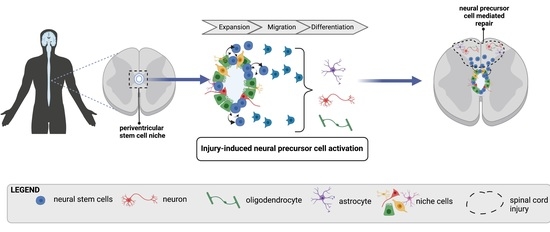
Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair
Abstract
:1. Spinal Cord Injury
2. Endogenous Neural Stem Cells
3. Adult Neural Stem Cells: A Heterogeneous Population of Cells
3.1. Definitive Neural Stem Cells (dNSCs)
3.2. Primitive Neural Stem Cells (pNSCs)
3.3. MSX1+ NSCs
3.4. Quiescent vs. Activated NSC States
4. The NSC Niche
4.1. Ependymal Cells: A Population of NSCs?
4.2. Tanycytes—A Population of Glial Progenitors?
4.3. Cerebrospinal Fluid (CSF) Contacting Cells: Immature Neurons, NSCs or Both?
4.4. Endothelial Cells—Paracrine Modulators of NSCs
4.5. The Extracellular Matrix: A Regulator of NSC Function
5. Regulating Neural Precursors to Enhance Neurorepair
5.1. Microglia, Astrocytes and Oligodendrocytes: Parenchymal “Influencers” on NSCs
5.2. Bloodborne and Extracellular Factors
5.3. The Pleiotropic Power of Repurposed Pharmaceuticals
5.4. Regulating Neural Stem Cell Behaviour with Electric Fields
6. Future Directions
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kwon, B.K.; Tetzlaff, W.; Grauer, J.N.; Beiner, J.; Vaccaro, A.R. Pathophysiology and Pharmacologic Treatment of Acute Spinal Cord Injury. Spine J. 2004, 4, 451–464. [Google Scholar] [CrossRef] [PubMed]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Sekhon, L.H.S.; Fehlings, M.G. Epidemiology, Demographics, and Pathophysiology of Acute Spinal Cord Injury. Spine 2001, 26, S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The Role of Excitotoxicity in Secondary Mechanisms of Spinal Cord Injury: A Review with an Emphasis on the Implications for White Matter Degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Brockie, S.; Hong, J.; Fehlings, M.G. The Role of Microglia in Modulating Neuroinflammation after Spinal Cord Injury. Int. J. Mol. Sci. 2021, 22, 9706. [Google Scholar] [CrossRef]
- Oyinbo, C.A. Secondary Injury Mechanisms in Traumatic Spinal Cord Injury: A Nugget of This Multiply Cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Kumar, R.; Lim, J.; Mekary, R.A.; Rattani, A.; Dewan, M.C.; Sharif, S.Y.; Osorio-Fonseca, E.; Park, K.B. Traumatic Spinal Injury: Global Epidemiology and Worldwide Volume. World Neurosurg. 2018, 113, e345–e363. [Google Scholar] [CrossRef]
- Pan, W.; Kastin, A.J. Cytokine Transport across the Injured Blood-Spinal Cord Barrier. Curr. Pharm. Des. 2008, 14, 1620–1624. [Google Scholar] [CrossRef] [Green Version]
- Rezvan, M.; Meknatkhah, S.; Hassannejad, Z.; Sharif-Alhoseini, M.; Zadegan, S.A.; Shokraneh, F.; Vaccaro, A.R.; Lu, Y.; Rahimi-Movaghar, V. Time-Dependent Microglia and Macrophages Response after Traumatic Spinal Cord Injury in Rat: A Systematic Review. Injury 2020, 51, 2390–2401. [Google Scholar] [CrossRef]
- Bellver-Landete, V.; Bretheau, F.; Mailhot, B.; Vallières, N.; Lessard, M.; Janelle, M.-E.; Vernoux, N.; Tremblay, M.-È.; Fuehrmann, T.; Shoichet, M.S.; et al. Microglia Are an Essential Component of the Neuroprotective Scar That Forms after Spinal Cord Injury. Nat. Commun. 2019, 10, 518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, D.J.; Popovich, P.G. Inflammation and Its Role in Neuroprotection, Axonal Regeneration and Functional Recovery after Spinal Cord Injury. Exp. Neurol. 2008, 209, 378–388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pukos, N.; Goodus, M.T.; Sahinkaya, F.R.; McTigue, D.M. Myelin Status and Oligodendrocyte Lineage Cells over Time after Spinal Cord Injury: What Do We Know and What Still Needs to Be Unwrapped? Glia 2019, 67, 2178–2202. [Google Scholar] [CrossRef]
- Tripathi, R.; McTigue, D.M. Prominent Oligodendrocyte Genesis along the Border of Spinal Contusion Lesions. Glia 2007, 55, 698–711. [Google Scholar] [CrossRef]
- Lytle, J.M.; Chittajallu, R.; Wrathall, J.R.; Gallo, V. NG2 Cell Response in the CNP-EGFP Mouse after Contusive Spinal Cord Injury. Glia 2009, 57, 270–285. [Google Scholar] [CrossRef] [Green Version]
- Okada, S.; Hara, M.; Kobayakawa, K.; Matsumoto, Y.; Nakashima, Y. Astrocyte Reactivity and Astrogliosis after Spinal Cord Injury. Neurosci. Res. 2018, 126, 39–43. [Google Scholar] [CrossRef]
- Yang, T.; Dai, Y.; Chen, G.; Cui, S. Dissecting the Dual Role of the Glial Scar and Scar-Forming Astrocytes in Spinal Cord Injury. Front. Cell Neurosci. 2020, 14, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hackett, A.R.; Lee, J.K. Understanding the NG2 Glial Scar after Spinal Cord Injury. Front. Neurol. 2016, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Not Everything Is Scary about a Glial Scar. Nature 2016, 532, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Jiang, M.; Li, H. Functional Requirement of Dicer1 and MiR-17-5p in Reactive Astrocyte Proliferation after Spinal Cord Injury in the Mouse. Glia 2014, 62, 2044–2060. [Google Scholar] [CrossRef]
- Goldshmit, Y.; Kanner, S.; Zacs, M.; Frisca, F.; Pinto, A.R.; Currie, P.D.; Pinkas-Kramarski, R. Rapamycin Increases Neuronal Survival, Reduces Inflammation and Astrocyte Proliferation after Spinal Cord Injury. Mol. Cell Neurosci. 2015, 68, 82–91. [Google Scholar] [CrossRef]
- Okada, S.; Nakamura, M.; Katoh, H.; Miyao, T.; Shimazaki, T.; Ishii, K.; Yamane, J.; Yoshimura, A.; Iwamoto, Y.; Toyama, Y.; et al. Conditional Ablation of Stat3 or Socs3 Discloses a Dual Role for Reactive Astrocytes after Spinal Cord Injury. Nat. Med. 2006, 12, 829–834. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte Reactivity: Subtypes, States, and Functions in CNS Innate Immunity. Trends Immunol. 2020, 41, 758–770. [Google Scholar] [CrossRef] [PubMed]
- Silver, J. The Glial Scar Is More than Just Astrocytes. Exp. Neurol. 2016, 286, 147–149. [Google Scholar] [CrossRef] [PubMed]
- Lukovic, D.; Stojkovic, M.; Moreno-Manzano, V.; Jendelova, P.; Sykova, E.; Bhattacharya, S.S.; Erceg, S. Concise Review: Reactive Astrocytes and Stem Cells in Spinal Cord Injury: Good Guys or Bad Guys? Stem Cells 2015, 33, 1036–1041. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; Burda, J.E.; Ren, Y.; Ao, Y.; O’Shea, T.M.; Kawaguchi, R.; Coppola, G.; Khakh, B.S.; Deming, T.J.; Sofroniew, M.V. Astrocyte Scar Formation Aids Central Nervous System Axon Regeneration. Nature 2016, 532, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.Z.; Neumann, B.; Förster, S.; Franklin, R.J.M. Schwann Cell Remyelination of the Central Nervous System: Why Does It Happen and What Are the Benefits? Open Biol. 2021, 11, 200352. [Google Scholar] [CrossRef]
- Meletis, K.; Barnabé-Heider, F.; Carlén, M.; Evergren, E.; Tomilin, N.; Shupliakov, O.; Frisén, J. Spinal Cord Injury Reveals Multilineage Differentiation of Ependymal Cells. PLoS Biol. 2008, 6, e182. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Göritz, C.; Sabelström, H.; Takebayashi, H.; Pfrieger, F.W.; Meletis, K.; Frisén, J. Origin of New Glial Cells in Intact and Injured Adult Spinal Cord. Cell Stem. Cell 2010, 7, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Ren, Y.; Ao, Y.; O’Shea, T.M.; Burda, J.E.; Bernstein, A.M.; Brumm, A.J.; Muthusamy, N.; Ghashghaei, H.T.; Carmichael, S.T.; Cheng, L.; et al. Ependymal Cell Contribution to Scar Formation after Spinal Cord Injury Is Minimal, Local and Dependent on Direct Ependymal Injury. Sci. Rep. 2017, 7, 41122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sabelström, H.; Stenudd, M.; Réu, P.; Dias, D.O.; Elfineh, M.; Zdunek, S.; Damberg, P.; Göritz, C.; Frisén, J. Resident Neural Stem Cells Restrict Tissue Damage and Neuronal Loss after Spinal Cord Injury in Mice. Science 2013, 342, 637–640. [Google Scholar] [CrossRef] [Green Version]
- Becker, C.G.; Becker, T. Adult Zebrafish as a Model for Successful Central Nervous System Regeneration. Restor. Neurol. Neurosci. 2008, 26, 71–80. [Google Scholar]
- Gilbert, E.A.B.; Vickaryous, M.K. Neural Stem/Progenitor Cells Are Activated during Tail Regeneration in the Leopard Gecko (Eublepharis Macularius). J. Comp. Neurol. 2018, 526, 285–309. [Google Scholar] [CrossRef] [PubMed]
- Mchedlishvili, L.; Mazurov, V.; Grassme, K.S.; Goehler, K.; Robl, B.; Tazaki, A.; Roensch, K.; Duemmler, A.; Tanaka, E.M. Reconstitution of the Central and Peripheral Nervous System during Salamander Tail Regeneration. Proc. Natl. Acad. Sci. USA 2012, 109, E2258–E2266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mchedlishvili, L.; Epperlein, H.H.; Telzerow, A.; Tanaka, E.M. A Clonal Analysis of Neural Progenitors during Axolotl Spinal Cord Regeneration Reveals Evidence for Both Spatially Restricted and Multipotent Progenitors. Development 2007, 134, 2083–2093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, C.G.; Becker, T. Neuronal Regeneration from Ependymo-Radial Glial Cells: Cook, Little Pot, Cook! Dev. Cell 2015, 32, 516–527. [Google Scholar] [CrossRef] [Green Version]
- Harel, N.Y.; Strittmatter, S.M. Can Regenerating Axons Recapitulate Developmental Guidance during Recovery from Spinal Cord Injury? Nat. Rev. Neurosci. 2006, 7, 603–616. [Google Scholar] [CrossRef] [Green Version]
- Ferretti, P.; Zhang, F.; O’Neill, P. Changes in Spinal Cord Regenerative Ability through Phylogenesis and Development: Lessons to Be Learnt. Dev. Dyn. 2003, 226, 245–256. [Google Scholar] [CrossRef]
- Becker, C.G.; Corral, R.D.D. Neural Development and Regeneration: It’s All in Your Spinal Cord. Development 2015, 142, 811–816. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, E.M.; Ferretti, P. Considering the Evolution of Regeneration in the Central Nervous System. Nat. Rev. Neurosci. 2009, 10, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Tazaki, A.; Tanaka, E.M.; Fei, J.-F. Salamander Spinal Cord Regeneration: The Ultimate Positive Control in Vertebrate Spinal Cord Regeneration. Dev. Biol. 2017, 432, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, E.B.; Morshead, C. Stem Cell Heterogeneity and Regenerative Competence: The Enormous Potential of Rare Cells. Neural. Regen Res. 2021, 16, 285. [Google Scholar] [CrossRef] [PubMed]
- Morshead, C.M.; Reynolds, B.A.; Craig, C.G.; McBurney, M.W.; Staines, W.A.; Morassutti, D.; Weiss, S.; van der Kooy, D. Neural Stem Cells in the Adult Mammalian Forebrain: A Relatively Quiescent Subpopulation of Subependymal Cells. Neuron 1994, 13, 1071–1082. [Google Scholar] [CrossRef]
- Reynolds, B.; Weiss, S. Generation of Neurons and Astrocytes from Isolated Cells of the Adult Mammalian Central Nervous System. Science 1992, 255, 1707–1710. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Dunne, C.; Hewson, J.; Wohl, C.; Wheatley, M.; Peterson, A.C.; Reynolds, B.A. Multipotent CNS Stem Cells Are Present in the Adult Mammalian Spinal Cord and Ventricular Neuroaxis. J. Neurosci. 1996, 16, 7599–7609. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Frisén, J. Stem Cells for Spinal Cord Repair. Cell Stem. Cell 2008, 3, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Xu, W.; Sachewsky, N.; Azimi, A.; Hung, M.; Gappasov, A.; Morshead, C.M. Myelin Basic Protein Regulates Primitive and Definitive Neural Stem Cell Proliferation from the Adult Spinal Cord. Stem. Cells 2017, 35, 485–496. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.; Reynolds, B.A.; Vescovi, A.L.; Morshead, C.; Craig, C.G.; van der Kooy, D. Is There a Neural Stem Cell in the Mammalian Forebrain? Trends Neurosci. 1996, 19, 387–393. [Google Scholar] [CrossRef]
- Coles-Takabe, B.L.K.; Brain, I.; Purpura, K.A.; Karpowicz, P.; Zandstra, P.W.; Morshead, C.M.; van der Kooy, D. Don’t Look: Growing Clonal versus Nonclonal Neural Stem Cell Colonies. Stem. Cells 2008, 26, 2938–2944. [Google Scholar] [CrossRef]
- Johansson, C.B.; Momma, S.; Clarke, D.L.; Risling, M.; Lendahl, U.; Frisén, J. Identification of a Neural Stem Cell in the Adult Mammalian Central Nervous System. Cell 1999, 96, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Horner, P.J.; Power, A.E.; Kempermann, G.; Kuhn, H.G.; Palmer, T.D.; Winkler, J.; Thal, L.J.; Gage, F.H. Proliferation and Differentiation of Progenitor Cells throughout the Intact Adult Rat Spinal Cord. J. Neurosci. 2000, 20, 2218–2228. [Google Scholar] [CrossRef]
- Shihabuddin, L.S.; Horner, P.J.; Ray, J.; Gage, F.H. Adult Spinal Cord Stem Cells Generate Neurons after Transplantation in the Adult Dentate Gyrus. J. Neurosci. 2000, 20, 8727–8735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Temple, S. The Development of Neural Stem Cells. Nature 2001, 414, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.G.; Becker, T.; Hugnot, J.-P. The Spinal Ependymal Zone as a Source of Endogenous Repair Cells across Vertebrates. Prog. Neurobiol. 2018, 170, 67–80. [Google Scholar] [CrossRef]
- Horky, L.L.; Galimi, F.; Gage, F.H.; Horner, P.J. Fate of Endogenous Stem/Progenitor Cells Following Spinal Cord Injury. J. Comp. Neurol. 2006, 498, 525–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reynolds, B.A.; Weiss, S. Clonal and Population Analyses Demonstrate That an EGF-Responsive Mammalian Embryonic CNS Precursor Is a Stem Cell. Dev. Biol. 1996, 175, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghazale, H.; Ripoll, C.; Leventoux, N.; Jacob, L.; Azar, S.; Mamaeva, D.; Glasson, Y.; Calvo, C.-F.; Thomas, J.-L.; Meneceur, S.; et al. RNA Profiling of the Human and Mouse Spinal Cord Stem Cell Niches Reveals an Embryonic-like Regionalization with MSX1+ Roof-Plate-Derived Cells. Stem. Cell Rep. 2019, 12, 1159–1177. [Google Scholar] [CrossRef] [Green Version]
- Chevreau, R.; Ghazale, H.; Ripoll, C.; Chalfouh, C.; Delarue, Q.; Hemonnot-Girard, A.L.; Mamaeva, D.; Hirbec, H.; Rothhut, B.; Wahane, S.; et al. RNA Profiling of Mouse Ependymal Cells after Spinal Cord Injury Identifies the Oncostatin Pathway as a Potential Key Regulator of Spinal Cord Stem Cell Fate. Cells 2021, 10, 3332. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; He, Y.; Zhang, H.; Chen, L.; Cao, L.; Yang, L.; Wang, C.; Pan, Y.; Tang, Q.; Tan, W.; et al. The Neural Stem Cell Properties of PKD2L1+ Cerebrospinal Fluid-Contacting Neurons in Vitro. Front. Cell Neurosci. 2021, 15, 630882. [Google Scholar] [CrossRef]
- Wang, Y.-Z.; Plane, J.M.; Jiang, P.; Zhou, C.J.; Deng, W. Concise Review: Quiescent and Active States of Endogenous Adult Neural Stem Cells: Identification and Characterization. Stem. Cells 2011, 29, 907–912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempermann, G. Activity Dependency and Aging in the Regulation of Adult Neurogenesis. Csh. Perspect. Biol. 2015, 7, a018929. [Google Scholar] [CrossRef] [Green Version]
- Fabel, K.; Kempermann, G. Physical Activity and the Regulation of Neurogenesis in the Adult and Aging Brain. Neuromol. Med. 2008, 10, 59–66. [Google Scholar] [CrossRef]
- Redmond, S.A.; Figueres-Oñate, M.; Obernier, K.; Nascimento, M.A.; Parraguez, J.I.; López-Mascaraque, L.; Fuentealba, L.C.; Alvarez-Buylla, A. Development of Ependymal and Postnatal Neural Stem Cells and Their Origin from a Common Embryonic Progenitor. Cell Rep. 2019, 27, 429–441.e3. [Google Scholar] [CrossRef] [Green Version]
- Furutachi, S.; Miya, H.; Watanabe, T.; Kawai, H.; Yamasaki, N.; Harada, Y.; Imayoshi, I.; Nelson, M.; Nakayama, K.I.; Hirabayashi, Y.; et al. Slowly Dividing Neural Progenitors Are an Embryonic Origin of Adult Neural Stem Cells. Nat. Neurosci. 2015, 18, 657–665. [Google Scholar] [CrossRef]
- Marqués-Torrejón, M.Á.; Williams, C.A.C.; Southgate, B.; Alfazema, N.; Clements, M.P.; Garcia-Diaz, C.; Blin, C.; Arranz-Emparan, N.; Fraser, J.; Gammoh, N.; et al. LRIG1 Is a Gatekeeper to Exit from Quiescence in Adult Neural Stem Cells. Nat. Commun. 2021, 12, 2594. [Google Scholar] [CrossRef] [PubMed]
- Codega, P.; Silva-Vargas, V.; Paul, A.; Maldonado-Soto, A.R.; Deleo, A.M.; Pastrana, E.; Doetsch, F. Prospective Identification and Purification of Quiescent Adult Neural Stem Cells from Their in Vivo Niche. Neuron 2014, 82, 545–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imura, T.; Nakano, I.; Kornblum, H.I.; Sofroniew, M.V. Phenotypic and Functional Heterogeneity of GFAP-expressing Cells in Vitro: Differential Expression of LeX/CD15 by GFAP-expressing Multipotent Neural Stem Cells and Non-neurogenic Astrocytes. Glia 2006, 53, 277–293. [Google Scholar] [CrossRef]
- Ellis, P.; Fagan, B.M.; Magness, S.T.; Hutton, S.; Taranova, O.; Hayashi, S.; McMahon, A.; Rao, M.; Pevny, L. SOX2, a Persistent Marker for Multipotential Neural Stem Cells Derived from Embryonic Stem Cells, the Embryo or the Adult. Dev. Neurosci. 2004, 26, 148–165. [Google Scholar] [CrossRef]
- Liu, S.; Chen, Z. Employing Endogenous NSCs to Promote Recovery of Spinal Cord Injury. Stem. Cells Int. 2019, 2019, 1958631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakshman, N.; Xu, W.; Morshead, C.M. A Neurosphere Assay to Evaluate Endogenous Neural Stem Cell Activation in a Mouse Model of Minimal Spinal Cord Injury. J. Vis. Exp. 2018, 139, e57727. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, M.; Xue, X.; Nie, H.; Wu, X.; Sun, M.; Qiao, L.; Li, X.; Xu, B.; Xiao, Z.; Zhao, Y.; et al. Single-Cell RNA Sequencing Reveals Nestin+ Active Neural Stem Cells Outside the Central Canal after Spinal Cord Injury. Sci. China Life Sci. 2021, 65, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Stenudd, M.; Sabelström, H.; Frisén, J. Role of Endogenous Neural Stem Cells in Spinal Cord Injury and Repair. JAMA Neurol. 2015, 72, 235–237. [Google Scholar] [CrossRef] [PubMed]
- Mothe, A.J.; Tator, C.H. Proliferation, Migration, and Differentiation of Endogenous Ependymal Region Stem/Progenitor Cells Following Minimal Spinal Cord Injury in the Adult Rat. Neuroscience 2005, 131, 177–187. [Google Scholar] [CrossRef]
- Hitoshi, S.; Seaberg, R.M.; Koscik, C.; Alexson, T.; Kusunoki, S.; Kanazawa, I.; Tsuji, S.; van der Kooy, D. Primitive Neural Stem Cells from the Mammalian Epiblast Differentiate to Definitive Neural Stem Cells under the Control of Notch Signaling. Genes Dev. 2004, 18, 1806–1811. [Google Scholar] [CrossRef] [Green Version]
- Sachewsky, N.; Xu, W.; Fuehrmann, T.; Kooy, D.V.D.; Morshead, C.M. Lineage Tracing Reveals the Hierarchical Relationship between Neural Stem Cell Populations in the Mouse Forebrain. Sci Rep. 2019, 9, 17730. [Google Scholar] [CrossRef]
- Sachewsky, N.; Leeder, R.; Xu, W.; Rose, K.L.; Yu, F.; van der Kooy, D.; Morshead, C.M. Primitive Neural Stem Cells in the Adult Mammalian Brain Give Rise to GFAP-Expressing Neural Stem Cells. Stem. Cell Rep. 2014, 2, 810–824. [Google Scholar] [CrossRef] [Green Version]
- Reeve, R.L.; Yammine, S.Z.; Morshead, C.M.; van der Kooy, D. Quiescent Oct4+ Neural Stem Cells (NSCs) Repopulate Ablated Glial Fibrillary Acidic Protein+ NSCs in the Adult Mouse Brain. Stem. Cells 2017, 35, 2071–2082. [Google Scholar] [CrossRef] [Green Version]
- Reeve, R.L.; Yammine, S.Z.; DeVeale, B.; van der Kooy, D. Targeted Activation of Primitive Neural Stem Cells in the Mouse Brain. Eur. J. Neurosci. 2016, 43, 1474–1485. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells That Become Activated upon Brain Injury. Cell Stem. Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Beck, C.W.; Christen, B.; Slack, J.M.W. Molecular Pathways Needed for Regeneration of Spinal Cord and Muscle in a Vertebrate. Dev. Cell 2003, 5, 429–439. [Google Scholar] [CrossRef] [Green Version]
- Gengatharan, A.; Malvaut, S.; Marymonchyk, A.; Ghareghani, M.; Snapyan, M.; Fischer-Sternjak, J.; Ninkovic, J.; Götz, M.; Saghatelyan, A. Adult Neural Stem Cell Activation in Mice Is Regulated by the Day/Night Cycle and Intracellular Calcium Dynamics. Cell 2021, 184, 709–722.e13. [Google Scholar] [CrossRef] [PubMed]
- Linnarsson, S. Sequencing Single Cells Reveals Sequential Stem Cell States. Cell Stem. Cell 2015, 17, 251–252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urbán, N.; Blomfield, I.M.; Guillemot, F. Quiescence of Adult Mammalian Neural Stem Cells: A Highly Regulated Rest. Neuron 2019, 104, 834–848. [Google Scholar] [CrossRef]
- Mira, H.; Andreu, Z.; Suh, H.; Lie, D.C.; Jessberger, S.; Consiglio, A.; Emeterio, J.S.; Hortigüela, R.; Marqués-Torrejón, M.Á.; Nakashima, K.; et al. Signaling through BMPR-IA Regulates Quiescence and Long-Term Activity of Neural Stem Cells in the Adult Hippocampus. Cell Stem. Cell 2010, 7, 78–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Clevers, H. Coexistence of Quiescent and Active Adult Stem Cells in Mammals. Science 2010, 327, 542–545. [Google Scholar] [CrossRef] [Green Version]
- Piccin, D.; Yu, F.; Morshead, C.M. Notch Signaling Imparts and Preserves Neural Stem Characteristics in the Adult Brain. Stem. Cells Dev. 2013, 22, 1541–1550. [Google Scholar] [CrossRef]
- Morshead, C.; van der Kooy, D. Postmitotic Death Is the Fate of Constitutively Proliferating Cells in the Subependymal Layer of the Adult Mouse Brain. J. Neurosci. 1992, 12, 249–256. [Google Scholar] [CrossRef]
- Morizur, L.; Chicheportiche, A.; Gauthier, L.R.; Daynac, M.; Boussin, F.D.; Mouthon, M.-A. Distinct Molecular Signatures of Quiescent and Activated Adult Neural Stem Cells Reveal Specific Interactions with Their Microenvironment. Stem. Cell Rep. 2018, 11, 565–577. [Google Scholar] [CrossRef] [Green Version]
- Suh, H.; Deng, W.; Gage, F.H. Signaling in Adult Neurogenesis. Annu Rev. Cell Dev. Bi 2009, 25, 253–275. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Chell, J.M.; Merre, P.L.; Wu, Y.; Zamboni, M.; Bergenstråhle, J.; Stenudd, M.; Sopova, E.; Lundeberg, J.; Shupliakov, O.; et al. A Latent Lineage Potential in Resident Neural Stem Cells Enables Spinal Cord Repair. Science 2020, 370, eabb8795. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up Quiescent Neural Stem Cells: Molecular Mechanisms and Implications in Neurodevelopmental Disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, K.J.; Turnley, A.M. Regulation of Endogenous Neural Stem/Progenitor Cells for Neural Repair—Factors That Promote Neurogenesis and Gliogenesis in the Normal and Damaged Brain. Front. Cell Neurosci. 2013, 6, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belenguer, G.; Duart-Abadia, P.; Jordán-Pla, A.; Domingo-Muelas, A.; Blasco-Chamarro, L.; Ferrón, S.R.; Morante-Redolat, J.M.; Fariñas, I. Adult Neural Stem Cells Are Alerted by Systemic Inflammation through TNF-α Receptor Signaling. Cell Stem. Cell 2021, 28, 285–299.e9. [Google Scholar] [CrossRef]
- Miller, F.D.; Kaplan, D.R. Mobilizing Endogenous Stem Cells for Repair and Regeneration: Are We There Yet? Cell Stem. Cell 2012, 10, 650–652. [Google Scholar] [CrossRef] [Green Version]
- Daynac, M.; Chicheportiche, A.; Pineda, J.R.; Gauthier, L.R.; Boussin, F.D.; Mouthon, M.-A. Quiescent Neural Stem Cells Exit Dormancy upon Alteration of GABAAR Signaling Following Radiation Damage. Stem. Cell Res. 2013, 11, 516–528. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gu, S.; Gan, J.; Tian, Y.; Zhang, F.; Zhao, H.; Lei, D. Neural Stem Cells Overexpressing Nerve Growth Factor Improve Functional Recovery in Rats Following Spinal Cord Injury via Modulating Microenvironment and Enhancing Endogenous Neurogenesis. Front. Cell Neurosci. 2021, 15, 773375. [Google Scholar] [CrossRef]
- Dadwal, P.; Mahmud, N.; Sinai, L.; Azimi, A.; Fatt, M.; Wondisford, F.E.; Miller, F.D.; Morshead, C.M. Activating Endogenous Neural Precursor Cells Using Metformin Leads to Neural Repair and Functional Recovery in a Model of Childhood Brain Injury. Stem. Cell Rep. 2015, 5, 166–173. [Google Scholar] [CrossRef] [Green Version]
- Ayoub, R.; Ruddy, R.M.; Cox, E.; Oyefiade, A.; Derkach, D.; Laughlin, S.; Ades-aron, B.; Shirzadi, Z.; Fieremans, E.; MacIntosh, B.J.; et al. Assessment of Cognitive and Neural Recovery in Survivors of Pediatric Brain Tumors in a Pilot Clinical Trial Using Metformin. Nat. Med. 2020, 26, 1285–1294. [Google Scholar] [CrossRef]
- Hugnot, J.-P.; Franzen, R. The Spinal Cord Ependymal Region: A Stem Cell Niche in the Caudal Central Nervous System. Front. Biosci. (Landmark Ed.) 2011, 16, 1044–1059. [Google Scholar] [CrossRef]
- Marichal, N.; Reali, C.; Trujillo-Cenóz, O.; Russo, R.E. Spinal Cord Stem Cells In Their Microenvironment: The Ependyma as a Stem Cell Niche. Adv. Exp. Med. Biol. 2017, 1041, 55–79. [Google Scholar] [CrossRef]
- Lane, S.W.; Williams, D.A.; Watt, F.M. Modulating the Stem Cell Niche for Tissue Regeneration. Nat. Biotechnol. 2014, 32, 795–803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural Stem Cell Niche Heterogeneity. Semin. Cell Dev. Biol. 2019, 95, 42–53. [Google Scholar] [CrossRef]
- Kazanis, I.; ffrench-Constant, C. Extracellular Matrix and the Neural Stem Cell Niche. Dev. Neurobiol. 2011, 71, 1006–1017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruddy, R.M.; Morshead, C.M. Home Sweet Home: The Neural Stem Cell Niche throughout Development and after Injury. Cell Tissue Res. 2017, 371, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Dai, J. Bridging the Gap with Functional Collagen Scaffolds: Tuning Endogenous Neural Stem Cells for Severe Spinal Cord Injury Repair. Biomater Sci.-UK 2017, 6, 265–271. [Google Scholar] [CrossRef]
- Suzuki, H.; Ahuja, C.S.; Salewski, R.P.; Li, L.; Satkunendrarajah, K.; Nagoshi, N.; Shibata, S.; Fehlings, M.G. Neural Stem Cell Mediated Recovery Is Enhanced by Chondroitinase ABC Pretreatment in Chronic Cervical Spinal Cord Injury. PLoS ONE 2017, 12, e0182339. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, L.K.; Truong, M.K.V.; Bednarczyk, M.R.; Aumont, A.; Fernandes, K.J.L. Cellular Organization of the Central Canal Ependymal Zone, a Niche of Latent Neural Stem Cells in the Adult Mammalian Spinal Cord. Neuroscience 2009, 164, 1044–1056. [Google Scholar] [CrossRef]
- Muthusamy, N.; Brumm, A.; Zhang, X.; Carmichael, S.T.; Ghashghaei, H.T. Foxj1 Expressing Ependymal Cells Do Not Contribute New Cells to Sites of Injury or Stroke in the Mouse Forebrain. Sci. Rep.-UK 2018, 8, 1766. [Google Scholar] [CrossRef] [Green Version]
- Abdi, K.; Lai, C.-H.; Paez-Gonzalez, P.; Lay, M.; Pyun, J.; Kuo, C.T. Uncovering Inherent Cellular Plasticity of Multiciliated Ependyma Leading to Ventricular Wall Transformation and Hydrocephalus. Nat. Commun. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Shah, P.T.; Stratton, J.A.; Stykel, M.G.; Abbasi, S.; Sharma, S.; Mayr, K.A.; Koblinger, K.; Whelan, P.J.; Biernaskie, J. Single-Cell Transcriptomics and Fate Mapping of Ependymal Cells Reveals an Absence of Neural Stem Cell Function. Cell 2018, 173, 1045–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Worthington, W.C., Jr.; Cathcart, R.S., III. Ependymal Cilia: Distribution and Activity in the Adult Human Brain. Science 1963, 139, 221–222. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Manzano, V. Ependymal Cells in the Spinal Cord as Neuronal Progenitors. Curr. Opin. Pharm. 2020, 50, 82–87. [Google Scholar] [CrossRef]
- Furube, E.; Ishii, H.; Nambu, Y.; Kurganov, E.; Nagaoka, S.; Morita, M.; Miyata, S. Neural Stem Cell Phenotype of Tanycyte-like Ependymal Cells in the Circumventricular Organs and Central Canal of Adult Mouse Brain. Sci. Rep.-UK 2020, 10, 2826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alfaro-Cervello, C.; Soriano-Navarro, M.; Mirzadeh, Z.; Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Biciliated Ependymal Cell Proliferation Contributes to Spinal Cord Growth. J. Comp. Neurol. 2012, 520, 3528–3552. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzadeh, Z.; Han, Y.-G.; Soriano-Navarro, M.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Cilia Organize Ependymal Planar Polarity. J. Neurosci. 2010, 30, 2600–2610. [Google Scholar] [CrossRef] [Green Version]
- Alfaro-Cervello, C.; Cebrian-Silla, A.; Soriano-Navarro, M.; Garcia-Tarraga, P.; Matías-Guiu, J.; Gomez-Pinedo, U.; Aguilar, P.M.; Alvarez-Buylla, A.; Luquin, M.-R.; Garcia-Verdugo, J.M. The Adult Macaque Spinal Cord Central Canal Zone Contains Proliferative Cells and Closely Resembles the Human. J. Comp. Neurol. 2014, 522, 1800–1817. [Google Scholar] [CrossRef]
- Malatesta, P.; Götz, M. Radial Glia—From Boring Cables to Stem Cell Stars. Development 2013, 140, 483–486. [Google Scholar] [CrossRef] [Green Version]
- Fei, J.-F.; Schuez, M.; Tazaki, A.; Taniguchi, Y.; Roensch, K.; Tanaka, E.M. CRISPR-Mediated Genomic Deletion of Sox2 in the Axolotl Shows a Requirement in Spinal Cord Neural Stem Cell Amplification during Tail Regeneration. Stem. Cell Rep. 2014, 3, 444–459. [Google Scholar] [CrossRef] [Green Version]
- Bruni, J.E. Ependymal Development, Proliferation, and Functions: A Review. Microsc. Res. Tech. 1998, 41, 2–13. [Google Scholar] [CrossRef]
- Petracca, Y.L.; Sartoretti, M.M.; Bella, D.J.D.; Marin-Burgin, A.; Carcagno, A.L.; Schinder, A.F.; Lanuza, G.M. The Late and Dual Origin of Cerebrospinal Fluid-Contacting Neurons in the Mouse Spinal Cord. Development 2016, 143, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Christenson, J.; Alford, S.; Grillner, S.; Hökfelt, T. Co-Localized GABA and Somatostatin Use Different Ionic Mechanisms to Hyperpolarize Target Neurons in the Lamprey Spinal Cord. Neurosci. Lett. 1991, 134, 93–97. [Google Scholar] [CrossRef]
- Jalalvand, E.; Robertson, B.; Wallén, P.; Hill, R.H.; Grillner, S. Laterally Projecting Cerebrospinal Fluid-Contacting Cells in the Lamprey Spinal Cord Are of Two Distinct Types. J. Comp. Neurol. 2014, 522, 1753–1768. [Google Scholar] [CrossRef] [PubMed]
- Russo, R.E.; Fernández, A.; Reali, C.; Radmilovich, M.; Trujillo-Cenóz, O. Functional and Molecular Clues Reveal Precursor-like Cells and Immature Neurones in the Turtle Spinal Cord. J. Physiol. 2004, 560, 831–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jalalvand, E.; Robertson, B.; Wallén, P.; Grillner, S. Ciliated Neurons Lining the Central Canal Sense Both Fluid Movement and PH through ASIC3. Nat. Commun. 2016, 7, 10002. [Google Scholar] [CrossRef] [Green Version]
- Busch, T.; Köttgen, M.; Hofherr, A. TRPP2 Ion Channels: Critical Regulators of Organ Morphogenesis in Health and Disease. Cell Calcium. 2017, 66, 25–32. [Google Scholar] [CrossRef]
- Kútna, V.; Ševc, J.; Gombalová, Z.; Matiašová, A.; Daxnerová, Z. Enigmatic Cerebrospinal Fluid-Contacting Neurons Arise Even after the Termination of Neurogenesis in the Rat Spinal Cord during Embryonic Development and Retain Their Immature-like Characteristics until Adulthood. Acta Histochem. 2014, 116, 278–285. [Google Scholar] [CrossRef]
- Orts-Del’Immagine, A.; Trouslard, J.; Airault, C.; Hugnot, J.-P.; Cordier, B.; Doan, T.; Kastner, A.; Wanaverbecq, N. Postnatal Maturation of Mouse Medullo-Spinal Cerebrospinal Fluid-Contacting Neurons. Neuroscience 2017, 343, 39–54. [Google Scholar] [CrossRef]
- Pontes, A.; Zhang, Y.; Hu, W. Novel Functions of GABA Signaling in Adult Neurogenesis. Front. Biol. 2013, 8, 496–507. [Google Scholar] [CrossRef]
- Giachino, C.; Barz, M.; Tchorz, J.S.; Tome, M.; Gassmann, M.; Bischofberger, J.; Bettler, B.; Taylor, V. GABA Suppresses Neurogenesis in the Adult Hippocampus through GABAB Receptors. J. Cell Sci. 2014, 127, e1. [Google Scholar] [CrossRef]
- Azevedo, P.O.; Lousado, L.; Paiva, A.E.; Andreotti, J.P.; Santos, G.S.P.; Sena, I.F.G.; Prazeres, P.H.D.M.; Filev, R.; Mintz, A.; Birbrair, A. Endothelial Cells Maintain Neural Stem Cells Quiescent in Their Niche. Neuroscience 2017, 363, 62–65. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Ma, D.; Yang, Y. Endothelial Cells Promote Neural Stem Cell Proliferation and Differentiation Associated with VEGF Activated Notch and Pten Signaling. Dev. Dynam 2010, 239, 2345–2353. [Google Scholar] [CrossRef]
- Liu, S.; Xiao, Z.; Li, X.; Zhao, Y.; Wu, X.; Han, J.; Chen, B.; Li, J.; Fan, C.; Xu, B.; et al. Vascular Endothelial Growth Factor Activates Neural Stem Cells through Epidermal Growth Factor Receptor Signal after Spinal Cord Injury. Cns. Neurosci. 2019, 25, 375–385. [Google Scholar] [CrossRef]
- Sato, Y.; Uchida, Y.; Hu, J.; Young-Pearse, T.L.; Niikura, T.; Mukouyama, Y. Soluble APP Functions as a Vascular Niche Signal That Controls Adult Neural Stem Cell Number. Development 2017, 144, 2730–2736. [Google Scholar] [CrossRef] [Green Version]
- Tucker, R.; Drabikowski, K.; Hess, J.; Ferralli, J.; Chiquet-Ehrismann, R.; Adams, J. Phylogenetic Analysis of the Tenascin Gene Family: Evidence of Origin Early in the Chordate Lineage. BMC Evol. Biol. 2006, 6, 60. [Google Scholar] [CrossRef] [Green Version]
- Karus, M.; Denecke, B.; ffrench-Constant, C.; Wiese, S.; Faissner, A. The Extracellular Matrix Molecule Tenascin C Modulates Expression Levels and Territories of Key Patterning Genes during Spinal Cord Astrocyte Specification. Development 2011, 138, 5321–5331. [Google Scholar] [CrossRef] [Green Version]
- Imbeault, S.; Gauvin, L.G.; Toeg, H.D.; Pettit, A.; Sorbara, C.D.; Migahed, L.; DesRoches, R.; Menzies, A.S.; Nishii, K.; Paul, D.L.; et al. The Extracellular Matrix Controls Gap Junction Protein Expression and Function in Postnatal Hippocampal Neural Progenitor Cells. BMC Neurosci. 2009, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ Stem Cells Lie in a Vascular Niche: A Quantitative Analysis of Niche Cell-Cell Interactions. Cell Stem. Cell 2008, 3, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Carvalho-Paulo, D.; Neto, J.B.T.; Filho, C.S.; de Oliveira, T.C.G.; de Sousa, A.A.; dos Reis, R.R.; dos Santos, Z.A.; de Lima, C.M.; de Oliveira, M.A.; Said, N.M.; et al. Microglial Morphology Across Distantly Related Species: Phylogenetic, Environmental and Age Influences on Microglia Reactivity and Surveillance States. Front. Immunol. 2021, 12, 683026. [Google Scholar] [CrossRef]
- David, S.; Kroner, A. Repertoire of Microglial and Macrophage Responses after Spinal Cord Injury. Nat. Rev. Neurosci. 2011, 12, 388–399. [Google Scholar] [CrossRef]
- Akhmetzyanova, E.; Kletenkov, K.; Mukhamedshina, Y.; Rizvanov, A. Different Approaches to Modulation of Microglia Phenotypes after Spinal Cord Injury. Front. Syst. Neurosci. 2019, 13, 37. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sato, K. Effects of Microglia on Neurogenesis. Glia 2015, 63, 1394–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shechter, R.; Schwartz, M. Harnessing Monocyte-derived Macrophages to Control Central Nervous System Pathologies: No Longer ‘If’ but ‘How’. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Town, T.; Nikolic, V.; Tan, J. The Microglial “Activation” Continuum: From Innate to Adaptive Responses. J. Neuroinflamm. 2005, 2, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vay, S.U.; Flitsch, L.J.; Rabenstein, M.; Rogall, R.; Blaschke, S.; Kleinhaus, J.; Reinert, N.; Bach, A.; Fink, G.R.; Schroeter, M.; et al. The Plasticity of Primary Microglia and Their Multifaceted Effects on Endogenous Neural Stem Cells in Vitro and in Vivo. J. Neuroinflamm. 2018, 15, 226. [Google Scholar] [CrossRef]
- Osman, A.M.; Rodhe, J.; Shen, X.; Dominguez, C.A.; Joseph, B.; Blomgren, K. The Secretome of Microglia Regulate Neural Stem Cell Function. Neuroscience 2019, 405, 92–102. [Google Scholar] [CrossRef]
- Kumar, A.; Stoica, B.A.; Loane, D.J.; Yang, M.; Abulwerdi, G.; Khan, N.; Kumar, A.; Thom, S.R.; Faden, A.I. Microglial-Derived Microparticles Mediate Neuroinflammation after Traumatic Brain Injury. J. Neuroinflamm. 2017, 14, 47. [Google Scholar] [CrossRef] [Green Version]
- Hou, B.-R.; Jiang, C.; Wang, Z.-N.; Ren, H.-J. Exosome-Mediated Crosstalk between Microglia and Neural Stem Cells in the Repair of Brain Injury. Neural. Regen Res. 2019, 15, 1023–1024. [Google Scholar] [CrossRef]
- Butovsky, O.; Jedrychowski, M.P.; Cialic, R.; Krasemann, S.; Murugaiyan, G.; Fanek, Z.; Greco, D.J.; Wu, P.M.; Doykan, C.E.; Kiner, O.; et al. Targeting MiR-155 Restores Abnormal Microglia and Attenuates Disease in SOD1 Mice. Ann. Neurol. 2015, 77, 75–99. [Google Scholar] [CrossRef]
- Huang, S.; Ge, X.; Yu, J.; Han, Z.; Yin, Z.; Li, Y.; Chen, F.; Wang, H.; Zhang, J.; Lei, P. Increased MiR-124-3p in Microglial Exosomes Following Traumatic Brain Injury Inhibits Neuronal Inflammation and Contributes to Neurite Outgrowth via Their Transfer into Neurons. Faseb J. 2018, 32, 512–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.E.; Parikshak, N.N.; Belgard, T.G.; Geschwind, D.H. Genome-Wide, Integrative Analysis Implicates MicroRNA Dysregulation in Autism Spectrum Disorder. Nat. Neurosci. 2016, 19, 1463–1476. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, X.; Kawaguchi, R.; Zhang, Y.; Wang, Q.; Monavarfeshani, A.; Yang, Z.; Chen, B.; Shi, Z.; Meng, H.; et al. Microglia-Organized Scar-Free Spinal Cord Repair in Neonatal Mice. Nature 2020, 587, 613–618. [Google Scholar] [CrossRef] [PubMed]
- Nunan, R.; Sivasathiaseelan, H.; Khan, D.; Zaben, M.; Gray, W. Microglial VPAC1R Mediates a Novel Mechanism of Neuroimmune-Modulation of Hippocampal Precursor Cells via IL-4 Release. Glia 2014, 62, 1313–1327. [Google Scholar] [CrossRef] [Green Version]
- Tsarouchas, T.M.; Wehner, D.; Cavone, L.; Munir, T.; Keatinge, M.; Lambertus, M.; Underhill, A.; Barrett, T.; Kassapis, E.; Ogryzko, N.; et al. Dynamic Control of Proinflammatory Cytokines Il-1β and Tnf-α by Macrophages in Zebrafish Spinal Cord Regeneration. Nat. Commun. 2018, 9, 4670. [Google Scholar] [CrossRef] [Green Version]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of Two Distinct Macrophage Subsets with Divergent Effects Causing Either Neurotoxicity or Regeneration in the Injured Mouse Spinal Cord. J. Neurosci. Off. J. Soc. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [Green Version]
- Ling, E.; Leblond, C.P. Investigation of Glial Cells in Semithin Sections. II. Variation with Age in the Numbers of the Various Glial Cell Types in Rat Cortex and Corpus Callosum. J. Comp. Neurol. 1973, 149, 73–81. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and Pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [Green Version]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef] [Green Version]
- Ridet, J.L.; Privat, A.; Malhotra, S.K.; Gage, F.H. Reactive Astrocytes: Cellular and Molecular Cues to Biological Function. Trends Neurosci. 1997, 20, 570–577. [Google Scholar] [CrossRef]
- Lau, L.T.; Yu, A.C.-H. Astrocytes Produce and Release Interleukin-1, Interleukin-6, Tumor Necrosis Factor Alpha and Interferon-Gamma Following Traumatic and Metabolic Injury. J. Neurotraum. 2001, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pöyhönen, S.; Er, S.; Domanskyi, A.; Airavaara, M. Effects of Neurotrophic Factors in Glial Cells in the Central Nervous System: Expression and Properties in Neurodegeneration and Injury. Front. Physiol. 2019, 10, 486. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, J.R.; Herrmann, J.E.; Woo, M.J.; Tansey, K.E.; Doan, N.B.; Sofroniew, M.V. Reactive Astrocytes Protect Tissue and Preserve Function after Spinal Cord Injury. J. Neurosci. 2004, 24, 2143–2155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tica, J.; Didangelos, A. Comparative Transcriptomics of Rat and Axolotl After Spinal Cord Injury Dissects Differences and Similarities in Inflammatory and Matrix Remodeling Gene Expression Patterns. Front. Neurosci.-Switz 2018, 12, 808. [Google Scholar] [CrossRef] [Green Version]
- Antos, C.L.; Tanaka, E.M. Vertebrates That Regenerate As Models for Guiding Stem Cells. In The Cell Biology of Stem Cells; Springer: Boston, MA, USA, 2010; Volume 695, pp. 184–214. ISBN 978-1-4419-7036-7. [Google Scholar]
- Zambusi, A.; Ninkovic, J. Regeneration of the Central Nervous System-Principles from Brain Regeneration in Adult Zebrafish. World J. Stem Cells 2020, 12, 8–24. [Google Scholar] [CrossRef]
- Miller, S.J. Astrocyte Heterogeneity in the Adult Central Nervous System. Front. Cell Neurosci. 2018, 12, 401. [Google Scholar] [CrossRef] [Green Version]
- Emsley, J.G.; Arlotta, P.; Macklis, J.D. Star-Cross’d Neurons: Astroglial Effects on Neural Repair in the Adult Mammalian CNS. Trends Neurosci. 2004, 27, 238–240. [Google Scholar] [CrossRef]
- Horner, P.J.; Palmer, T.D. New Roles for Astrocytes: The Nightlife of an ‘Astrocyte’. La Vida Loca! Trends Neurosci. 2003, 26, 597–603. [Google Scholar] [CrossRef]
- Song, H.; Stevens, C.F.; Gage, F.H. Astroglia Induce Neurogenesis from Adult Neural Stem Cells. Nature 2002, 417, 39–44. [Google Scholar] [CrossRef]
- Faijerson, J.; Tinsley, R.B.; Apricó, K.; Thorsell, A.; Nodin, C.; Nilsson, M.; Blomstrand, F.; Eriksson, P.S. Reactive Astrogliosis Induces Astrocytic Differentiation of Adult Neural Stem/Progenitor Cells in Vitro. J. Neurosci. Res. 2006, 84, 1415–1424. [Google Scholar] [CrossRef]
- Lim, D.A.; Tramontin, A.D.; Trevejo, J.M.; Herrera, D.G.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Noggin Antagonizes BMP Signaling to Create a Niche for Adult Neurogenesis. Neuron 2000, 28, 713–726. [Google Scholar] [CrossRef] [Green Version]
- Jovanovic, V.M.; Salti, A.; Tilleman, H.; Zega, K.; Jukic, M.M.; Zou, H.; Friedel, R.H.; Prakash, N.; Blaess, S.; Edenhofer, F.; et al. BMP/SMAD Pathway Promotes Neurogenesis of Midbrain Dopaminergic Neurons In Vivo and in Human Induced Pluripotent and Neural Stem Cells. J. Neurosci. 2018, 38, 1662–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Zhang, W.; Yang, P. Current States of Endogenous Stem Cells in Adult Spinal Cord. J. Neurosci. Res. 2015, 93, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, J.; Zhou, L.; Pollard, S.M.; Smith, A. Interplay between FGF2 and BMP Controls the Self-Renewal, Dormancy and Differentiation of Rat Neural Stem Cells. J. Cell Sci. 2011, 124, 1867–1877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Regan, M.R.; Huang, Y.H.; Kim, Y.S.; Dykes-Hoberg, M.I.; Jin, L.; Watkins, A.M.; Bergles, D.E.; Rothstein, J.D. Variations in Promoter Activity Reveal a Differential Expression and Physiology of Glutamate Transporters by Glia in the Developing and Mature CNS. J. Neurosci. 2007, 27, 6607–6619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, H.; Walters, G.; Paulsen, A.R.; Scarisbrick, I.A. Astrocyte Heterogeneity across the Brain and Spinal Cord Occurs Developmentally, in Adulthood and in Response to Demyelination. PLoS ONE 2017, 12, e0180697. [Google Scholar] [CrossRef] [Green Version]
- Molofsky, A.V.; Kelley, K.W.; Tsai, H.-H.; Redmond, S.A.; Chang, S.M.; Madireddy, L.; Chan, J.R.; Baranzini, S.E.; Ullian, E.M.; Rowitch, D.H. Astrocyte-Encoded Positional Cues Maintain Sensorimotor Circuit Integrity. Nature 2014, 509, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Barkho, B.Z.; Song, H.; Aimone, J.B.; Smrt, R.D.; Kuwabara, T.; Nakashima, K.; Gage, F.H.; Zhao, X. Identification of Astrocyte-Expressed Factors That Modulate Neural Stem/Progenitor Cell Differentiation. Stem. Cells Dev. 2006, 15, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Hao, H.; Zhao, S.; Zhang, Y.; Liu, Q.; Liu, H.; Liu, S.; Yuan, Q.; Bing, L.; Ling, E.-A.; et al. Roles of Activated Astrocyte in Neural Stem Cell Proliferation and Differentiation. Stem. Cell Res. 2011, 7, 41–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.-W.; Jia, D.-Y.; Du, Z.-H.; Fu, J.; Zhao, S.-D.; Liu, S.-M.; Zhang, Y.-M.; Ling, E.-A.; Hao, A.-J. Roles of Activated Astrocytes in Bone Marrow Stromal Cell Proliferation and Differentiation. Neuroscience 2009, 160, 319–329. [Google Scholar] [CrossRef]
- Lakshman, N.; Bourget, C.; Siu, R.; Bamm, V.V.; Xu, W.; Harauz, G.; Morshead, C.M. Niche-dependent Inhibition of Neural Stem Cell Proliferation and Oligodendrogenesis Is Mediated by the Presence of Myelin Basic Protein. Stem. Cells 2021, 39, 776–786. [Google Scholar] [CrossRef]
- Almad, A.; Sahinkaya, F.R.; McTigue, D.M. Oligodendrocyte Fate after Spinal Cord Injury. Neurotherapeutics 2011, 8, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Lamers, K.J.; de Reus, H.P.; Jongen, P.J. Myelin Basic Protein in CSF as Indicator of Disease Activity in Multiple Sclerosis. Mult. Scler. 1998, 4, 124–126. [Google Scholar] [CrossRef]
- Karakatsani, A.; Shah, B.; de Almodovar, C.R. Blood Vessels as Regulators of Neural Stem Cell Properties. Front. Mol. Neurosci. 2019, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Androutsellis-Theotokis, A.; Rueger, M.A.; Park, D.M.; Boyd, J.D.; Padmanabhan, R.; Campanati, L.; Stewart, C.V.; LeFranc, Y.; Plenz, D.; Walbridge, S.; et al. Angiogenic Factors Stimulate Growth of Adult Neural Stem Cells. PLoS ONE 2010, 5, e9414. [Google Scholar] [CrossRef] [Green Version]
- Katsimpardi, L.; Litterman, N.K.; Schein, P.A.; Miller, C.M.; Loffredo, F.S.; Wojtkiewicz, G.R.; Chen, J.W.; Lee, R.T.; Wagers, A.J.; Rubin, L.L. Vascular and Neurogenic Rejuvenation of the Aging Mouse Brain by Young Systemic Factors. Science 2014, 344, 630–634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villeda, S.A.; Luo, J.; Mosher, K.I.; Zou, B.; Britschgi, M.; Bieri, G.; Stan, T.M.; Fainberg, N.; Ding, Z.; Eggel, A.; et al. The Ageing Systemic Milieu Negatively Regulates Neurogenesis and Cognitive Function. Nature 2011, 477, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Fang, X.; Huang, D.; Luo, Q.; Zheng, M.; Wang, K.; Cao, L.; Yin, Z. Erythropoietin Signaling Increases Neurogenesis and Oligodendrogenesis of Endogenous Neural Stem Cells Following Spinal Cord Injury Both in Vivo and in Vitro. Mol. Med. Rep. 2018, 17, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Pathipati, P.; Gorba, T.; Scheepens, A.; Goffin, V.; Sun, Y.; Fraser, M. Growth Hormone and Prolactin Regulate Human Neural Stem Cell Regenerative Activity. Neuroscience 2011, 190, 409–427. [Google Scholar] [CrossRef]
- Kolb, B.; Morshead, C.; Gonzalez, C.; Kim, M.; Gregg, C.; Shingo, T.; Weiss, S. Growth Factor-Stimulated Generation of New Cortical Tissue and Functional Recovery after Stroke Damage to the Motor Cortex of Rats. J. Cereb. Blood Flow Metab. 2007, 27, 983–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shingo, T.; Gregg, C.; Enwere, E.; Fujikawa, H.; Hassam, R.; Geary, C.; Cross, J.C.; Weiss, S. Pregnancy-Stimulated Neurogenesis in the Adult Female Forebrain Mediated by Prolactin. Science 2003, 299, 117–120. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.R.; Lovett, S.J.; Majda, B.T.; Yoon, J.H.; Wheeler, L.P.G.; Hodgetts, S.I. Neurotrophic Factors for Spinal Cord Repair: Which, Where, How and When to Apply, and for What Period of Time? Brain Res. 2015, 1619, 36–71. [Google Scholar] [CrossRef]
- Hodgetts, S.I.; Harvey, A.R. Neurotrophic Factors Used to Treat Spinal Cord Injury. Vitam. Horm. 2016, 104, 405–457. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.A.; O’Shea, T.M.; Burda, J.E.; Ao, Y.; Barlatey, S.L.; Bernstein, A.M.; Kim, J.H.; James, N.D.; Rogers, A.; Kato, B.; et al. Required Growth Facilitators Propel Axon Regeneration across Complete Spinal Cord Injury. Nature 2018, 561, 396–400. [Google Scholar] [CrossRef]
- Chen, S.-Q.; Cai, Q.; Shen, Y.-Y.; Cai, X.-Y.; Lei, H.-Y. Combined Use of NGF/BDNF/BFGF Promotes Proliferation and Differentiation of Neural Stem Cells in Vitro. Int. J. Dev. Neurosci. 2014, 38, 74–78. [Google Scholar] [CrossRef] [PubMed]
- McTigue, D.M.; Horner, P.J.; Stokes, B.T.; Gage, F.H. Neurotrophin-3 and Brain-Derived Neurotrophic Factor Induce Oligodendrocyte Proliferation and Myelination of Regenerating Axons in the Contused Adult Rat Spinal Cord. J. Neurosci. 1998, 18, 5354–5365. [Google Scholar] [CrossRef]
- Schnell, L.; Schneider, R.; Kolbeck, R.; Barde, Y.-A.; Schwab, M.E. Neurotrophin-3 Enhances Sprouting of Corticospinal Tract during Development and after Adult Spinal Cord Lesion. Nature 1994, 367, 170–173. [Google Scholar] [CrossRef]
- Li, Q.; Ford, M.C.; Lavik, E.B.; Madri, J.A. Modeling the Neurovascular Niche: VEGF- and BDNF-mediated Cross-talk between Neural Stem Cells and Endothelial Cells: An in Vitro Study. J. Neurosci. Res. 2006, 84, 1656–1668. [Google Scholar] [CrossRef]
- Tavazoie, M.; der Veken, L.V.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A Specialized Vascular Niche for Adult Neural Stem Cells. Cell Stem. Cell 2008, 3, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Sun, G.J.; Zhou, Y.; Stadel, R.P.; Moss, J.; Yong, J.H.A.; Ito, S.; Kawasaki, N.K.; Phan, A.T.; Oh, J.H.; Modak, N.; et al. Tangential Migration of Neuronal Precursors of Glutamatergic Neurons in the Adult Mammalian Brain. PNAS 2015, 112, 9484–9489. [Google Scholar] [CrossRef] [Green Version]
- Chang, D.-J.; Cho, H.-Y.; Hwang, S.; Lee, N.; Choi, C.; Lee, H.; Hong, K.S.; Oh, S.-H.; Kim, H.S.; Shin, D.A.; et al. Therapeutic Effect of BDNF-Overexpressing Human Neural Stem Cells (F3.BDNF) in a Contusion Model of Spinal Cord Injury in Rats. Int. J. Mol. Sci. 2021, 22, 6970. [Google Scholar] [CrossRef] [PubMed]
- Xiong, L.-L.; Zou, Y.; Shi, Y.; Zhang, P.; Zhang, R.-P.; Dai, X.-J.; Liu, B.; Wang, T.-H. Tree Shrew Neural Stem Cell Transplantation Promotes Functional Recovery of Tree Shrews with a Hemi-Sectioned Spinal Cord Injury by Upregulating Nerve Growth Factor Expression. Int. J. Mol. Med. 2018, 41, 3267–3277. [Google Scholar] [CrossRef]
- Yang, Z.; Duan, H.; Mo, L.; Qiao, H.; Li, X. The Effect of the Dosage of NT-3/Chitosan Carriers on the Proliferation and Differentiation of Neural Stem Cells. Biomaterials 2010, 31, 4846–4854. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xiang, Z.; Ying, Y.; Huang, Z.; Tu, Y.; Chen, M.; Ye, J.; Dou, H.; Sheng, S.; Li, X.; et al. Nerve Growth Factor (NGF) with Hypoxia Response Elements Loaded by Adeno-Associated Virus (AAV) Combined with Neural Stem Cells Improve the Spinal Cord Injury Recovery. Cell Death Discov. 2021, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Kojima, A.; Tator, C.H. Intrathecal Administration of Epidermal Growth Factor and Fibroblast Growth Factor 2 Promotes Ependymal Proliferation and Functional Recovery after Spinal Cord Injury in Adult Rats. J. Neurotraum. 2002, 19, 223–238. [Google Scholar] [CrossRef]
- Martens, D.J.; Seaberg, R.M.; Kooy, D.V.D. In Vivo Infusions of Exogenous Growth Factors into the Fourth Ventricle of the Adult Mouse Brain Increase the Proliferation of Neural Progenitors around the Fourth Ventricle and the Central Canal of the Spinal Cord. Eur. J. Neurosci. 2002, 16, 1045–1057. [Google Scholar] [CrossRef]
- Mah, A.T.; Yan, K.S.; Kuo, C.J. Wnt Pathway Regulation of Intestinal Stem Cells. J. Physiol. 2016, 594, 4837–4847. [Google Scholar] [CrossRef] [Green Version]
- Famili, F.; Perez, L.G.; Naber, B.A.; Noordermeer, J.N.; Fradkin, L.G.; Staal, F.J. The Non-Canonical Wnt Receptor Ryk Regulates Hematopoietic Stem Cell Repopulation in Part by Controlling Proliferation and Apoptosis. Cell Death Dis. 2016, 7, e2479. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.; Gascón, S.; Masserdotti, G.; Deshpande, A.; Simon, C.; Fischer, J.; Dimou, L.; Lie, D.C.; Schroeder, T.; Berninger, B. Oligodendrogliogenic and Neurogenic Adult Subependymal Zone Neural Stem Cells Constitute Distinct Lineages and Exhibit Differential Responsiveness to Wnt Signalling. Nat. Cell Biol. 2013, 15, 602–613. [Google Scholar] [CrossRef] [Green Version]
- Piccin, D.; Morshead, C.M. Wnt Signaling Regulates Symmetry of Division of Neural Stem Cells in the Adult Brain and in Response to Injury. Stem. Cells 2011, 29, 528–538. [Google Scholar] [CrossRef]
- Reya, T.; Duncan, A.W.; Ailles, L.; Domen, J.; Scherer, D.C.; Willert, K.; Hintz, L.; Nusse, R.; Weissman, I.L. A Role for Wnt Signalling in Self-Renewal of Haematopoietic Stem Cells. Nature 2003, 423, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.-S.; Zhang, H.; Wang, W.; Hua, X.-Y.; Hu, Y.; Zhang, S.-Q.; Li, G.-W. Wnt-3a Protein Promote Neuronal Differentiation of Neural Stem Cells Derived from Adult Mouse Spinal Cord. Neurol. Res. 2013, 29, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Fan, C.; Xiao, Z.; Zhao, Y.; Zhang, H.; Sun, J.; Zhuang, Y.; Wu, X.; Shi, J.; Chen, Y.; et al. A Collagen Microchannel Scaffold Carrying Paclitaxel-Liposomes Induces Neuronal Differentiation of Neural Stem Cells through Wnt/β-Catenin Signaling for Spinal Cord Injury Repair. Biomaterials 2018, 183, 114–127. [Google Scholar] [CrossRef] [PubMed]
- Perale, G.; Rossi, F.; Sundstrom, E.; Bacchiega, S.; Masi, M.; Forloni, G.; Veglianese, P. Hydrogels in Spinal Cord Injury Repair Strategies. ACS Chem. Neurosci. 2011, 2, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Shultz, R.B.; Zhong, Y. Hydrogel-Based Local Drug Delivery Strategies for Spinal Cord Repair. Neural. Regen Res. 2020, 16, 247–253. [Google Scholar] [CrossRef]
- Hlavac, N.; Kasper, M.; Schmidt, C.E. Progress toward Finding the Perfect Match: Hydrogels for Treatment of Central Nervous System Injury. Mater. Today Adv. 2020, 6, 100039. [Google Scholar] [CrossRef]
- Tom, V.J.; Sandrow-Feinberg, H.R.; Miller, K.; Domitrovich, C.; Bouyer, J.; Zhukareva, V.; Klaw, M.C.; Lemay, M.A.; Houlé, J.D. Exogenous BDNF Enhances the Integration of Chronically Injured Axons That Regenerate through a Peripheral Nerve Grafted into a Chondroitinase-Treated Spinal Cord Injury Site. Exp. Neurol. 2013, 239, 91–100. [Google Scholar] [CrossRef] [Green Version]
- Hayflick, L. Human Cells and Aging. Sci. Am. 1968, 218, 32–37. [Google Scholar] [CrossRef]
- Chan, M.; Yuan, H.; Soifer, I.; Maile, T.M.; Wang, R.Y.; Ireland, A.; O’Brien, J.; Goudeau, J.; Chan, L.; Vijay, T.; et al. Revisiting the Hayflick Limit: Insights from an Integrated Analysis of Changing Transcripts, Proteins, Metabolites and Chromatin. Biorxiv 2021. [Google Scholar] [CrossRef]
- Albors, A.R.; Tazaki, A.; Rost, F.; Nowoshilow, S.; Chara, O.; Tanaka, E.M. Planar Cell Polarity-Mediated Induction of Neural Stem Cell Expansion during Axolotl Spinal Cord Regeneration. Elife 2015, 4, e10230. [Google Scholar] [CrossRef]
- Chernoff, E.A.G.; Stocum, D.L.; Nye, H.L.D.; Cameron, J.A. Urodele Spinal Cord Regeneration and Related Processes. Dev. Dyn. 2003, 226, 295–307. [Google Scholar] [CrossRef]
- Lucini, C.; D’Angelo, L.; Cacialli, P.; Palladino, A.; de Girolamo, P. BDNF, Brain, and Regeneration: Insights from Zebrafish. Int. J. Mol. Sci. 2018, 19, 3155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tonge, D.A.; Leclere, P.G. Directed Axonal Growth towards Axolotl Limb Blastemas in Vitro. Neuroscience 2000, 100, 201–211. [Google Scholar] [CrossRef]
- Wehner, D.; Tsarouchas, T.M.; Michael, A.; Haase, C.; Weidinger, G.; Reimer, M.M.; Becker, T.; Becker, C.G. Wnt Signaling Controls Pro-Regenerative Collagen XII in Functional Spinal Cord Regeneration in Zebrafish. Nat. Commun. 2017, 8, 126. [Google Scholar] [CrossRef]
- O’Hara, C.M.; Chernoff, E.A.G. Growth Factor Modulation of Injury-Reactive Ependymal Cell Proliferation and Migration. Tissue Cell 1994, 26, 599–611. [Google Scholar] [CrossRef]
- Wagers, A.J. The Stem Cell Niche in Regenerative Medicine. Cell Stem. Cell 2012, 10, 362–369. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Gallagher, D.; DeVito, L.M.; Cancino, G.I.; Tsui, D.; He, L.; Keller, G.M.; Frankland, P.W.; Kaplan, D.R.; Miller, F.D. Metformin Activates an Atypical PKC-CBP Pathway to Promote Neurogenesis and Enhance Spatial Memory Formation. Cell Stem. Cell 2012, 11, 23–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatt, M.; Hsu, K.; He, L.; Wondisford, F.; Miller, F.D.; Kaplan, D.R.; Wang, J. Metformin Acts on Two Different Molecular Pathways to Enhance Adult Neural Precursor Proliferation/Self-Renewal and Differentiation. Stem. Cell Rep. 2015, 5, 988–995. [Google Scholar] [CrossRef] [Green Version]
- Erlandsson, A.; Morshead, C.M. Exploiting the Properties of Adult Stem Cells for the Treatment of Disease. Curr. Opin. Mol. 2006, 8, 331–337. [Google Scholar]
- Tedesco, D.; Haragsim, L. Cyclosporine: A Review. J. Transplant. 2012, 2012, 230386. [Google Scholar] [CrossRef] [Green Version]
- Hunt, J.; Cheng, A.; Hoyles, A.; Jervis, E.; Morshead, C.M. Cyclosporin A Has Direct Effects on Adult Neural Precursor Cells. J. Neurosci. 2010, 30, 2888–2896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, J.; Morshead, C. Cyclosporin A Enhances Cell Survival in Neural Precursor Populations in the Adult Central Nervous System. Mol. Cell. Pharmacol. 2010, 3, 2888–2896. [Google Scholar] [CrossRef]
- Erlandsson, A.; Lin, C.-H.A.; Yu, F.; Morshead, C.M. Immunosuppression Promotes Endogenous Neural Stem and Progenitor Cell Migration and Tissue Regeneration after Ischemic Injury. Exp. Neurol. 2011, 230, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Sachewsky, N.; Hunt, J.; Cooke, M.J.; Azimi, A.; Zarin, T.; Miu, C.; Shoichet, M.S.; Morshead, C.M. Cyclosporin A Enhances Neural Precursor Cell Survival in Mice through a Calcineurin-Independent Pathway. Dis. Model. Mech. 2014, 7, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Tuladhar, A.; Morshead, C.M.; Shoichet, M.S. Circumventing the Blood-Brain Barrier: Local Delivery of Cyclosporin A Stimulates Stem Cells in Stroke-Injured Rat Brain. J. Control. Release 2015, 215, 1–11. [Google Scholar] [CrossRef]
- Nusrat, L.; Livingston-Thomas, J.M.; Raguthevan, V.; Adams, K.; Vonderwalde, I.; Corbett, D.; Morshead, C.M. Cyclosporin A-Mediated Activation of Endogenous Neural Precursor Cells Promotes Cognitive Recovery in a Mouse Model of Stroke. Front. Aging Neurosci. 2018, 10, 93. [Google Scholar] [CrossRef] [Green Version]
- Potts, M.B.; Lim, D.A. An Old Drug for New Ideas: Metformin Promotes Adult Neurogenesis and Spatial Memory Formation. Cell Stem. Cell 2012, 11, 5–6. [Google Scholar] [CrossRef] [Green Version]
- Gilbert, E.; Kehtari, T.; Morshead, C. Metformin Activates Resident Neural Stem and Progenitor Cells, Reduces Inflammation and Improves Functional Recovery Following Spinal Cord Injury. FASEB J. 2021, 35. [Google Scholar] [CrossRef]
- Tao, L.; Li, D.; Liu, H.; Jiang, F.; Xu, Y.; Cao, Y.; Gao, R.; Chen, G. Neuroprotective Effects of Metformin on Traumatic Brain Injury in Rats Associated with NF-ΚB and MAPK Signaling Pathway. Brain Res. Bull. 2018, 140, 154–161. [Google Scholar] [CrossRef]
- Łabuzek, K.; Liber, S.; Gabryel, B.; Okopień, B. Metformin Has Adenosine-Monophosphate Activated Protein Kinase (AMPK)-Independent Effects on LPS-Stimulated Rat Primary Microglial Cultures. Pharm. Rep. 2010, 62, 827–848. [Google Scholar] [CrossRef]
- DiBona, V.L.; Shah, M.K.; Krause, K.J.; Zhu, W.; Voglewede, M.M.; Smith, D.M.; Crockett, D.P.; Zhang, H. Metformin Reduces Neuroinflammation and Improves Cognitive Functions after Traumatic Brain Injury. Neurosci. Res. 2021, 172, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Saisho, Y. Metformin and Inflammation: Its Potential Beyond Glucose-Lowering Effect. Endocr. Metab. Immune Disord. Targets 2015, 15, 196–205. [Google Scholar] [CrossRef] [PubMed]
- Hattori, Y.; Suzuki, K.; Hattori, S.; Kasai, K. Metformin Inhibits Cytokine-Induced Nuclear Factor KappaB Activation via AMP-Activated Protein Kinase Activation in Vascular Endothelial Cells. Hypertens Dallas Tex 1979 2006, 47, 1183–1188. [Google Scholar]
- Jin, Q.; Cheng, J.; Liu, Y.; Wu, J.; Wang, X.; Wei, S.; Zhou, X.; Qin, Z.; Jia, J.; Zhen, X. Improvement of Functional Recovery by Chronic Metformin Treatment Is Associated with Enhanced Alternative Activation of Microglia/Macrophages and Increased Angiogenesis and Neurogenesis Following Experimental Stroke. Brain Behav. Immun. 2014, 40, 131–142. [Google Scholar] [CrossRef]
- Liu, Y.; Tang, G.; Zhang, Z.; Wang, Y.; Yang, G.-Y. Metformin Promotes Focal Angiogenesis and Neurogenesis in Mice Following Middle Cerebral Artery Occlusion. Neurosci. Lett. 2014, 579, 46–51. [Google Scholar] [CrossRef]
- Livingston, J.M.; Syeda, T.; Christie, T.; Gilbert, E.A.B.; Morshead, C.M. Subacute Metformin Treatment Reduces Inflammation and Improves Functional Outcome Following Neonatal Hypoxia Ischemia. Brain Behav. Immun.—Health 2020, 7, 100119. [Google Scholar] [CrossRef]
- Ruddy, R.M.; Adams, K.V.; Morshead, C.M. Age- and Sex-Dependent Effects of Metformin on Neural Precursor Cells and Cognitive Recovery in a Model of Neonatal Stroke. Sci. Adv. 2019, 5, eaax1912. [Google Scholar] [CrossRef] [Green Version]
- Derkach, D.; Kehtari, T.; Renaud, M.; Heidari, M.; Lakshman, N.; Morshead, C.M. Metformin Pretreatment Rescues Olfactory Memory Associated with Subependymal Zone Neurogenesis in a Juvenile Model of Cranial Irradiation. Cell Rep. Med. 2021, 2, 100231. [Google Scholar] [CrossRef]
- Kosaraju, J.; Seegobin, M.; Gouveia, A.; Syal, C.; Sarma, S.N.; Lu, K.J.; Ilin, J.; He, L.; Wondisford, F.E.; Lagace, D.; et al. Metformin Promotes CNS Remyelination and Improves Social Interaction Following Focal Demyelination through CBP Ser436 Phosphorylation. Exp. Neurol. 2020, 334, 113454. [Google Scholar] [CrossRef]
- Ma, D.; Wang, B.; Zawadzka, M.; Gonzalez, G.; Wu, Z.; Yu, B.; Rawlins, E.L.; Franklin, R.J.M.; Zhao, C. A Subpopulation of Foxj1-Expressing, Nonmyelinating Schwann Cells of the Peripheral Nervous System Contribute to Schwann Cell Remyelination in the Central Nervous System. J. Neurosci. 2018, 38, 9228–9239. [Google Scholar] [CrossRef]
- Adams, D.S.; Robinson, K.R.; Fukumoto, T.; Yuan, S.; Albertson, R.C.; Yelick, P.; Kuo, L.; McSweeney, M.; Levin, M. Early, H+-V-ATPase-Dependent Proton Flux Is Necessary for Consistent Left-Right Patterning of Non-Mammalian Vertebrates. Development 2006, 133, 1657–1671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouleau, N.; Dotta, B.T. Electromagnetic Fields as Structure-Function Zeitgebers in Biological Systems: Environmental Orchestrations of Morphogenesis and Consciousness. Front. Integr. Neurosci. 2014, 8, 84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaig, C.D.; Rajnicek, A.M.; Song, B.; Zhao, M. Controlling Cell Behavior Electrically: Current Views and Future Potential. Physiol. Rev. 2005, 85, 943–978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuccitelli, R.; Nuccitelli, P.; Ramlatchan, S.; Sanger, R.; Smith, P.J.S. Imaging the Electric Field Associated with Mouse and Human Skin Wounds. Wound Repair Regen 2008, 16, 432–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariza, C.A.; Fleury, A.T.; Tormos, C.J.; Petruk, V.; Chawla, S.; Oh, J.; Sakaguchi, D.S.; Mallapragada, S.K. The Influence of Electric Fields on Hippocampal Neural Progenitor Cells. Stem. Cell Rev. Rep. 2010, 6, 585–600. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Droujinine, I.A.; Popovic, M.R.; Morshead, C.M. Adult Subependymal Neural Precursors, but Not Differentiated Cells, Undergo Rapid Cathodal Migration in the Presence of Direct Current Electric Fields. PLoS ONE 2011, 6, e23808. [Google Scholar] [CrossRef] [Green Version]
- Sefton, E.; Iwasa, S.N.; Morrison, T.; Naguib, H.E.; Popovic, M.R.; Morshead, C.M. Electric Field Application In Vivo Regulates Neural Precursor Cell Behavior in the Adult Mammalian Forebrain. ENeuro 2020, 7, ENEURO.0273-20.2020. [Google Scholar] [CrossRef]
- Meng, X.; Li, W.; Young, F.; Gao, R.; Chalmers, L.; Zhao, M.; Song, B. Electric Field-Controlled Directed Migration of Neural Progenitor Cells in 2D and 3D Environments. J. Vis. Exp. Jove 2012, 60, e3453. [Google Scholar] [CrossRef]
- Williamson, M.R.; Jones, T.A.; Drew, M.R. Functions of Subventricular Zone Neural Precursor Cells in Stroke Recovery. Behav. Brain Res. 2019, 376, 112209. [Google Scholar] [CrossRef]
- Jeong, S.H.; Jun, S.B.; Song, J.K.; Kim, S.J. Activity-Dependent Neuronal Cell Migration Induced by Electrical Stimulation. Med. Biol. Eng. Comput. 2009, 47, 93–99. [Google Scholar] [CrossRef]
- Babona-Pilipos, R.; Liu, N.; Pritchard-Oh, A.; Mok, A.; Badawi, D.; Popovic, M.R.; Morshead, C.M. Calcium Influx Differentially Regulates Migration Velocity and Directedness in Response to Electric Field Application. Exp. Cell Res. 2018, 368, 202–214. [Google Scholar] [CrossRef]
- Nakajima, K.; Zhu, K.; Sun, Y.-H.; Hegyi, B.; Zeng, Q.; Murphy, C.J.; Small, J.V.; Chen-Izu, Y.; Izumiya, Y.; Penninger, J.M.; et al. KCNJ15/Kir4.2 Couples with Polyamines to Sense Weak Extracellular Electric Fields in Galvanotaxis. Nat. Commun. 2015, 6, 8532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iwasa, S.N.; Rashidi, A.; Sefton, E.; Liu, N.X.; Popovic, M.R.; Morshead, C.M. Charge-Balanced Electrical Stimulation Can Modulate Neural Precursor Cell Migration in the Presence of Endogenous Electric Fields in Mouse Brains. ENeuro 2019, 6, ENEURO.0382-19.2019. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Lyon, J.G.; Alvarado-Velez, M.; Betancur, M.I.; Mokarram, N.; Shin, J.H.; Bellamkonda, R.V. Enriching Neural Stem Cell and Pro-Healing Glial Phenotypes with Electrical Stimulation after Traumatic Brain Injury in Male Rats. Biorxiv 2020. [Google Scholar] [CrossRef]
- Ayanwuyi, L.; Tokarska, N.; McLean, N.A.; Johnston, J.M.; Verge, V.M.K. Brief Electrical Nerve Stimulation Enhances Intrinsic Repair Capacity of the Focally Demyelinated Central Nervous System. Neural. Regen Res. 2021, 17, 1042–1050. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Straaten, M.G.V.; Drubach, D.I.; et al. Neuromodulation of Lumbosacral Spinal Networks Enables Independent Stepping after Complete Paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef] [PubMed]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of Over-Ground Walking after Chronic Motor Complete Spinal Cord Injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilbert, E.A.B.; Lakshman, N.; Lau, K.S.K.; Morshead, C.M. Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair. Cells 2022, 11, 846. https://doi.org/10.3390/cells11050846
Gilbert EAB, Lakshman N, Lau KSK, Morshead CM. Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair. Cells. 2022; 11(5):846. https://doi.org/10.3390/cells11050846
Chicago/Turabian StyleGilbert, Emily A. B., Nishanth Lakshman, Kylie S. K. Lau, and Cindi M. Morshead. 2022. "Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair" Cells 11, no. 5: 846. https://doi.org/10.3390/cells11050846
APA StyleGilbert, E. A. B., Lakshman, N., Lau, K. S. K., & Morshead, C. M. (2022). Regulating Endogenous Neural Stem Cell Activation to Promote Spinal Cord Injury Repair. Cells, 11(5), 846. https://doi.org/10.3390/cells11050846