a glutarimide intermediate at neutral or alkaline pH to yield α-Glu and γ-Glu [
50]. In general, the deamidation rate of Gln is much slower than the rate of Asn [
45].
The rate of deamidation of Asn and Gln residues in a protein or peptide depends on external conditions, such as buffer type, ionic strength, pH, temperature, and protein or peptide sequence [
47]. We speculated that the observed high deamidation ratio in phosphopeptides is likely due to three reasons. First, it has been reported that exposure to elevated pH (>10) increases the rate of the formation of succinimide or glutarimide intermediates due to the greater deprotonation of the peptide bond nitrogen at high pH values. The deamidation reaction proceeded more quickly at high pH, and ammonia was an effective general base catalyst for the deamidation of Asn and Gln residues in peptides [
48,
51]. The rate of deamidation was 6.5-fold faster in the solution which contained ammonia [
48]. Therefore, using a high pH ammonia elution buffer to elute phosphopeptides from TiO
2 beads would lead to a high deamidation ratio of phosphopeptides. Second, the sequence immediately around Asn and Gln residues significantly affects the deamidation rate. Deamidation proceeds much more quickly if Asn or Gln is followed by a polar amino acid with a relatively small side chain, such as Gly, Ala, Ser, Thr, Asp, Glu, or His; their low steric hindrance leaves the peptide group open for the attack [
47]. The rate of deamidation decreases as the size and steric bulk of the residues increase [
48]. Neighboring Ser and Thr increase the deamidation rate. In this study, we observed that phosphopeptides enriched by TiO
2 had a bias toward Ser, Pro, Glu, and Asp at the amino acid level (
Figure 3D) and a significant preference for Pro, Asp, Glu, and Ser at the +1 to +3 position of pS and pT at peptide sequence (
Figure 3C). From this point of view, the special amino acid sequences in phosphopeptides enriched by TiO
2 favored the deamidation of Asn and Gln in phosphopeptides. We observed a general preference for amino acids with small side chains in the deamidated phosphopeptides enriched by TiO
2. There was a high frequency of Gly and Ser at the n + 1 residue of deamidated Asn residue and Pro, Ser, and Glu at the n + 1 residue of deamidated Gln residue (
Figure 5B), while a high frequency of Ser, Pro, and Glu before deamidated Asn or Gln was observed (
Figure 5B). This indicates that amino acids following Asn or Gln and amino acids before Asn or Gln significantly affect phosphopeptide deamidation. Third, the sample loading buffer for phosphopeptide enrichment with TiO
2 was acidified with TFA to block the non-specific binding of acidic non-phosphopeptides. The acidic conditions (pH < 2) used during phosphopeptide enrichment could accelerate Asn and Gln deamidation in phosphopeptides by direct hydrolysis.
We then investigated why the deamidation ratio and the percentage of deamidation on Gln were higher in phosphopeptides purified from the glutamic acid method than from the lactic acid method. Since saturated glutamic acid (~0.14 M) was used in the sample loading buffer of the glutamic acid method, we speculate that a high concentration of glutamic acid would lead to the accumulation of glutarimide intermediate, which would accelerate deamidation on Gln in phosphopeptides purified in the glutamic acid method.
Due to the special amino acid sequence and pattern of phosphopeptides and specific conditions for phosphopeptide enrichment, the deamidation of phosphopeptides is a nonnegligible but neglected phenomenon in phosphoproteomic research. The deamidation of phosphopeptides can reduce the intensities of native phosphopeptides and lead to the inaccurate quantification of phosphopeptides, which is especially harmful to low-abundance phosphopeptides.
Due to the complexity of phosphoproteomes, the number of phosphopeptides identified in single-shot analysis is limited, and fractionation of phosphopeptides before LC-MS/MS is essential for increasing phosphoproteome coverage [
52]. However, for large-scale phosphoproteomic analysis, it is unclear whether it is better to fractionate peptides first and then perform phosphopeptide enrichment for each fraction (a “fractionation before enrichment” strategy) or to first enrich phosphopeptides from the whole sample and then fractionate purified phosphopeptides (a “fractionation after enrichment” strategy). The first strategy is often used in conventional phosphoproteomic analyses, likely because it has better enrichment specificity. However, this strategy is labor-intensive and time-consuming and requires relatively large amounts of starting material (typically 2–3 mg) since sample loss can occur during the fractionation process. The second strategy is more robust and time-efficient. Hydrophilic interaction liquid chromatography (HILIC) [
23] and HpH-RP chromatography [
53,
54] have been used to fractionate purified phosphopeptides due to their high orthogonality toward acidic RP-LC-MS/MS analysis. In this study, we compared ammonia-based and TEA-based HpH-RP phosphopeptide fractionation methods and found that the TEA-based method performed better than the ammonia-based method, likely because the ammonia-based method loaded samples in high pH conditions, which led to the loss of some multi-phosphorylated peptides since multi-phosphorylated peptides interact less with RP materials under high pH values. With the TEA-based method, on average, more than 30,000 phosphopeptides, corresponding to about 19,000 phosphopeptides, can be identified in three fractions of one replicate experiment (
Table 2). Therefore, HpH-RP fractionation could greatly expand phosphoproteome coverage compared with single-shot analysis (
Figure 7A).
Altogether, we comprehensively evaluated different phosphopeptide enrichment and fractionation methods, which provides a basis for choosing the best method of performing a comprehensive and in-depth phosphoproteomic analysis. We also provided a robust, efficient, and reproducible large-scale phosphoproteomic analysis workflow, which contributes to a better understanding of phosphorylation-related mechanisms.