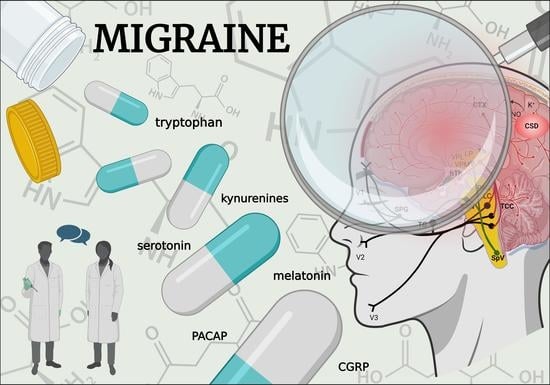
Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms
Abstract
:1. Migraine
2. Tryptophan and Its Role in Migraine
3. Role of the Tryptophan/Serotonin Pathway in Migraine
3.1. Serotonin Pathway
3.2. Serotonin Transporter
3.3. Serotonin Receptors
3.4. Melatonin
4. Role of Tryptophan/Kynurenine Pathway in Migraine
4.1. Kynurenine Pathway
4.2. Kynurenines
5. Neuropeptides in Migraine
5.1. Pituitary Adenylate Cyclase-Activating Polypeptide
5.2. Calcitonin Gene-Related Peptide
5.3. The Relationship between Neuropeptides and the Kynurenine System
6. Clinical Studies
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3-HANA | 3-hydroxyanthranilic acid |
| 3-HAO | 3-hydroxyanthranilate oxidase |
| 3-HK | 3-hydroxy-L-kynurenine |
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | serotonin |
| 5-HTP | 5-hydroxytryptophan |
| 5-HTT | serotonin transporter |
| AA | anthranilic acid |
| AADC | L-aromatic amino acid decarboxylase |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid |
| ATP | adenosine triphosphate |
| BBB | blood–brain barrier |
| BDNF | brain-derived neurotrophic factor |
| CFA | complete Freund’s adjuvant |
| CGRP | calcitonin gene-related peptide |
| CNS | central nervous system |
| CSD | cortical spreading depression |
| DRG | dorsal root ganglion |
| ERK | extracellular signal-regulated kinase |
| FHM | familial hemiplegic migraine |
| GABA | gamma-aminobutyric acid |
| GFAP | glial fibrillary acidic protein |
| GPR35 | G-protein-coupled receptor 35 |
| Iba1 | ionized calcium-binding adapter molecule 1 |
| IDO | indoleamine-2,3-dioxygenase |
| IL-1 β | interleukin-1 |
| KAT | kynurenine aminotransferase |
| KMO | kynurenine-3-monooxygenase |
| KP | kynurenine pathway |
| KYNA | kynurenic acid |
| KYNU | kynureninase |
| L-KYN | L-kynurenine |
| MAO | monoamine oxidase |
| MAPK | mitogen-activated protein kinases |
| MCA | middle cerebral artery |
| mCPP | m-chlorophenylpiperazine |
| MMA | middle meningeal artery |
| MT1/2 | melatonin receptors 1/2 |
| NAD | nicotinamide adenine dinucleotide |
| NADP | nicotinamide adenine dinucleotide phosphate |
| NMDA | N-methyl-D-aspartate |
| NO | nitric oxide |
| NOS | nitric oxide synthases |
| NTG | nitroglycerin |
| PACAP | pituitary adenylate cyclase-activating polypeptide |
| PIC | picolinic acid |
| PPE | plasma protein extravasation |
| QUIN | quinolinic acid |
| SP | substance P |
| TDO | tryptophan-2,3-dioxygenase |
| TG | trigeminal ganglia |
| TMJ | temporomandibular joint |
| TNC | trigeminal nucleus caudalis |
| TPH | L-tryptophan-hydroxylase |
| TRP | tryptophan |
| TRPV1 | transient receptor potential vanilloid 1 |
| TS | trigeminal system |
| TSH | thyroid-stimulating hormone |
| VIP | vasoactive intestinal peptide |
| VMAT2 | vesicular monoamine transporter isoform 2 |
| XA | xanthurenic acid |
References
- Steiner, T.J.; Stovner, L.J.; Vos, T. GBD 2015: Migraine is the third cause of disability in under 50s. J. Headache Pain 2016, 17, 104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cerbo, R.; Pesare, M.; Aurilia, C.; Rondelli, V.; Barbanti, P. Socio–economic costs of migraine. J. Headache Pain 2001, 2 (Suppl. S1), s15–s19. [Google Scholar] [CrossRef] [Green Version]
- Yucel, A.; Thach, A.; Kumar, S.; Loden, C.; Bensink, M.; Goldfarb, N. Estimating the Economic Burden of Migraine on US Employers. Am. J. Manag. Care 2020, 26, e403–e408. [Google Scholar] [CrossRef]
- Headache Classification Committee of the International Headache Society (IHS). The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013, 33, 629–808. [Google Scholar] [CrossRef] [Green Version]
- Goadsby, P.; Holland, P.; Martins-Oliveira, M.; Hoffmann, J.; Schankin, C.; Akerman, S. Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 2017, 97, 553–622. [Google Scholar] [CrossRef]
- Edvinsson, L. Tracing neural connections to pain pathways with relevance to primary headaches. Cephalalgia 2011, 31, 737–747. [Google Scholar] [CrossRef]
- Spekker, E.; Tanaka, M.; Szabó, A.; Vécsei, L. Neurogenic Inflammation: The Participant in Migraine and Recent Advancements in Translational Research. Biomedicines 2021, 10, 76. [Google Scholar] [CrossRef]
- Lauritzen, M.; Hansen, A.J. The Effect of Glutamate Receptor Blockade on Anoxic Depolarization and Cortical Spreading Depression. J. Cereb. Blood Flow Metab. 1992, 12, 223–229. [Google Scholar] [CrossRef] [Green Version]
- Kramer, D.R.; Fujii, T.; Ohiorhenuan, I.; Liu, C.Y. Cortical spreading depolarization: Pathophysiology, implications, and future directions. J. Clin. Neurosci. 2016, 24, 22–27. [Google Scholar] [CrossRef]
- Hoffmann, J.; Charles, A. Glutamate and Its Receptors as Therapeutic Targets for Migraine. Neurotherapeutics 2018, 15, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, M.D.; Odink, J.; Bos, K.D.; Malessy, M.; Bruyn, G.W. Neuroexcitatory plasma amino acids are elevated in migraine. Neurology 1990, 40, 1582–1586. [Google Scholar] [CrossRef] [PubMed]
- Martínez, F.; Castillo, J.; Rodríguez, J.R.; Leira, R.; Noya, M. Neuroexcitatory Amino Acid Levels in Plasma and Cerebrospinal Fluid During Migraine Attacks. Cephalalgia 1993, 13, 89–93. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.; Zukerman, E.; Soares, C.S.; Alonso, E.; Santos, B.; Faulhaber, M. Cerebrospinal Fluid Glutamate Levels in Chronic Migraine. Cephalalgia 2004, 24, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Taylor, B.K.; Basbaum, A.I. Neurochemical Characterization of Extracellular Serotonin in the Rostral Ventromedial Medulla and Its Modulation by Noxious Stimuli. J. Neurochem. 1995, 65, 578–589. [Google Scholar] [CrossRef]
- Rebeck, G.W.; Maynard, K.I.; Hyman, B.T.; Moskowitz, M.A. Selective 5-HT1Da serotonin receptor gene expression in trigeminal ganglia: Implications for antimigraine drug development. Proc. Natl. Acad. Sci. USA 1994, 91, 3666–3669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berman, N.E.; Puri, V.; Chandrala, S.; Puri, S.; MacGregor, R.; Liverman, C.S.; Klein, R.M. Serotonin in Trigeminal Ganglia of Female Rodents: Relevance to Menstrual Migraine. Headache 2006, 46, 1230–1245. [Google Scholar] [CrossRef] [PubMed]
- Saxena, P.; De Vries, P.; Villalón, C. 5-HT 1-like receptors: A time to bid goodbye. Trends Pharmacol. Sci. 1998, 19, 311–316. [Google Scholar] [CrossRef]
- Cameron, C.; Kelly, S.; Hsieh, S.-C.; Murphy, M.; Chen, L.; Kotb, A.; Peterson, J.; Coyle, D.; Skidmore, B.; Gomes, T.; et al. Triptans in the Acute Treatment of Migraine: A Systematic Review and Network Meta-Analysis. Headache 2015, 55 (Suppl. S4), 221–235. [Google Scholar] [CrossRef] [Green Version]
- Párdutz, A.; Fejes, A.; Bohár, Z.; Tar, L.; Toldi, J.; Vécsei, L. Kynurenines and headache. J. Neural Transm. 2012, 119, 285–296. [Google Scholar] [CrossRef]
- Stone, T.W.; Darlington, L.G. Endogenous kynurenines as targets for drug discovery and development. Nat. Rev. Drug Discov. 2002, 1, 609–620. [Google Scholar] [CrossRef]
- Comai, S.; Bertazzo, A.; Brughera, M.; Crotti, S. Tryptophan in health and disease. Adv. Clin. Chem. 2020, 95, 165–218. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Liu, J.; Zhou, J.; Liang, H.; Wang, Y.; Sun, Y.; Ma, B.; Yin, Y. Low levels of serum serotonin and amino acids identified in migraine patients. Biochem. Biophys. Res. Commun. 2018, 496, 267–273. [Google Scholar] [CrossRef] [PubMed]
- D’Andrea, G.; D’Amico, D.; Bussone, G.; Bolner, A.; Aguggia, M.; Saracco, M.G.; Galloni, E.; De Riva, V.; D’Arrigo, A.; Colavito, D.; et al. Tryptamine levels are low in plasma of chronic migraine and chronic tension-type headache. Neurol. Sci. 2014, 35, 1941–1945. [Google Scholar] [CrossRef]
- Alam, Z.; Coombes, N.; Waring, R.H.; Williams, A.C.; Steventon, G.B. Plasma levels of neuroexcitatory amino acids in patients with migraine or tension headache. J. Neurol. Sci. 1998, 156, 102–106. [Google Scholar] [CrossRef]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered kynurenine pathway metabolites in serum of chronic migraine patients. J. Headache Pain 2015, 17, 47. [Google Scholar] [CrossRef] [Green Version]
- Curto, M.; Lionetto, L.; Negro, A.; Capi, M.; Perugino, F.; Fazio, F.; Giamberardino, M.A.; Simmaco, M.; Nicoletti, F.; Martelletti, P. Altered serum levels of kynurenine metabolites in patients affected by cluster headache. J. Headache Pain 2015, 17, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, S. Acute tryptophan depletion in humans: A review of theoretical, practical and ethical aspects. J. Psychiatry Neurosci. 2013, 38, 294–305. [Google Scholar] [CrossRef] [Green Version]
- Deen, M.; Christensen, C.E.; Hougaard, A.; Hansen, H.D.; Knudsen, G.M.; Ashina, M. Serotonergic mechanisms in the migraine brain—A systematic review. Cephalalgia 2017, 37, 251–264. [Google Scholar] [CrossRef]
- Carpenter, L.L.; Anderson, G.M.; Pelton, G.H.; Gudin, J.A.; Kirwin, P.D.S.; Price, L.H.; Heninger, G.R.; McDougle, C.J. Tryptophan Depletion During Continuous CSF Sampling in Healthy Human Subjects. Neuropsychopharmacology 1998, 19, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Williams, W.A.; Shoaf, S.E.; Hommer, D.; Rawlings, R.; Linnoila, M. Effects of acute tryptophan depletion on plasma and cerebrospinal fluid tryptophan and 5-hydroxyindoleacetic acid in normal volunteers. J. Neurochem. 1999, 72, 1641–1647. [Google Scholar] [CrossRef]
- Van Der Stelt, H.M.; Broersen, L.M.; Olivier, B.; Westenberg, H.G.M. Effects of dietary tryptophan variations on extracellular serotonin in the dorsal hippocampus of rats. Psychopharmacology 2004, 172, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Drummond, P. Tryptophan Depletion Increases Nausea, Headache and Photophobia in Migraine Sufferers. Cephalalgia 2006, 26, 1225–1233. [Google Scholar] [CrossRef] [Green Version]
- Jahromi, S.R.; Togha, M.; Ghorbani, Z.; Hekmatdoost, A.; Khorsha, F.; Rafiee, P.; Shirani, P.; Nourmohammadi, M.; Ansari, H. The association between dietary tryptophan intake and migraine. Neurol. Sci. 2019, 40, 2349–2355. [Google Scholar] [CrossRef] [PubMed]
- Gecse, K.; Dobos, D.; Aranyi, C.S.; Galambos, A.; Baksa, D.; Kocsel, N.; Szabó, E.; Pap, D.; Virág, D.; Ludányi, K.; et al. Association of plasma tryptophan concentration with periaqueductal gray matter functional connectivity in migraine patients. Sci. Rep. 2022, 12, 739. [Google Scholar] [CrossRef]
- Villalón, C.M.; vanDenBrink, A.M. The Role of 5-Hydroxytryptamine in the Pathophysiology of Migraine and its Relevance to the Design of Novel Treatments. Med. Chem. 2017, 17, 928–938. [Google Scholar] [CrossRef]
- Comings, D.E. Serotonin: A Key to Migraine Disorders? Health Fit. Mag. 1994. [Google Scholar]
- Nichols, D.E.; Nichols, C.D. Serotonin Receptors. Chem. Rev. 2008, 108, 1614–1641. [Google Scholar] [CrossRef]
- Varnäs, K.; Halldin, C.; Hall, H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum. Brain Mapp. 2004, 22, 246–260. [Google Scholar] [CrossRef]
- Barnes, N.M.; Sharp, T. A review of central 5-HT receptors and their function. Neuropharmacology 1999, 38, 1083–1152. [Google Scholar] [CrossRef]
- Sicuteri, F.; Testi, A.; Anselmi, B. Biochemical investigations in headache: Increase in hydroxytryindoleacetic acid excretion during migraine attacks. Int. Arch. Allergy 1961, 19, 55–58. [Google Scholar] [CrossRef]
- Anthony, M.; Hinterberger, H.; Lance, J.W. Plasma Serotonin in Migraine and Stress. Arch. Neurol. 1967, 16, 544–552. [Google Scholar] [CrossRef]
- Curran, D.A.; Hinterberger, H.; Lance, J.W. Total plasma serotonin, 5-hydroxyindoleacetic acid and p-hydroxy-m-methoxymandelic acid excretion in normal and migrainous subjects. Brain 1965, 88, 997–1010. [Google Scholar] [CrossRef]
- Lance, J.W.; Anthony, M.; Hinterberger, H. The control of cranial arteries by humoral mechanisms and its relation to the migraine syndrome. Headache 1967, 7, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Kimball, R.W.; Friedman, A.P.; Vallejo, E. Effect of serotonin in migraine patients. Neurology 1960, 10, 107–111. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.D.; Odink, J.; Tapparelli, C.; Van Kempen, G.; Pennings, E.J.; Bruyn, G.W. Serotonin metabolism in migraine. Neurology 1989, 39, 1239–1242. [Google Scholar] [CrossRef]
- Rossi, C.; Pini, L.A.; Cupini, M.L.; Calabresi, P.; Sarchielli, P. Endocannabinoids in platelets of chronic migraine patients and medication-overuse headache patients: Relation with serotonin levels. Eur. J. Clin. Pharmacol. 2008, 64, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Best, J.; Nijhout, H.F.; Reed, M. Serotonin synthesis, release and reuptake in terminals: A mathematical model. Theor. Biol. Med. Model. 2010, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Höglund, E.; Øverli, Ø.; Winberg, S. Tryptophan Metabolic Pathways and Brain Serotonergic Activity: A Comparative Review. Front. Endocrinol. 2019, 10, 158. [Google Scholar] [CrossRef] [Green Version]
- Sandler, M. Changes in 5-hydroxytryptamine and its metabolites in neuropsychiatric disorders. Psychopharmacol. Bull. 1981, 17, 19–21. [Google Scholar] [PubMed]
- Mohammad-Zadeh, L.F.; Moses, L.; Gwaltney-Brant, S.M. Serotonin: A review. J. Vet.-Pharmacol. Ther. 2008, 31, 187–199. [Google Scholar] [CrossRef]
- Lesch, K.; Aulakh, C.S.; Wolozin, B.L.; Tolliver, T.J.; Hill, J.L.; Murphy, D.L. Regional brain expression of serotonin transporter mRNA and its regulation by reuptake inhibiting antidepressants. Mol. Brain Res. 1993, 17, 31–35. [Google Scholar] [CrossRef]
- Murphy, D.L.; Mueller, E.A.; Aulakh, C.S.; Bagdy, G.; Garrick, N.A. Serotoninergic Function in Neuropsychiatric Disorders. In Serotonin; Mylechrane, E.J., Angus, J.A., de la Lande, I.S., Humphrey, P.P.A., Eds.; Macmillan Press: London, UK, 1989; pp. 257–264. [Google Scholar]
- Schuh-Hofer, S.; Richter, M.; Geworski, L.; Villringer, A.; Israel, H.; Wenzel, R.; Munz, D.L.; Arnold, G. Increased serotonin transporter availability in the brainstem of migraineurs. J. Neurol. 2007, 254, 789–796. [Google Scholar] [CrossRef]
- Horvath, G.A.; Selby, K.; Poskitt, K.; Hyland, K.; Waters, P.J.; Coulter-Mackie, M.; Stockler-Ipsiroglu, S.G. Hemiplegic migraine, seizures, progressive spastic paraparesis, mood disorder, and coma in siblings with low systemic serotonin. Cephalalgia 2011, 31, 1580–1586. [Google Scholar] [CrossRef]
- Meneses, A. Physiological, pathophysiological and therapeutic roles of 5-HT systems in learning and memory. Rev. Neurosci. 1998, 9, 275–289. [Google Scholar] [CrossRef]
- Lambert, G. The Lack of Peripheral Pathology in Migraine Headache. Headache 2010, 50, 895–908. [Google Scholar] [CrossRef] [PubMed]
- Longmore, J.; Shaw, D.; Smith, D.; Hopkins, R.; McAlliste, G.; Pickard, J.; Sirinathsinghji, D.; Butler, A.; Hill, R. Differential Distribution of 5Ht1D-and 5HT1B-Immunoreactivity within the Human Trigemino-Cerebrovascular System: Implications for the Discovery of New Antimigraine Drugs. Cephalalgia 1997, 17, 833–842. [Google Scholar] [CrossRef]
- Hou, M.; Kanje, M.; Longmore, J.; Tajti, J.; Uddman, R.; Edvinsson, L. 5-HT1B and 5-HT1D receptors in the human trigeminal ganglion: Co-localization with calcitonin gene-related peptide, substance P and nitric oxide synthase. Brain Res. 2001, 909, 112–120. [Google Scholar] [CrossRef]
- Bouchelet, I.; Cohen, Z.; Case, B.; Séguéla, P.; Hamel, E. Differential expression of sumatriptan-sensitive 5-hydroxytryptamine receptors in human trigeminal ganglia and cerebral blood vessels. Mol. Pharmacol. 1996, 50, 219–223. [Google Scholar] [PubMed]
- Longmore, J.; Dowson, A.J.; Hill, R.G. Advances in migraine therapy—5-HT receptor subtype-specific agonist drugs. Curr. Opin. CPNS Investig. Drugs 1999, 1, 39–53. [Google Scholar]
- Dodick, D.; Lipton, R.B.; Martin, V.; Papademetriou, V.; Rosamond, W.; MaassenVanDenBrink, A.; Loutfi, H.; Welch, K.M.; Goadsby, P.J.; Hahn, S.; et al. Consensus Statement: Cardiovascular Safety Profile of Triptans (5-HT1B/1D Agonists) in the Acute Treatment of Migraine. Headache 2004, 44, 414–425. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L.; Schenck, K. Contractile responses to sumatriptan and ergotamine in the rabbit saphenous vein: Effect of selective 5-HT1F receptor agonists and PGF2α. J. Cereb. Blood Flow Metab. 2000, 131, 562–568. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, N.; Skljarevski, V.; Phebus, L.; Johnson, K. 5-HT1F Receptor Agonists in Acute Migraine Treatment: A Hypothesis. Cephalalgia 2003, 23, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Adham, N.; Kao, H.T.; Schecter, L.E.; Bard, J.; Olsen, M.; Urquhart, D.; Durkin, M.; Hartig, P.R.; Weinshank, R.L.; Branchek, T.A. Cloning of another human serotonin receptor (5-HT1F): A fifth 5-HT1 receptor subtype coupled to the inhibition of adenylate cyclase. Proc. Natl. Acad. Sci. USA 1993, 90, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Vila-Pueyo, M. Targeted 5-HT1F Therapies for Migraine. Neurotherapeutics 2018, 15, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.W.; Schaus, J.M.; Durkin, M.M.; Audia, J.E.; Kaldor, S.W.; Flaugh, M.E.; Adham, N.; Zgombick, J.M.; Cohen, M.L.; Branchek, T.A.; et al. 5-HT1F receptor agonists inhibit neurogenic dural inflammation in guinea pigs. NeuroReport 1997, 8, 2237–2239. [Google Scholar] [CrossRef] [PubMed]
- Mitsikostas, D.D.; del Rio, M.S.; Moskowitz, M.A.; Waeber, C. Both 5-HT1B and 5-HT1F receptors modulate c-fos expression within rat trigeminal nucleus caudalis. Eur. J. Pharmacol. 1999, 369, 271–277. [Google Scholar] [CrossRef]
- Mitsikostas, D.; del Rio, M.S.; Waeber, C. 5-Hydroxytryptamine1B/1D and 5-Hydroxytryptamine1FReceptors Inhibit Capsaicin-Induced C-Fos Immunoreactivity within Mouse Trigeminal Nucleus Caudalis. Cephalalgia 2002, 22, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Nelson, D.L.; Phebus, L.A.; Johnson, K.W.; Wainscott, D.B.; Cohen, M.L.; Calligaro, D.O.; Xu, Y.-C. Preclinical pharmacological profile of the selective 5-HT1F receptor agonist lasmiditan. Cephalalgia 2010, 30, 1159–1169. [Google Scholar] [CrossRef]
- Kovalchin, J.; Ghiglieri, A.; Zanelli, E.; Ings, R.; Mathers, T. Lasmiditan acts specifically on the 5-HT1F receptors in the central nervous system. Cephalalgia 2016, 36, 103. [Google Scholar]
- Akerman, S.; Romero-Reyes, M.; Holland, P.R. Current and novel insights into the neurophysiology of migraine and its implications for therapeutics. Pharmacol. Ther. 2017, 172, 151–170. [Google Scholar] [CrossRef]
- Huang, P.-C.; Yang, F.-C.; Chang, C.-M.; Yang, C.-P. Targeting the 5-HT1B/1D and 5-HT1F receptors for acute migraine treatment. Prog. Brain Res. 2020, 255, 99–121. [Google Scholar] [CrossRef] [PubMed]
- Callebert, J.; Esteve, J.M.; Hervé, P.; Peoc’H, K.; Tournois, C.; Drouet, L.; Launay, J.M.; Maroteaux, L. Evidence for a Control of Plasma Serotonin Levels by 5-Hydroxytryptamine2B Receptors in Mice. J. Pharmacol. Exp. Ther. 2006, 317, 724–731. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Richter, J.A.; Hurley, J.H. Release of Glutamate and CGRP from Trigeminal Ganglion Neurons: Role of Calcium Channels and 5-HT1 Receptor Signaling. Mol. Pain 2008, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Florian, J.A.; Watts, S.W. Integration of mitogen-activated protein kinase kinase activation in vascular 5-hydroxytryptamine2A receptor signal transduction. J. Pharmacol. Exp. Ther. 1998, 284, 346–355. [Google Scholar]
- Martin, R.S.; Martin, G.R. Investigations into migraine pathogenesis: Time course for effects of m-CPP, BW723C86 or glyceryl trinitrate on appearance of Fos-like immunoreactivity in rat trigeminal nucleus caudalis (TNC). Cephalalgia 2001, 21, 46–52. [Google Scholar] [CrossRef]
- Johnson, K.; Nelson, D.; Dieckman, D.; Wainscott, D.; Lucaites, V.; Audia, J.; Owton, W.; Phebus, L. Neurogenic Dural Protein Extravasation Induced by Meta-Chlorophenylpiperazine (mCPP) Involves Nitric Oxide and 5-HT2B Receptor Activation. Cephalalgia 2003, 23, 117–123. [Google Scholar] [CrossRef]
- Schmitz, B.; Ullmer, C.; Segelcke, D.; Gwarek, M.; Zhu, X.-R.; Lübbert, H. BF-1—A novel selective 5-HT2B receptor antagonist blocking neurogenic dural plasma protein extravasation in guinea pigs. Eur. J. Pharmacol. 2015, 751, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Brewerton, T.D.; Murphy, D.L.; Mueller, E.A.; Jimerson, D.C. Induction of migrainelike headaches by the serotonin agonist m-chlorophenylpiperazine. Clin. Pharmacol. Ther. 1988, 43, 605–609. [Google Scholar] [CrossRef] [PubMed]
- Silberstein, S.D. Methysergide. Cephalalgia 1998, 18, 421–435. [Google Scholar] [CrossRef]
- Bettahi, I.; Pozo, D.; Osuna, C.; Reiter, R.J.; Acuña-Castroviejo, D.; Guerrero, J.M. Melatonin reduces nitric oxide synthase activity in rat hypothalamus. J. Pineal Res. 1996, 20, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Lance, J.W.; Anthony, M.; Somerville, B. Comparative Trial of Serotonin Antagonists in the Management of Migraine. Br. Med. J. 1970, 2, 327–330. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Calvo, J.R.; Karbownik, M.; Qi, W.; Tan, D.X. Melatonin and Its Relation to the Immune System and Inflammation. Ann. N. Y. Acad. Sci. 2000, 917, 376–386. [Google Scholar] [CrossRef] [PubMed]
- Peres, M.F.P.; Masruha, M.R.; Zukerman, E.; Moreira-Filho, C.A.; Cavalheiro, E. Potential therapeutic use of melatonin in migraine and other headache disorders. Expert Opin. Investig. Drugs 2006, 15, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Adnyana, I.M.O.; Tertia, C.; Widyadharma, I.P.E.; Mahadewi, N.P.A.P. Melatonin as a treatment for migraine sufferers: A systematic review. Egypt. J. Neurol. Psychiatry Neurosurg. 2022, 58, 94. [Google Scholar] [CrossRef]
- Scarabelot, V.L.; Medeiros, L.F.; de Oliveira, C.; Adachi, L.N.S.; de Macedo, I.C.; Cioato, S.G.; de Freitas, J.S.; de Souza, A.; Quevedo, A.; Caumo, W.; et al. Melatonin Alters the Mechanical and Thermal Hyperalgesia Induced by Orofacial Pain Model in Rats. Inflammation 2016, 39, 1649–1659. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.L.; Laste, G.; de Macedo, I.C.; Rozisky, J.R.; da Silva, F.R.; Caumo, W. Melatonin administration reduces inflammatory pain in rats. J. Pain Res. 2012, 5, 359–362. [Google Scholar] [CrossRef] [Green Version]
- Noseda, R.; Hernández, A.; Valladares, L.; Mondaca, M.; Laurido, C.; Soto-Moyano, R. Melatonin-induced inhibition of spinal cord synaptic potentiation in rats is MT2 receptor-dependent. Neurosci. Lett. 2004, 360, 41–44. [Google Scholar] [CrossRef]
- Danilov, A.; Kurganova, J. Melatonin in Chronic Pain Syndromes. Pain Ther. 2016, 5, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Weaver, D.; Rivkees, S.; Reppert, S. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. J. Neurosci. 1989, 9, 2581–2590. [Google Scholar] [CrossRef]
- Williams, L.M.; Hannah, L.T.; Hastings, M.H.; Maywood, E.S. Melatonin receptors in the rat brain and pituitary. J. Pineal Res. 1995, 19, 173–177. [Google Scholar] [CrossRef]
- Yu, C.-X.; Zhu, C.-B.; Xu, S.-F.; Cao, X.-D.; Wu, G.-C. The analgesic effects of peripheral and central administration of melatonin in rats. Eur. J. Pharmacol. 2000, 403, 49–53. [Google Scholar] [CrossRef]
- Shavali, S.; Ho, B.; Govitrapong, P.; Sawlom, S.; Ajjimaporn, A.; Klongpanichapak, S.; Ebadi, M. Melatonin exerts its analgesic actions not by binding to opioid receptor subtypes but by increasing the release of β-endorphin an endogenous opioid. Brain Res. Bull. 2005, 64, 471–479. [Google Scholar] [CrossRef]
- Srinivasan, V.; Lauterbach, E.C.; Ho, K.Y.; Acuña-Castroviejo, D.; Zakaria, R.; Brzezinski, A. Melatonin in Antinociception: Its Therapeutic Applications. Curr. Neuropharmacol. 2012, 10, 167–178. [Google Scholar] [CrossRef] [Green Version]
- Mease, P. Fibromyalgia syndrome: Review of clinical presentation, pathogenesis, outcome measures, and treatment. J. Rheumatol. 2005, 75, 6–21. [Google Scholar]
- Reiter, R.J.; Acuna-Castroviejo, D.; Tan, D.-X. Melatonin therapy in fibromyalgia. Curr. Pain Headache Rep. 2007, 11, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Song, G.H.; Leng, P.H.; Gwee, K.A.; Moochhala, S.M.; Ho, K.Y. Melatonin improves abdominal pain in irritable bowel syndrome patients who have sleep disturbances: A randomised, double blind, placebo controlled study. Gut 2005, 54, 1402–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laste, G.; Rozisky, J.R.; Caumo, W.; Torres, I.L.D.S. Short- but Not Long-Term Melatonin Administration Reduces Central Levels of Brain-Derived Neurotrophic Factor in Rats with Inflammatory Pain. Neuroimmunomodulation 2015, 22, 358–364. [Google Scholar] [CrossRef] [PubMed]
- Masruha, M.R.; Lin, J.; Vieira, D.S.D.S.; Minett, T.S.; Cipolla-Neto, J.; Zukerman, E.; Vilanova, L.C.; Peres, M.F. Urinary 6-Sulphatoxymelatonin Levels Are Depressed in Chronic Migraine and Several Comorbidities. Headache 2010, 50, 413–419. [Google Scholar] [CrossRef]
- Claustrat, B.; Loisy, C.; Brun, J.; Beorchia, S.; Arnaud, J.L.; Chazot, G. Nocturnal Plasma Melatonin Levels in Migraine: A Preliminary Report. Headache 1989, 29, 242–245. [Google Scholar] [CrossRef] [PubMed]
- Masruha, M.R.; Vieira, D.S.D.S.; Minett, T.S.C.; Cipolla-Neto, J.; Zukerman, E.; Vilanova, L.C.P.; Peres, M.F.P. Low urinary 6-sulphatoxymelatonin concentrations in acute migraine. J. Headache Pain 2008, 9, 221–224. [Google Scholar] [CrossRef] [Green Version]
- Murialdo, G.; Fonzi, S.; Costelli, P.; Solinas, G.P.; Parodi, C.; Marabini, S.; Fanciullacci, M.; Polleri, A. Urinary Melatonin Excretion Throughout the Ovarian Cycle in Menstrually Related Migraine. Cephalalgia 1994, 14, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Brun, J.; Claustrat, B.; Saddier, P.; Chazot, G. Nocturnal Melatonin Excretion is Decreased in Patients with Migraine without Aura Attacks Associated with Menses. Cephalalgia 1995, 15, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Wolf, H. The effect of hormones and vitamin B6 on urinary excretion of metabolites of the kynurenine pathway. Scand. J. Clin. Lab. Investig. 1974, 136, 1–186. [Google Scholar]
- Vécsei, L.; Szalárdy, L.; Fülöp, F.; Toldi, J. Kynurenines in the CNS: Recent advances and new questions. Nat. Rev. Drug Discov. 2013, 12, 64–82. [Google Scholar] [CrossRef] [PubMed]
- Schwarcz, R.; Bruno, J.P.; Muchowski, P.J.; Wu, H.-Q. Kynurenines in the mammalian brain: When physiology meets pathology. Nat. Rev. Neurosci. 2012, 13, 465–477. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.; Boegman, R.; Beninger, R.; Jhamandas, K. Protection against quinolinic acid-mediated excitotoxicity in nigrostriatal dopaminergic neurons by endogenous kynurenic acid. Neuroscience 1997, 78, 967–975. [Google Scholar] [CrossRef]
- Silva-Adaya, D.; La Cruz, V.P.-D.; Villeda-Hernández, J.; Carrillo-Mora, P.; González-Herrera, I.G.; García, E.; Colín-Barenque, L.; Pedraza-Chaverrí, J.; Santamaría, A. Protective effect of l-kynurenine and probenecid on 6-hydroxydopamine-induced striatal toxicity in rats: Implications of modulating kynurenate as a protective strategy. Neurotoxicol. Teratol. 2011, 33, 303–312. [Google Scholar] [CrossRef]
- Knyihár-Csillik, E.; Toldi, J.; Krisztin-Péva, B.; Chadaide, Z.; Németh, H.; Fenyő, R.; Vécsei, L. Prevention of electrical stimulation-induced increase of c-fos immunoreaction in the caudal trigeminal nucleus by kynurenine combined with probenecid. Neurosci. Lett. 2007, 418, 122–126. [Google Scholar] [CrossRef]
- Vamos, E.; Párdutz, A.; Varga, H.; Bohár, Z.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L. l-kynurenine combined with probenecid and the novel synthetic kynurenic acid derivative attenuate nitroglycerin-induced nNOS in the rat caudal trigeminal nucleus. Neuropharmacology 2009, 57, 425–429. [Google Scholar] [CrossRef]
- Toldi, J.; Oláh, G.; Herédi, J.; Menyhárt, A.; Czinege, Z.; Nagy, D.; Fuzik, J.; Krucsó, E.; Kocsis, K.; Knapp, L.; et al. Unexpected effects of peripherally administered kynurenic acid on cortical spreading depression and related blood–brain barrier permeability. Drug Des. Dev. Ther. 2013, 7, 981–987. [Google Scholar] [CrossRef] [Green Version]
- Knyihár-Csillik, E.; Chadaide, Z.; Okuno, E.; Krisztin-Péva, B.; Toldi, J.; Varga, C.; Molnár, A.; Csillik, B.; Vécsei, L. Kynurenine aminotransferase in the supratentorial dura mater of the rat: Effect of stimulation of the trigeminal ganglion. Exp. Neurol. 2004, 186, 242–247. [Google Scholar] [CrossRef]
- Spekker, E.; Laborc, K.F.; Bohár, Z.; Nagy-Grócz, G.; Fejes-Szabó, A.; Szűcs, M.; Vécsei, L.; Párdutz, A. Effect of dural inflammatory soup application on activation and sensitization markers in the caudal trigeminal nucleus of the rat and the modulatory effects of sumatriptan and kynurenic acid. J. Headache Pain 2021, 22, 17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-Q.; Ji, G.-C.; Wu, G.-C.; Zhao, Z.-Q. Kynurenic acid enhances electroacupuncture analgesia in normal and carrageenan-injected rats. Brain Res. 2003, 966, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mecs, L.; Tuboly, G.; Nagy, E.; Benedek, G.; Horvath, G. The Peripheral Antinociceptive Effects of Endomorphin-1 and Kynurenic Acid in the Rat Inflamed Joint Model. Anesthesia Analg. 2009, 109, 1297–1304. [Google Scholar] [CrossRef] [PubMed]
- Fukui, S.; Schwarcz, R.; Rapoport, S.I.; Takada, Y.; Smith, Q.R. Blood?Brain Barrier Transport of Kynurenines: Implications for Brain Synthesis and Metabolism. J. Neurochem. 1991, 56, 2007–2017. [Google Scholar] [CrossRef]
- Arimura, A. PACAP: The road to discovery. Peptides 2007, 28, 1617–1619. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Hashimoto, H.; Shintani, N.; Tanida, M.; Hayata, A.; Hashimoto, R.; Baba, A. PACAP is implicated in the stress axes. Curr. Pharm. Des. 2011, 17, 985–989. [Google Scholar] [CrossRef] [Green Version]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the Protective Effects of PACAP in Models of Neurodegenerative Diseases In Vitro and In Vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef]
- Seaborn, T.; Masmoudi-Kouli, O.; Fournier, A.; Vaudry, H.; Vaudry, D. Protective effects of pituitary adenylate cyclase-activating polypeptide (PACAP) against apoptosis. Curr. Pharm. Des. 2011, 17, 204–214. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Fukumitsu, H.; Furukawa, S. PACAP decides neuronal laminar fate via PKA signaling in the developing cerebral cortex. Biochem. Biophys. Res. Commun. 2008, 369, 1144–1149. [Google Scholar] [CrossRef]
- Palkovits, M.; Somogyvári-Vigh, A.; Arimura, A. Concentrations of pituitary adenylate cyclase activating polypeptide (PACAP) in human brain nuclei. Brain Res. 1995, 699, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Vécsei, L.; Tuka, B.; Tajti, J. Role of PACAP in migraine headaches. Brain 2014, 137 Pt 3, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Laburthe, M.; Couvineau, A.; Tan, V. Class II G protein-coupled receptors for VIP and PACAP: Structure, models of activation and pharmacology. Peptides 2007, 28, 1631–1639. [Google Scholar] [CrossRef]
- Fukuchi, M.; Kuwana, Y.; Tabuchi, A.; Tsuda, M. Balance between cAMP and Ca2+ signals regulates expression levels of pituitary adenylate cyclase-activating polypeptide gene in neurons. Genes Cells 2016, 21, 921–929. [Google Scholar] [CrossRef]
- Körtési, T.; Tuka, B.; Tajti, J.; Bagoly, T.; Fülöp, F.; Helyes, Z.; Vécsei, L. Kynurenic Acid Inhibits the Electrical Stimulation Induced Elevated Pituitary Adenylate Cyclase-Activating Polypeptide Expression in the TNC. Front. Neurol. 2018, 8, 745. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; De Molliens, M.P.; Schneebeli, S.T.; Brewer, M.; Song, G.; Chatenet, D.; Braas, K.M.; May, V.; Li, J. Targeting the PAC1 Receptor for Neurological and Metabolic Disorders. Curr. Top. Med. Chem. 2019, 19, 1399–1417. [Google Scholar] [CrossRef] [PubMed]
- MMarkovics, A.; Kormos, V.; Gaszner, B.; Lashgarara, A.; Szoke, E.; Sandor, K.; Szabadfi, K.; Tuka, B.; Tajti, J.; Szolcsanyi, J.; et al. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol. Dis. 2012, 45, 633–644. [Google Scholar] [CrossRef]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Németh, J.; Márk, L.; Brubel, R.; Reglődi, D.; Párdutz, A.; Szolcsányi, J.; et al. Peripheral and central alterations of pituitary adenylate cyclase activating polypeptide-like immunoreactivity in the rat in response to activation of the trigeminovascular system. Peptides 2012, 33, 307–316. [Google Scholar] [CrossRef]
- Tuka, B.; Helyes, Z.; Markovics, A.; Bagoly, T.; Szolcsányi, J.; Szabó, N.; Tóth, E.; Kincses, Z.T.; Vécsei, L.; Tajti, J. Alterations in PACAP-38-like immunoreactivity in the plasma during ictal and interictal periods of migraine patients. Cephalalgia 2013, 33, 1085–1095. [Google Scholar] [CrossRef] [Green Version]
- Schytz, H.W.; Birk, S.; Wienecke, T.; Kruuse, C.; Olesen, J.; Ashina, M. PACAP38 induces migraine-like attacks in patients with migraine without aura. Brain 2009, 132 Pt 1, 16–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schytz, H.W.; Olesen, J.; Ashina, M. The PACAP receptor: A novel target for migraine treatment. Neurotherapeutics 2010, 7, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, S.; Christensen, A.F.; Liu, M.L.; Janjooa, B.N.; Olesen, J.; Ashina, M. Calcitonin gene-related peptide induced migraine attacks in patients with and without familial aggregation of migraine. Cephalalgia 2017, 37, 114–124. [Google Scholar] [CrossRef]
- VVeréb, D.; Szabó, N.; Tuka, B.; Tajti, J.; Király, A.; Faragó, P.; Kocsis, K.; Tóth, E.; Kincses, B.; Bagoly, T.; et al. Correlation of neurochemical and imaging markers in migraine: PACAP38 and DTI measures. Neurology 2018, 91, e1166–e1174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, F.M.; Asghar, M.S.; Guo, S.; Hougaard, A.; Hansen, A.E.; Schytz, H.W.; van der Geest, R.; de Koning, P.J.; Larsson, H.B.; Olesen, J.; et al. Headache and prolonged dilatation of the middle meningeal artery by PACAP38 in healthy volunteers. Cephalalgia 2012, 32, 140–149. [Google Scholar] [CrossRef]
- Goadsby, P.J.; Edvinsson, L.; Ekman, R. Release of vasoactive peptides in the extracerebral circulation of humans and the cat during activation of the trigeminovascular system. Ann. Neurol. 1988, 23, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Tajti, J.; Szok, D.; Majláth, Z.; Tuka, B.; Csáti, A.; Vécsei, L. Migraine and neuropeptides. Neuropeptides 2015, 52, 19–30. [Google Scholar] [CrossRef] [Green Version]
- Romero-Reyes, M.; Pardi, V.; Akerman, S. A potent and selective calcitonin gene-related peptide (CGRP) receptor antagonist, MK-8825, inhibits responses to nociceptive trigeminal activation: Role of CGRP in orofacial pain. Exp. Neurol. 2015, 271, 95–103. [Google Scholar] [CrossRef]
- Jansen-Olesen, I.; Baun, M.; Amrutkar, D.V.; Ramachandran, R.; Christophersen, D.V.; Olesen, J. PACAP-38 but not VIP induces release of CGRP from trigeminal nucleus caudalis via a receptor distinct from the PAC1 receptor. Neuropeptides 2014, 48, 53–64. [Google Scholar] [CrossRef]
- Eftekhari, S.; Salvatore, C.A.; Johansson, S.; Chen, T.-B.; Zeng, Z.; Edvinsson, L. Localization of CGRP, CGRP receptor, PACAP and glutamate in trigeminal ganglion. Relation to the blood–brain barrier. Brain Res. 2015, 1600, 93–109. [Google Scholar] [CrossRef]
- Mahmoudi, J.; Mohaddes, G.; Erfani, M.; Sadigh-Eteghad, S.; Karimi, P.; Rajabi, M.; Reyhani-Rad, S.; Farajdokht, F. Cerebrolysin attenuates hyperalgesia, photophobia, and neuroinflammation in a nitroglycerin-induced migraine model in rats. Brain Res. Bull. 2018, 140, 197–204. [Google Scholar] [CrossRef] [PubMed]
- Körtési, T.; Tuka, B.; Nyári, A.; Vécsei, L.; Tajti, J. The effect of orofacial complete Freund’s adjuvant treatment on the expression of migraine-related molecules. J. Headache Pain 2019, 20, 43. [Google Scholar] [CrossRef] [PubMed]
- Romero-Reyes, M.; Uyanik, J.M. Orofacial pain management: Current perspectives. J. Pain Res. 2014, 7, 99–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Csáti, A.; Edvinsson, L.; Vécsei, L.; Toldi, J.; Fulop, F.; Tajti, J.; Warfvinge, K. Kynurenic acid modulates experimentally induced inflammation in the trigeminal ganglion. J. Headache Pain 2015, 16, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukács, M.; Warfvinge, K.; Tajti, J.; Fülöp, F.; Toldi, J.; Vécsei, L.; Edvinsson, L. Topical dura mater application of CFA induces enhanced expression of c-fos and glutamate in rat trigeminal nucleus caudalis: Attenuated by KYNA derivate (SZR72). J. Headache Pain 2017, 18, 39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aczél, T.; Kun, J.; Szőke, E.; Rauch, T.; Junttila, S.; Gyenesei, A.; Bölcskei, K.; Helyes, Z. Transcriptional Alterations in the Trigeminal Ganglia, Nucleus and Peripheral Blood Mononuclear Cells in a Rat Orofacial Pain Model. Front. Mol. Neurosci. 2018, 11, 219. [Google Scholar] [CrossRef] [PubMed]
- Veres, G.; Fejes-Szabó, A.; Zádori, D.; Nagy-Grócz, G.; László, A.M.; Bajtai, A.; Mándity, I.; Szentirmai, M.; Bohár, Z.; Laborc, K.; et al. A comparative assessment of two kynurenic acid analogs in the formalin model of trigeminal activation: A behavioral, immunohistochemical and pharmacokinetic study. J. Neural Transm. 2017, 124, 99–112. [Google Scholar] [CrossRef] [Green Version]
- Nagy-Grócz, G.; Tar, L.; Bohár, Z.; Fejes-Szabó, A.; Laborc, K.F.; Spekker, E.; Vécsei, L.; Párdutz, A. The modulatory effect of anandamide on nitroglycerin-induced sensitization in the trigeminal system of the rat. Cephalalgia 2016, 36, 849–861. [Google Scholar] [CrossRef] [Green Version]
- Kuburas, A.; Mason, B.N.; Hing, B.; Wattiez, A.-S.; Reis, A.S.; Sowers, L.P.; Loomis, C.M.; Garcia-Martinez, L.F.; Russo, A.F. PACAP Induces Light Aversion in Mice by an Inheritable Mechanism Independent of CGRP. J. Neurosci. 2021, 41, 4697–4715. [Google Scholar] [CrossRef]
- Ernstsen, C.; Christensen, S.L.; Rasmussen, R.H.; Nielsen, B.S.; Jansen-Olesen, I.; Olesen, J.; Kristensen, D.M. The PACAP pathway is independent of CGRP in mouse models of migraine: Possible new drug target? Brain 2022, 145, 2450–2460. [Google Scholar] [CrossRef]
- Tuka, B.; Nyári, A.; Cseh, E.K.; Körtési, T.; Veréb, D.; Tömösi, F.; Kecskeméti, G.; Janáky, T.; Tajti, J.; Vécsei, L. Clinical relevance of depressed kynurenine pathway in episodic migraine patients: Potential prognostic markers in the peripheral plasma during the interictal period. J. Headache Pain 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Al-Karagholi, M.A.; Hansen, J.M.; Abou-Kassem, D.; Hansted, A.K.; Ubhayasekera, K.; Bergquist, J.; Vécsei, L.; Jansen-Olesen, I.; Ashina, M. Phase 1 study to access safety, tolerability, pharmacokinetics, and pharmacodynamics of kynurenine in healthy volunteers. Pharmacol. Res. Perspect. 2021, 9, e00741. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Körtési, T.; Spekker, E.; Vécsei, L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells 2022, 11, 3795. https://doi.org/10.3390/cells11233795
Körtési T, Spekker E, Vécsei L. Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells. 2022; 11(23):3795. https://doi.org/10.3390/cells11233795
Chicago/Turabian StyleKörtési, Tamás, Eleonóra Spekker, and László Vécsei. 2022. "Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms" Cells 11, no. 23: 3795. https://doi.org/10.3390/cells11233795
APA StyleKörtési, T., Spekker, E., & Vécsei, L. (2022). Exploring the Tryptophan Metabolic Pathways in Migraine-Related Mechanisms. Cells, 11(23), 3795. https://doi.org/10.3390/cells11233795