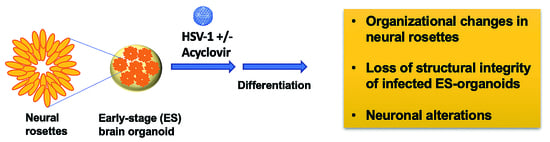
The Impaired Neurodevelopment of Human Neural Rosettes in HSV-1-Infected Early Brain Organoids
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Virus Strains
2.3. Virus Preparation
2.4. HSV-1 Plaque Assay
2.5. Acyclovir Resistance Assay
2.6. Generation and Infection of Early-Stage Organoids from Neural Rosettes
2.7. Generation and Infection of Early-Stage Organoids from iPSCs
2.8. Generation of A-3D Cultures
2.9. Immunofluorescence
2.10. Quantitative Confocal Analysis
3. Results
3.1. Neural Rosettes-Based Culture Systems
3.2. A-3D Culture System
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kraemer, J.M.; Marquez, D.X. Psychosocial correlates and outcomes of yoga or walking among older adults. J. Psychol. 2009, 143, 390–404. [Google Scholar] [CrossRef] [PubMed]
- Stegmann, B.J.; Carey, J.C. TORCH Infections. Toxoplasmosis, Other (syphilis, varicella-zoster, parvovirus B19), Rubella, Cytomegalovirus (CMV), and Herpes infections. Curr. Womens Health Rep. 2002, 2, 253–258. [Google Scholar] [PubMed]
- Manjunathachar, H.; Singh, K.N.; Chouksey, V.; Kumar, R.; Sharma, R.K.; Barde, P.V. Prevalence of torch infections and its associated poor outcome in high-risk pregnant women of Central India: Time to think for prevention strategies. Indian J. Med. Microbiol. 2020, 38, 379–384. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Newman, L.M.; Gottlieb, S.L. First estimates of the global and regional incidence of neonatal herpes infection. Lancet Glob. Heal. 2017, 5, e300–e309. [Google Scholar] [CrossRef] [Green Version]
- Whitley, R.; Arvin, A.; Prober, C.; Burchett, S.; Corey, L.; Powell, D.; Plotkin, S.; Starr, S.; Alford, C.; Connor, J.; et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N. Engl. J. Med. 1991, 324, 444–449. [Google Scholar] [CrossRef]
- Whitley, R.; Arvin, A.; Prober, C.; Corey, L.; Burchett, S.; Plotkin, S.; Starr, S.; Jacobs, R.; Powell, D.; Nahmias, A.; et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N. Engl. J. Med. 1991, 324, 450–454. [Google Scholar] [CrossRef]
- Engman, M.-L.; Adolfsson, I.; Lewensohn-Fuchs, I.; Forsgren, M.; Mosskin, M.; Malm, G. Neuropsychologic outcomes in children with neonatal herpes encephalitis. Pediatr. Neurol. 2008, 38, 398–405. [Google Scholar] [CrossRef]
- Whitley, R.J.; Corey, L.; Arvin, A.; Mintz, E.; Lakeman, F.D.; Sumaya, C.V.; Wright, P.F.; Dunkle, L.M.; Steele, R.W.; Soong, S.-J.; et al. Changing presentation of herpes simplex virus infection in neonates. J. Infect. Dis. 1988, 158, 109–116. [Google Scholar] [CrossRef]
- Marquez, L.; Levy, M.L.; Munoz, F.M.; Palazzi, D.L. A report of three cases and review of intrauterine herpes simplex virus infection. Pediatr. Infect. Dis. J. 2011, 30, 153–157. [Google Scholar] [CrossRef] [Green Version]
- D’Aiuto, L.; Prasad, K.M.; Upton, C.H.; Viggiano, L.; Milosevic, J.; Raimondi, G.; McClain, L.; Chowdari, K.; Tischfield, J.; Sheldon, M.; et al. Persistent Infection by HSV-1 Is Associated With Changes in Functional Architecture of iPSC-Derived Neurons and Brain Activation Patterns Underlying Working Memory Performance. Schizophr. Bull. 2015, 41, 123–132. [Google Scholar] [CrossRef]
- Qiao, H.; Guo, M.; Shang, J.; Zhao, W.; Wang, Z.; Liu, N.; Li, B.; Zhou, Y.; Wu, Y.; Chen, P. Herpes simplex virus type 1 infection leads to neurodevelopmental disorder-associated neuropathological changes. PLoS Pathog. 2020, 16, e1008899. [Google Scholar] [CrossRef]
- Krenn, V.; Bosone, C.; Burkard, T.R.; Spanier, J.; Kalinke, U.; Calistri, A.; Salata, C.; Christoff, R.R.; Garcez, P.P.; Mirazimi, A.; et al. Organoid modeling of Zika and herpes simplex virus 1 infections reveals virus-specific responses leading to microcephaly. Cell Stem Cell 2021, 28, 1362–1379.e7. [Google Scholar] [CrossRef]
- D’Aiuto, L.; Naciri, J.; Radio, N.; Tekur, S.; Clayton, D.; Apodaca, G.; Di Maio, R.; Zhi, Y.; Dimitrion, P.; Piazza, P.; et al. Generation of three-dimensional human neuronal cultures: Application to modeling CNS viral infections. Stem Cell Res. Ther. 2018, 9, 134. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Klammer, A.M.; Naciri, J.N.; Yeung, J.; Demers, M.; Milosevic, J.; Kinchington, P.R.; Bloom, D.C.; Nimgaonkar, V.L.; D’Aiuto, L. Patterns of HSV-1 infection in neural progenitor cells. J. Virol. 2020, 94, e00994-20. [Google Scholar] [CrossRef]
- Zheng, W.; D’Aiuto, L.; Demers, M.J.; Muralidaran, V.; Wood, J.A.; Wesesky, M.; Chattopadhyay, A.; Nimgaonkar, V.L. Insights into bioinformatic approaches for repurposing compounds as anti-viral drugs. Antivir. Chem. Chemother. 2021, 29, 20402066211036822. [Google Scholar] [CrossRef]
- Ziv, O.; Zaritsky, A.; Yaffe, Y.; Mutukula, N.; Edri, R.; Elkabetz, Y. Quantitative Live Imaging of Human Embryonic Stem Cell Derived Neural Rosettes Reveals Structure-Function Dynamics Coupled to Cortical Development. PLoS Comput. Biol. 2015, 11, e1004453. [Google Scholar] [CrossRef] [Green Version]
- Elkabetz, Y.; Studer, L. Human ESC-derived neural rosettes and neural stem cell progression. Cold Spring Harb. Symp. Quant. Biol. 2008, 73, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Valensisi, C.; Andrus, C.; Buckberry, S.; Doni Jayavelu, N.; Lund, R.J.; Lister, R.; Hawkins, R.D. Epigenomic Landscapes of hESC-Derived Neural Rosettes: Modeling Neural Tube Formation and Diseases. Cell Rep. 2017, 20, 1448–1462. [Google Scholar] [CrossRef] [Green Version]
- D’Aiuto, L.; McNulty, J.; Hartline, C.; Demers, M.; Kalkeri, R.; Wood, J.; McClain, L.; Chattopadhyay, A.; Zhi, Y.; Naciri, J.; et al. R430: A potent inhibitor of DNA and RNA viruses. Sci. Rep. 2018, 8, 16662. [Google Scholar] [CrossRef] [Green Version]
- Pichler, M.; Staffler, A.; Bonometti, N.; Messner, H.; Deluca, J.; Thuile, T.; Kluge, R.; Schmuth, M.; Eisendle, K. Premature newborns with fatal intrauterine herpes simplex virus-1 infection: First report of twins and review of the literature. J. Eur. Acad. Dermatol. Venereol. 2014, 29, 1216–1220. [Google Scholar] [CrossRef]
- Straface, G.; Selmin, A.; Zanardo, V.; De Santis, M.; Ercoli, A.; Scambia, G. Herpes simplex virus infection in pregnancy. Infect. Dis. Obstet. Gynecol. 2012, 2012, 385697. [Google Scholar] [CrossRef] [PubMed]
- Kimberlin, D.W.; Lin, C.Y.; Jacobs, R.F.; Powell, D.A.; Corey, L.; Gruber, W.C.; Rathore, M.; Bradley, J.S.; Diaz, P.S.; Kumar, M.; et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 2001, 108, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Kakiuchi, S.; Nonoyama, S.; Wakamatsu, H.; Kogawa, K.; Wang, L.; Kinoshita-Yamaguchi, H.; Takayama-Ito, M.; Lim, C.-K.; Inoue, N.; Mizuguchi, M.; et al. Neonatal herpes encephalitis caused by a virologically confirmed acyclovir-resistant herpes simplex virus 1 strain. J. Clin. Microbiol. 2013, 51, 356–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitley, R.J.; Kimberlin, D.W. Kimberlin, Herpes simplex encephalitis: Children and adolescents. Semin. Pediatr. Infect. Dis. 2005, 16, 17–23. [Google Scholar] [CrossRef]
- Mejías, A.; Bustos, R.; Ardura, M.I.; Ramírez, C.; Sánchez, P.J. Persistence of herpes simplex virus DNA in cerebrospinal fluid of neonates with herpes simplex virus encephalitis. J. Perinatol. 2009, 29, 290–296. [Google Scholar]
- Nyquist, A.-C.; Rotbart, H.A.; Cotton, M.; Robinson, C.; Weinberg, A.; Hayward, A.R.; Berens, R.L.; Levin, M.J. Acyclovir-resistant neonatal herpes simplex virus infection of the larynx. J. Pediatr. 1994, 124, 967–971. [Google Scholar] [CrossRef]
- Levin, M.J.; Weinberg, A.; Leary, J.J.; Sarisky, R.T. Development of acyclovir-resistant herpes simplex virus early during the treatment of herpes neonatorum. Pediatr. Infect. Dis. J. 2001, 20, 1094–1097. [Google Scholar] [CrossRef]
- Agid, Y.; Buzsáki, G.; Diamond, D.M.; Frackowiak, R.; Giedd, J.; Girault, J.A.; Grace, A.; Lambert, J.J.; Manji, H.; Mayberg, H.; et al. How can drug discovery for psychiatric disorders be improved? Nat. Rev. Drug Discov. 2007, 6, 189–201. [Google Scholar] [CrossRef]
- Harris, J.B.; Holmes, A.P. Neonatal Herpes Simplex Viral Infections and Acyclovir: An Update. J. Pediatr. Pharmacol. Ther. 2017, 22, 88–93. [Google Scholar] [CrossRef] [Green Version]
- Kimberlin, D.W.; Kern, E.R.; Sidwell, R.W.; North, T.W.; Whitley, R.J. Models of antiviral resistance. Antivir. Res. 1995, 26, 415–422. [Google Scholar] [CrossRef]
- Fan, W.; Christian, K.M.; Song, H.; Ming, G.-L. Applications of Brain Organoids for Infectious Diseases. J. Mol. Biol. 2021, 434, 167243. [Google Scholar] [CrossRef] [PubMed]
- Broccoli, V.; Giannelli, S.G.; Mazzara, P.G. Modeling physiological and pathological human neurogenesis in the dish. Front. Neurosci. 2014, 8, 183. [Google Scholar]
- Zhao, Y.; Ji, S.; Wang, J.; Huang, J.; Zheng, P. mRNA-Seq and microRNA-Seq whole-transcriptome analyses of rhesus monkey embryonic stem cell neural differentiation revealed the potential regulators of rosette neural stem cells. DNA Res. 2014, 21, 541–554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zambrano, A.; Solis, L.; Salvadores, N.; Cortés, M.; Lerchundi, R.; Otth, C. Neuronal cytoskeletal dynamic modification and neurodegeneration induced by infection with herpes simplex virus type 1. J. Alzheimer’s Dis. 2008, 14, 259–269. [Google Scholar]
- Wang, J.-Z.; Xia, Y.-Y.; Grundke-Iqbal, I.; Iqbal, K. Abnormal hyperphosphorylation of tau: Sites, regulation, and molecular mechanism of neurofibrillary degeneration. J. Alzheimer’s Dis. 2013, 33, S123–S139. [Google Scholar] [CrossRef]
- Alonso, A.C.; Zaidi, T.; Grundke-Iqbal, I.; Iqbal, K. Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimer disease. Proc. Natl. Acad. Sci. USA 1994, 91, 5562–5566. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.; Sun, X.-B.; Wang, H.-Q.; Zhao, H.; Zhao, X.-Y.; Xu, Y.-X.; Guo, J.-C.; Zhu, C.-Q. Chronic restraint stress alters the expression and distribution of phosphorylated tau and MAP2 in cortex and hippocampus of rat brain. Brain Res. 2010, 1347, 132–141. [Google Scholar] [CrossRef]
- Zhang, J.; Dong, X.P. Dysfunction of microtubule-associated proteins of MAP2/tau family in Prion disease. Prion 2012, 6, 334–338. [Google Scholar]
- Bloom, D.C. Alphaherpesvirus Latency: A Dynamic State of Transcription and Reactivation. Adv. Virus Res. 2016, 94, 53–80. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Aiuto, L.; Caldwell, J.K.; Wallace, C.T.; Grams, T.R.; Wesesky, M.A.; Wood, J.A.; Watkins, S.C.; Kinchington, P.R.; Bloom, D.C.; Nimgaonkar, V.L. The Impaired Neurodevelopment of Human Neural Rosettes in HSV-1-Infected Early Brain Organoids. Cells 2022, 11, 3539. https://doi.org/10.3390/cells11223539
D’Aiuto L, Caldwell JK, Wallace CT, Grams TR, Wesesky MA, Wood JA, Watkins SC, Kinchington PR, Bloom DC, Nimgaonkar VL. The Impaired Neurodevelopment of Human Neural Rosettes in HSV-1-Infected Early Brain Organoids. Cells. 2022; 11(22):3539. https://doi.org/10.3390/cells11223539
Chicago/Turabian StyleD’Aiuto, Leonardo, Jill K. Caldwell, Callen T. Wallace, Tristan R. Grams, Maribeth A. Wesesky, Joel A. Wood, Simon C. Watkins, Paul R. Kinchington, David C. Bloom, and Vishwajit L. Nimgaonkar. 2022. "The Impaired Neurodevelopment of Human Neural Rosettes in HSV-1-Infected Early Brain Organoids" Cells 11, no. 22: 3539. https://doi.org/10.3390/cells11223539
APA StyleD’Aiuto, L., Caldwell, J. K., Wallace, C. T., Grams, T. R., Wesesky, M. A., Wood, J. A., Watkins, S. C., Kinchington, P. R., Bloom, D. C., & Nimgaonkar, V. L. (2022). The Impaired Neurodevelopment of Human Neural Rosettes in HSV-1-Infected Early Brain Organoids. Cells, 11(22), 3539. https://doi.org/10.3390/cells11223539