Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Serological Analyses
2.3. Peripheral Blood Mononuclear Cell (PBMC) Analyses
2.4. Statistics
3. Results
3.1. Patient Characteristics
3.2. Global Vaccination Characteristics
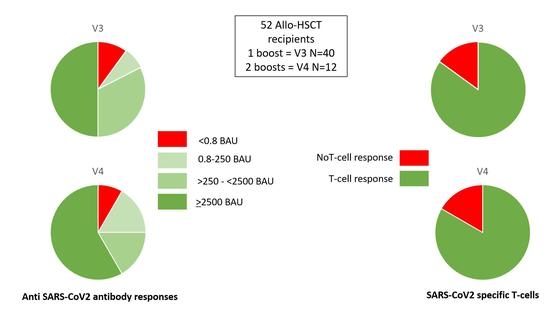
3.3. Humoral Responses
3.4. Cellular Responses
3.5. Follow-Up of Humoral and Cellular Specific Responses
3.6. Specific Responses and Protection
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xhaard, A.; Xhaard, C.; D’Aveni, M.; Salvator, H.; Chabi, M.L.; Berceanu, A.; Coman, T.; Beguin, Y.; Chalandon, Y.; Poiré, X.; et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A Société Francophone de Greffe de Moelle et de Thérapie cellulaire (SFGM-TC) multicentre cohort study. Br. J. Haematol. 2021, 192, e121–e124. [Google Scholar] [CrossRef]
- Bailey, A.J.M.; Kirkham, A.M.; Monaghan, M.; Shorr, R.; Buchan, C.A.; Bredeson, C.; Allan, D.S. A portrait of SARS-CoV-2 infection in patients undergoing hematopoietic cell transplantation: A systematic review of the literature. Curr. Oncol. 2022, 29, 337–349. [Google Scholar] [CrossRef]
- Bettini, E.; Locci, M. SARS-CoV-2 mRNA vaccines: Immunological mechanism and beyond. Vaccines 2021, 9, 147. [Google Scholar] [CrossRef]
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Béné, M.C.; Chevallier, P. Safety and antibody response after 1 and 2 doses of BNT162b2 mRNA vaccine in recipients of allogeneic hematopoietic stem cell transplant. JAMA Netw. Open 2021, 4, e2126344. [Google Scholar] [CrossRef]
- Maillard, A.; Redjoul, R.; Klemencie, M.; Labussiere-Wallet, H.; Le Bourgeois, A.; D’Aveni, M.; Huynh, A.; Berceanu, A.; Marchand, T.; Chantepie, S.P.; et al. Antibody response after 2 and 3 doses of SARS-CoV-2 mRNA vaccine in allogeneic hematopoietic cell transplant recipients. Blood 2022, 139, 134–137. [Google Scholar] [CrossRef]
- Jullien, M.; Le Bourgeois, A.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Rimbert, M.; Imbert, B.M.; Le Gouill, S.; Moreau, P.; Mahe, B.; et al. B cell aplasia is the most powerful predictive marker for poor humoral response after BNT162b2 mRNA SARS-CoV-2 vaccination in recipients of allogeneic hematopoietic stem cell transplantation. Transplant. Cell. Ther. 2022, 28, 279.e1–279.e4. [Google Scholar] [CrossRef]
- Krammer, F. The human antibody response to influenza A virus infection and vaccination. Nat. Rev. Immunol. 2019, 19, 383–397. [Google Scholar] [CrossRef]
- Béné, M.C.; de Carvalho Bittencourt, M.; Eveillard, M.; Le Bris, Y. Good IgA bad IgG in SARS-CoV-2 Infection? Clin. Infect. Dis. 2020, 71, 897–898. [Google Scholar] [CrossRef]
- Ram, R.; Hagin, D.; Kikozashvilli, N.; Freund, T.; Amit, O.; Bar-On, Y.; Beyar-Katz, O.; Shefer, G.; Moshiashvili, M.M.; Karni, C.; et al. Safety and immunogenicity of the BNT162b2 mRNA COVID-19 vaccine in patients after allogeneic HCT or CD19-based CART therapy-A single-center prospective cohort study. Transplant. Cell. Ther. 2021, 27, 788–794. [Google Scholar] [CrossRef]
- Lindemann, M.; Klisanin, V.; Thümmler, L.; Fisenkci, N.; Tsachakis-Mück, N.; Ditschkowski, M.; Schwarzkopf, S.; Klump, H.; Reinhardt, H.C.; Horn, P.A.; et al. Humoral and cellular vaccination responses against SARS-CoV-2 in hematopoietic stem cell transplant recipients. Vaccines 2021, 9, 1075. [Google Scholar] [CrossRef]
- Harrington, P.; Doores, K.J.; Saha, C.; Saunders, J.; Child, F.; Dillon, R.; Saglam, S.; Raj, K.; McLornan, D.; Avenoso, D.; et al. Repeated vaccination against SARS-CoV-2 elicits robust polyfunctional T cell response in allogeneic stem cell transplantation recipients. Cancer Cell 2021, 39, 1448–1449. [Google Scholar] [CrossRef] [PubMed]
- Clémenceau, B.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. SARS-CoV-2 T-cell responses in allogeneic hematopoietic stem cell recipients following two doses of BNT162b2 mRNA vaccine. Vaccines 2022, 10, 448. [Google Scholar] [CrossRef] [PubMed]
- Redjoul, R.; Le Bouter, A.; Parinet, V.; Fourati, S.; Maury, S. Antibody response after third BNT162b2 dose in recipients of allogeneic HSCT. Lancet Haematol. 2021, 8, e681–e683. [Google Scholar] [CrossRef]
- Le Bourgeois, A.; Coste-Burel, M.; Guillaume, T.; Peterlin, P.; Garnier, A.; Imbert, B.M.; Drumel, T.; Mahé, B.; Dubruille, V.; Blin, N.; et al. Interest of a third dose of BNT162b2 anti-SARS-CoV-2 messenger RNA vaccine after allotransplant. Br. J. Haematol. 2022, 196, e38–e40. [Google Scholar] [CrossRef]
- Chevallier, P.; Jullien, M.; Peterlin, P.; Garnier, A.; Le Bourgeois, A.; Coste-Burel, M.; Béné, M.C.; Guillaume, T. Effectiveness of a third dose of BNT162b2 anti-SARS-CoV-2 mRNA vaccine over a 6-month follow-up period in allogenic hematopoietic stem cells recipients. Hematol. Oncol. 2022; Epub ahead of print. [Google Scholar] [CrossRef]
- Kimura, M.; Ferreira, V.H.; Kothari, S.; Pasic, I.; Mattsson, J.I.; Kulasingam, V.; Humar, A.; Mah, A.; Delisle, J.S.; Ierullo, M.; et al. Safety and immunogenicity after a three-dose SARS-CoV-2 vaccine schedule in allogeneic stem cell transplant recipients. Transplant. Cell. Ther. 2022, 29, S2666–S6367. [Google Scholar] [CrossRef]
- Zuo, J.; Dowell, A.C.; Pearce, H.; Verma, K.; Long, H.M.; Begum, J.; Aiano, F.; Amin-Chowdhury, Z.; Hoschler, K.; Brooks, T.; et al. Robust SARS-CoV-2-specific T cell immunity is maintained at 6 months following primary infection. Nat. Immunol. 2021, 22, 620–626. [Google Scholar] [CrossRef]
- Bilich, T.; Nelde, A.; Heitmann, J.S.; Maringer, Y.; Roerden, M.; Bauer, J.; Rieth, J.; Wacker, M.; Peter, A.; Hörber, S.; et al. T cell and antibody kinetics delineate SARS-CoV-2 peptides mediating long-term immune responses in COVID-19 convalescent individuals. Sci. Transl. Med. 2021, 13, eabf7517. [Google Scholar] [CrossRef]
- Wellington, D.; Yin, Z.; Kessler, B.M.; Dong, T. Immunodominance complexity: Lessons yet to be learned from dominant T cell responses to SARS-CoV-2. Curr. Opin. Virol. 2021, 50, 183–191. [Google Scholar] [CrossRef]
- Schwarz, M.; Torre, D.; Lozano-Ojalvo, D.; Tan, A.T.; Tabaglio, T.; Mzoughi, S.; Sanchez-Tarjuelo, R.; Le Bert, N.; Lim, J.M.E.; Hatem, S.; et al. Rapid, scalable assessment of SARS-CoV-2 cellular immunity by whole-blood PCR. Nat. Biotechnol. 2022, 1–10. [Google Scholar] [CrossRef]
- Dalai, S.C.; Dines, J.N.; Snyder, T.M.; Gittelman, R.M.; Eerkes, T.; Vaney, P.; Howard, S.; Akers, K.; Skewis, L.; Monteforte, A.; et al. Clinical validation of a novel T-cell receptor sequencing assay for identification of recent or prior SARS-CoV-2 infection. Clin. Infect. Dis. 2022; Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Tarke, A.; Coelho, C.H.; Zhang, Z.; Dan, J.M.; Yu, E.D.; Methot, N.; Bloom, N.I.; Goodwin, B.; Phillips, E.; Mallal, S.; et al. SARS-CoV-2 vaccination induces immunological T cell memory able to cross-recognize variants from Alpha to Omicron. Cell 2022, 185, 847–859.e11. [Google Scholar] [CrossRef]
- Liu, J.; Chandrashekar, A.; Sellers, D.; Barrett, J.; Jacob-Dolan, C.; Lifton, M.; McMahan, K.; Sciacca, M.; VanWyk, H.; Wu, C.; et al. Vaccines elicit highly conserved cellular immunity to SARS-CoV-2 Omicron. Nature 2022, 603, 493–496. [Google Scholar] [CrossRef] [PubMed]
- Keeton, R.; Tincho, M.B.; Ngomti, A.; Baguma, R.; Benede, N.; Suzuki, A.; Khan, K.; Cele, S.; Bernstein, M.; Karim, F.; et al. T cell responses to SARS-CoV-2 spike cross-recognize Omicron. Nature 2022, 603, 488–492. [Google Scholar] [CrossRef]
- Emmelot, M.E.; Vos, M.; Boer, M.C.; Rots, N.Y.; de Wit, J.; van Els, C.A.C.M.; Kaaijk, P. Omicron BA.1 mutations in SARS-CoV-2 spike lead to reduced t-cell response in vaccinated and convalescent individuals. Viruses 2022, 14, 1570. [Google Scholar] [CrossRef]
- Hillus, D.; Schwarz, T.; Tober-Lau, P.; Vanshylla, K.; Hastor, H.; Thibeault, C.; Jentzsch, S.; Helbig, E.T.; Lippert, L.J.; Tscheak, P.; et al. Safety, reactogenicity, and immunogenicity of homologous and heterologous prime-boost immunisation with ChAdOx1 nCoV-19 and BNT162b2: A prospective cohort study. Lancet Respir. Med. 2021, 9, 1255–1265. [Google Scholar] [CrossRef]
- Tan, C.S.; Collier, A.Y.; Yu, J.; Liu, J.; Chandrashekar, A.; McMahan, K.; Jacob-Dolan, C.; He, X.; Roy, V.; Hauser, B.M.; et al. Durability of heterologous and homologous COVID-19 vaccine boosts. JAMA Netw. Open 2022, 5, e2226335. [Google Scholar] [CrossRef] [PubMed]

| Patients | Controls | |||
|---|---|---|---|---|
| Whole Cohort N = 52 | One Booster (V3) N = 40 | Two Boosters (V4) N = 12 | One Booster (V3) N = 12 | |
| Period of analysis | From 18 January 2022 to 3 March 2022 | 26 January 2022 to 4 February 2022 | ||
| Gender: Male/female | 33/19 | 26/14 | 7/5 | 3/9 |
| Median age: years (range) | 54 (20–74) | 54 (20–74) | 55 (25–69) | 54 (39–63) |
| Underlying disease: | NA | |||
| AML/MDS/MPS (myeloid) | 20/10/6 (36) | 11/7/6 (24) | 9/3/0 (12) | |
| ALL/NHL/HL/MM (lymphoid) | 8/4/3/1 (16) | 8/4/3/1 (16) | 0/0/0 (0) | |
| Donor type: | NA | |||
| Geno-identical/MUD/haplo/9/10 mis-MUD | 15/22/13/2 | 12/18/8/2 | 3/4/5/0 | |
| Conditioning: | NA | |||
| Myeloablative/reduced-intensity/sequential | 8/41/3 | 8/30/2 | 0/11/1 | |
| GVHD prophylaxis: | NA | |||
| CsA+ ATG (+-MMF or methotrexate) | 28 | 26 | 2 | |
| CsA + MMF + PTCY | 13 | 7 | 6 | |
| PTCY only | 11 | 7 | 4 | |
| Previous GVHD: No/Yes | 20/32 | 15/25 | 5/7 | NA |
| Ongoing treatment: No/Yes * | 41/11 | 32/8 | 9/3 | |
| Patients | Controls | |||
|---|---|---|---|---|
| Whole Cohort N = 52 | One Booster (V3) N = 40 | Two Boosters (V4) N = 12 | One Booster (V3) N = 12 | |
| Type of vaccine | 6/6 | |||
| BNT162b1/ mRNA-1273 | 52/0 | 40/0 | 12/0 | |
| Median time from transplant to V1 | NA | |||
| days (range) | 389 (86–4939) | 692 (91–4939) | 126.5 (86–1000) p = 0.001 | |
| Median time from transplant to: | NA | |||
| V3 days (range) | 487 (143–5160) | 889 (167–5160) | 267 (143–1090) p = 0.001 | |
| V4: days (range) | 459 (239–1306) | |||
| Median time from transplant to humoral and T-cell responses analyses: days (range) | 755 (189–5293) | 1045 (189–5293) | 517 (265–1376) p = 0.008 | NA |
| <1 year | 9 | 5 | 4 | |
| 1–2 years | 18 | 11 | 7 | |
| >2 years | 25 | 24 | 1 | |
| Median time from V1 to humoral and T-cell responses analyses: days (range) | 330 (84–404) | 324 (84–398) | 367 (175–404) p = 0.44 | 381 (358–391) p = 0.004 |
| Median time from V3 to humoral and T-cell responses analyses: days (range) | 229 (12–298) | 224 (12–298) | 255 (113–296) p = 0.19 | 55 (18–74) |
| Median time from last vaccine to humoral and T-cell responses analyses: days (range) | 125 (12–298) | 224 (12–298) | 60 (16–117) p = 0.0006 | 55 (18–74) p = 0.002 |
| Humoral response after V3/V4 N (%): | ||||
| Antibodies < 0.8 BAU/mL | 5 (9.5%) | 4 (10%) | 1 (8%) | 0 |
| Antibodies 0.8–250 BAU/mL | 5 (9.5%) | 3 (7.5%) | 2 (17%) | 0 |
| Antibodies > 250 BAU/mL | 42 (81%) | 33 (82.5%) | 9 (75%) p = 0.63 | 12 (100%) p = 0.25 |
| Antibodies > 2500 BAU/mL | 27 (52%) | 20 (50%) | 7 (58%) p = 0.85 | 12 (100%) p = 0.005 |
| T-cell response after V3/V4: N (%) | 44 (85%) | 34 (85%) | 10 (83%) p = 1 | 12 (100%) p = 0.33 |
| Median % of anti-spike T-cells (range) ** | 0.034 (0–1.143) | 0.028 (0–0.771) | 0.063 (0–1.143) p = 0.21 | 0.127 (0.006–0.235) p = 0.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clémenceau, B.; Le Bourgeois, A.; Guillaume, T.; Coste-Burel, M.; Peterlin, P.; Garnier, A.; Jullien, M.; Ollier, J.; Grain, A.; Béné, M.C.; et al. Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients. Cells 2022, 11, 3010. https://doi.org/10.3390/cells11193010
Clémenceau B, Le Bourgeois A, Guillaume T, Coste-Burel M, Peterlin P, Garnier A, Jullien M, Ollier J, Grain A, Béné MC, et al. Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients. Cells. 2022; 11(19):3010. https://doi.org/10.3390/cells11193010
Chicago/Turabian StyleClémenceau, Béatrice, Amandine Le Bourgeois, Thierry Guillaume, Marianne Coste-Burel, Pierre Peterlin, Alice Garnier, Maxime Jullien, Jocelyn Ollier, Audrey Grain, Marie C. Béné, and et al. 2022. "Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients" Cells 11, no. 19: 3010. https://doi.org/10.3390/cells11193010
APA StyleClémenceau, B., Le Bourgeois, A., Guillaume, T., Coste-Burel, M., Peterlin, P., Garnier, A., Jullien, M., Ollier, J., Grain, A., Béné, M. C., & Chevallier, P. (2022). Strong SARS-CoV-2 T-Cell Responses after One or Two COVID-19 Vaccine Boosters in Allogeneic Hematopoietic Stem Cell Recipients. Cells, 11(19), 3010. https://doi.org/10.3390/cells11193010