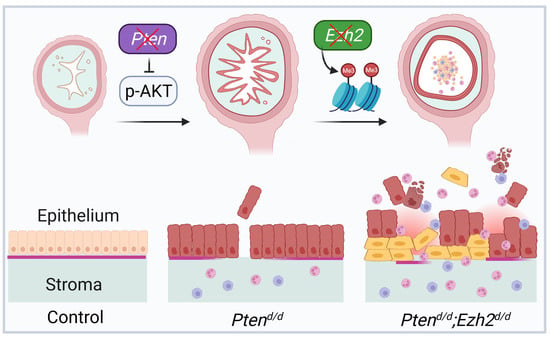
EZH2 and Endometrial Cancer Development: Insights from a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Ethics
2.2. Genotyping and DNA Recombination Analysis
2.3. Histology, Immunohistochemistry, and Immunofluorescence
2.4. Western Blot
2.5. Hypoxia Staining
2.6. Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Hormone Assays
2.8. Quantitative Reverse Transcription-PCR
2.9. Statistical Analysis
3. Results
3.1. Generation of Mice with Conditional Deletion of Ezh2 and Pten
3.2. Loss of EZH2 Reduces Tumor Burden during Early Carcinogenesis but Negatively Impacts Disease Outcome
3.3. Ezh2 and Pten Deletion Enhances the Accumulation of Intraluminal Neutrophils Compared with Pten Deletion Alone
3.4. Factors Contributing to the Developmental Trajectory of Endometrial Cancer Lacking PTEN and EZH2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2021. CA Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Di Cristofano, A.; Ellenson, L.H. Endometrial carcinoma. Annu. Rev. Pathol. 2007, 2, 57–85. [Google Scholar] [CrossRef] [PubMed]
- Malik, T.Y.; Chishti, U.; Aziz, A.B.; Sheikh, I. Comparison of risk factors and survival of type 1 and type ii endometrial cancers. Pak. J. Med. Sci. 2016, 32, 886–890. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.N.; Fader, A.N. Uterine papillary serous carcinoma. Clin. Obstet. Gynecol. 2011, 54, 278–291. [Google Scholar] [CrossRef] [PubMed]
- Lobo, F.D.; Thomas, E. Type ii endometrial cancers: A case series. J. Midlife Health 2016, 7, 69–72. [Google Scholar]
- Horn, L.C.; Meinel, A.; Handzel, R.; Einenkel, J. Histopathology of endometrial hyperplasia and endometrial carcinoma: An update. Ann. Diagn. Pathol. 2007, 11, 297–311. [Google Scholar] [CrossRef]
- Sherman, M.E.; Sturgeon, S.; Brinton, L.A.; Potischman, N.; Kurman, R.J.; Berman, M.L.; Mortel, R.; Twiggs, L.B.; Barrett, R.J.; Wilbanks, G.D. Risk factors and hormone levels in patients with serous and endometrioid uterine carcinomas. Mod. Pathol. 1997, 10, 963–968. [Google Scholar]
- Levine, D.A.; The Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature 2013, 497, 67–73. [Google Scholar] [CrossRef] [Green Version]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Li-Chang, H.H.; Kwon, J.S.; Melnyk, N.; Yang, W.; Senz, J.; Boyd, N.; Karnezis, A.N.; et al. A clinically applicable molecular-based classification for endometrial cancers. Br. J. Cancer 2015, 113, 299–310. [Google Scholar] [CrossRef] [Green Version]
- Talhouk, A.; McConechy, M.K.; Leung, S.; Yang, W.; Lum, A.; Senz, J.; Boyd, N.; Pike, J.; Anglesio, M.; Kwon, J.S.; et al. Confirmation of promise: A simple, genomics-based clinical classifier for endometrial cancer. Cancer 2017, 123, 802–813. [Google Scholar] [CrossRef] [Green Version]
- Kommoss, S.; McConechy, M.K.; Kommoss, F.; Leung, S.; Bunz, A.; Magrill, J.; Britton, H.; Kommoss, F.; Grevenkamp, F.; Karnezis, A.; et al. Final validation of the promise molecular classifier for endometrial carcinoma in a large population-based case series. Ann. Oncol. 2018, 29, 1180–1188. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Travaglino, A.; Raimondo, D.; Boccellino, M.P.; Maletta, M.; Borghese, G.; Casadio, P.; Insabato, L.; Mollo, A.; Zullo, F.; et al. Tumor-infiltrating lymphocytes and pole mutation in endometrial carcinoma. Gynecol. Oncol. 2021, 161, 621–628. [Google Scholar] [CrossRef] [PubMed]
- Huvila, J.; Orte, K.; Vainio, P.; Mettala, T.; Joutsiniemi, T.; Hietanen, S. Molecular subtype diagnosis of endometrial carcinoma: Comparison of the next-generation sequencing panel and proactive molecular risk classifier for endometrial cancer classifier. Hum. Pathol. 2021, 111, 98–109. [Google Scholar] [CrossRef] [PubMed]
- Urick, M.E.; Bell, D.W. Clinical actionability of molecular targets in endometrial cancer. Nat. Rev. Cancer 2019, 19, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Soumerai, T.E.; Donoghue, M.T.A.; Bandlamudi, C.; Srinivasan, P.; Chang, M.T.; Zamarin, D.; Cadoo, K.A.; Grisham, R.N.; O’Cearbhaill, R.E.; Tew, W.P.; et al. Clinical utility of prospective molecular characterization in advanced endometrial cancer. Clin. Cancer Res. 2018, 24, 5939–5947. [Google Scholar] [CrossRef] [Green Version]
- Tsimberidou, A.M.; Iskander, N.G.; Hong, D.S.; Wheler, J.J.; Falchook, G.S.; Fu, S.; Piha-Paul, S.; Naing, A.; Janku, F.; Luthra, R.; et al. Personalized medicine in a phase i clinical trials program: The md anderson cancer center initiative. Clin. Cancer Res. 2012, 18, 6373–6383. [Google Scholar] [CrossRef] [Green Version]
- Tashiro, H.; Blazes, M.S.; Wu, R.; Cho, K.R.; Bose, S.; Wang, S.I.; Li, J.; Parsons, R.; Ellenson, L.H. Mutations in pten are frequent in endometrial carcinoma but rare in other common gynecological malignancies. Cancer Res. 1997, 57, 3935–3940. [Google Scholar]
- Peiffer, S.L.; Herzog, T.J.; Tribune, D.J.; Mutch, D.G.; Gersell, D.J.; Goodfellow, P.J. Allelic loss of sequences from the long arm of chromosome 10 and replication errors in endometrial cancers. Cancer Res. 1995, 55, 1922–1926. [Google Scholar]
- Risinger, J.I.; Hayes, K.; Maxwell, G.L.; Carney, M.E.; Dodge, R.K.; Barrett, J.C.; Berchuck, A. Pten mutation in endometrial cancers is associated with favorable clinical and pathologic characteristics. Clin. Cancer Res. 1998, 4, 3005–3010. [Google Scholar]
- Daikoku, T.; Hirota, Y.; Tranguch, S.; Joshi, A.R.; DeMayo, F.J.; Lydon, J.P.; Ellenson, L.H.; Dey, S.K. Conditional loss of uterine pten unfailingly and rapidly induces endometrial cancer in mice. Cancer Res. 2008, 68, 5619–5627. [Google Scholar] [CrossRef] [Green Version]
- O’Hara, A.J.; Bell, D.W. The genomics and genetics of endometrial cancer. Adv. Genom. Genet. 2012, 2012, 33–47. [Google Scholar]
- Jani, K.S.; Jain, S.U.; Ge, E.J.; Diehl, K.L.; Lundgren, S.M.; Muller, M.M.; Lewis, P.W.; Muir, T.W. Histone h3 tail binds a unique sensing pocket in ezh2 to activate the prc2 methyltransferase. Proc. Natl. Acad. Sci. USA 2019, 116, 8295–8300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, K.H.; Roberts, C.W. Targeting ezh2 in cancer. Nat. Med. 2016, 22, 128–134. [Google Scholar] [CrossRef] [PubMed]
- Oki, S.; Sone, K.; Oda, K.; Hamamoto, R.; Ikemura, M.; Maeda, D.; Takeuchi, M.; Tanikawa, M.; Mori-Uchino, M.; Nagasaka, K.; et al. Oncogenic histone methyltransferase ezh2: A novel prognostic marker with therapeutic potential in endometrial cancer. Oncotarget 2017, 8, 40402–40411. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, I.M.; Halvorsen, O.J.; Collett, K.; Stefansson, I.M.; Straume, O.; Haukaas, S.A.; Salvesen, H.B.; Otte, A.P.; Akslen, L.A. Ezh2 expression is associated with high proliferation rate and aggressive tumor subgroups in cutaneous melanoma and cancers of the endometrium, prostate, and breast. J. Clin. Oncol. 2006, 24, 268–273. [Google Scholar] [CrossRef]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P.; et al. The polycomb group protein ezh2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef]
- Sashida, G.; Harada, H.; Matsui, H.; Oshima, M.; Yui, M.; Harada, Y.; Tanaka, S.; Mochizuki-Kashio, M.; Wang, C.; Saraya, A.; et al. Ezh2 loss promotes development of myelodysplastic syndrome but attenuates its predisposition to leukaemic transformation. Nat. Commun. 2014, 5, 4177. [Google Scholar] [CrossRef] [Green Version]
- Ernst, T.; Chase, A.J.; Score, J.; Hidalgo-Curtis, C.E.; Bryant, C.; Jones, A.V.; Waghorn, K.; Zoi, K.; Ross, F.M.; Reiter, A.; et al. Inactivating mutations of the histone methyltransferase gene ezh2 in myeloid disorders. Nat. Genet. 2010, 42, 722–726. [Google Scholar] [CrossRef]
- Yan, K.S.; Lin, C.Y.; Liao, T.W.; Peng, C.M.; Lee, S.C.; Liu, Y.J.; Chan, W.P.; Chou, R.H. Ezh2 in cancer progression and potential application in cancer therapy: A friend or foe? Int. J. Mol. Sci. 2017, 18, 1172. [Google Scholar] [CrossRef]
- Fang, X.; Ni, N.; Lydon, J.P.; Ivanov, I.; Bayless, K.J.; Rijnkels, M.; Li, Q. Enhancer of zeste 2 polycomb repressive complex 2 subunit is required for uterine epithelial integrity. Am. J. Pathol. 2019, 189, 1212–1225. [Google Scholar] [CrossRef] [Green Version]
- Soyal, S.M.; Mukherjee, A.; Lee, K.Y.; Li, J.; Li, H.; DeMayo, F.J.; Lydon, J.P. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis 2005, 41, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Liu, Y.; Hsu, Y.J.; Fujiwara, Y.; Kim, J.; Mao, X.; Yuan, G.C.; Orkin, S.H. Ezh1 mediates methylation on histone h3 lysine 27 and complements ezh2 in maintaining stem cell identity and executing pluripotency. Mol. Cell 2008, 32, 491–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Lin, P.; Lydon, J.P.; Li, Q. Conditional abrogation of transforming growth factor-beta receptor 1 in pten-inactivated endometrium promotes endometrial cancer progression in mice. J. Pathol. 2017, 243, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Ni, N.; Gao, Y.; Vincent, D.F.; Bartholin, L.; Li, Q. A novel mouse model of testicular granulosa cell tumors. Mol. Hum. Reprod. 2018, 24, 343–356. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative pcr and the 2(-delta delta c(t)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wang, X.; Spandidos, A.; Wang, H.; Seed, B. Primerbank: A pcr primer database for quantitative gene expression analysis, 2012 update. Nucleic Acids Res. 2012, 40, D1144–D1149. [Google Scholar] [CrossRef] [Green Version]
- Naganuma, T.; Takagi, S.; Kanetake, T.; Kitamura, T.; Hattori, S.; Miyakawa, T.; Sassa, T.; Kihara, A. Disruption of the sjogren-larsson syndrome gene aldh3a2 in mice increases keratinocyte growth and retards skin barrier recovery. J. Biol. Chem. 2016, 291, 11676–11688. [Google Scholar] [CrossRef] [Green Version]
- Blanco-Aparicio, C.; Renner, O.; Leal, J.F.M.; Carnero, A. Pten, more than the akt pathway. Carcinogenesis 2007, 28, 1379–1386. [Google Scholar] [CrossRef] [Green Version]
- Blaisdell, A.; Crequer, A.; Columbus, D.; Daikoku, T.; Mittal, K.; Dey, S.K.; Erlebacher, A. Neutrophils oppose uterine epithelial carcinogenesis via debridement of hypoxic tumor cells. Cancer Cell 2015, 28, 785–799. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.H.; Utsunomiya, H.; Pavone, M.E.; Yin, P.; Bulun, S.E. Retinoic acid inhibits endometrial cancer cell growth via multiple genomic mechanisms. J. Mol. Endocrinol. 2011, 46, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Saxena, S.; Awaji, M.; Singh, R.K. Tumor-associated neutrophils in cancer: Going pro. Cancers 2019, 11, 564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Korkmaz, B.; Horwitz, M.S.; Jenne, D.E.; Gauthier, F. Neutrophil elastase, proteinase 3, and cathepsin g as therapeutic targets in human diseases. Pharmacol. Rev. 2010, 62, 726–759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pijnenborg, J.M.A.; Wijnakker, M.; Hagelstein, J.; Delvoux, B.; Groothuis, P.G. Hypoxia contributes to development of recurrent endometrial carcinoma. Int. J. Gynecol. Cancer 2007, 17, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Mahiddine, K.; Blaisdell, A.; Ma, S.; Crequer-Grandhomme, A.; Lowell, C.A.; Erlebacher, A. Relief of tumor hypoxia unleashes the tumoricidal potential of neutrophils. J. Clin. Investig. 2020, 130, 389–403. [Google Scholar] [CrossRef] [PubMed]
- Tangen, I.L.; Werner, H.M.; Berg, A.; Halle, M.K.; Kusonmano, K.; Trovik, J.; Hoivik, E.A.; Mills, G.B.; Krakstad, C.; Salvesen, H.B. Loss of progesterone receptor links to high proliferation and increases from primary to metastatic endometrial cancer lesions. Eur. J. Cancer 2014, 50, 3003–3010. [Google Scholar] [CrossRef]
- Kim, J.J.; Chapman-Davis, E. Role of progesterone in endometrial cancer. Semin. Reprod. Med. 2010, 28, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Chung, H.H.; Or, Y.Z.; Shrestha, S.; Loh, J.T.; Lim, C.L.; Ong, Z.; Woo, A.R.E.; Su, I.H.; Lin, V.C.L. Estrogen reprograms the activity of neutrophils to foster protumoral microenvironment during mammary involution. Sci. Rep. 2017, 7, 46485. [Google Scholar] [CrossRef]
- Vazquez Rodriguez, G.; Abrahamsson, A.; Jensen, L.D.; Dabrosin, C. Estradiol promotes breast cancer cell migration via recruitment and activation of neutrophils. Cancer Immunol. Res. 2017, 5, 234–247. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, G.L.; Risinger, J.I.; Gumbs, C.; Shaw, H.; Bentley, R.C.; Barrett, J.C.; Berchuck, A.; Futreal, P.A. Mutation of the pten tumor suppressor gene in endometrial hyperplasias. Cancer Res. 1998, 58, 2500–2503. [Google Scholar]
- Gu, Y.; Zhang, J.; Guan, H. Expression of ezh2 in endometrial carcinoma and its effects on proliferation and invasion of endometrial carcinoma cells. Oncol. Lett. 2017, 14, 7191–7196. [Google Scholar] [CrossRef] [Green Version]
- Roh, J.W.; Choi, J.E.; Han, H.D.; Hu, W.; Matsuo, K.; Nishimura, M.; Lee, J.S.; Kwon, S.Y.; Cho, C.H.; Kim, J.; et al. Clinical and biological significance of ezh2 expression in endometrial cancer. Cancer Biol. Ther. 2020, 21, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Ihira, K.; Dong, P.; Xiong, Y.; Watari, H.; Konno, Y.; Hanley, S.J.; Noguchi, M.; Hirata, N.; Suizu, F.; Yamada, T.; et al. Ezh2 inhibition suppresses endometrial cancer progression via mir-361/twist axis. Oncotarget 2017, 8, 13509–13520. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Koyama, D.; Wada, T.; Izumi, T.; Hofgaard, P.O.; Bogen, B.; Furukawa, Y. Phosphorylation-mediated ezh2 inactivation promotes drug resistance in multiple myeloma. J. Clin. Investig. 2015, 125, 4375–4390. [Google Scholar] [CrossRef] [PubMed]
- Mallen-St Clair, J.; Soydaner-Azeloglu, R.; Lee, K.E.; Taylor, L.; Livanos, A.; Pylayeva-Gupta, Y.; Miller, G.; Margueron, R.; Reinberg, D.; Bar-Sagi, D. Ezh2 couples pancreatic regeneration to neoplastic progression. Genes Dev. 2012, 26, 439–444. [Google Scholar] [CrossRef] [Green Version]
- Nanjappa, M.K.; Mesa, A.M.; Medrano, T.I.; Jefferson, W.N.; DeMayo, F.J.; Williams, C.J.; Lydon, J.P.; Levin, E.R.; Cooke, P.S. The histone methyltransferase ezh2 is required for normal uterine development and function in mice. Biol. Reprod. 2019, 101, 306–317. [Google Scholar] [CrossRef]
- Mesa, A.M.; Mao, J.D.; Nanjappa, M.K.; Medrano, T.I.; Tevosian, S.; Yu, F.H.; Kinkade, J.; Lyu, Z.; Liu, Y.; Joshi, T.; et al. Mice lacking uterine enhancer of zeste homolog 2 have transcriptomic changes associated with uterine epithelial proliferation. Physiol. Genom. 2020, 52, 81–95. [Google Scholar] [CrossRef]
- Mesa, A.M.; Mao, J.; Medrano, T.I.; Bivens, N.J.; Jurkevich, A.; Tuteja, G.; Cooke, P.S.; Rosenfeld, C.S. Spatial transcriptomics analysis of uterine gene expression in enhancer of zeste homolog 2 conditional knockout micedagger. Biol. Reprod. 2021, 105, 1126–1139. [Google Scholar] [CrossRef]
- Xie, Y.; Naizabekov, S.; Chen, Z.; Tokay, T. Power of pten/akt: Molecular switch between tumor suppressors and oncogenes. Oncol. Lett. 2016, 12, 375–378. [Google Scholar] [CrossRef]
- Sirohi, V.K.; Medrano, T.I.; Mesa, A.M.; Kannan, A.; Bagchi, I.C.; Cooke, P.S. Regulation of akt signaling in mouse uterus. Endocrinology 2022, 163, bqab233. [Google Scholar] [CrossRef]
- Kruger, P.; Saffarzadeh, M.; Weber, A.N.; Rieber, N.; Radsak, M.; von Bernuth, H.; Benarafa, C.; Roos, D.; Skokowa, J.; Hartl, D. Neutrophils: Between host defence, immune modulation, and tissue injury. PLoS Pathog. 2015, 11, e1004651. [Google Scholar] [CrossRef] [Green Version]
- Masucci, M.T.; Minopoli, M.; Carriero, M.V. Tumor associated neutrophils. Their role in tumorigenesis, metastasis, prognosis and therapy. Front. Oncol. 2019, 9, 1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Hou, N.; Cheng, X.; Zhang, J.; Tan, X.; Zhang, C.; Tang, Y.; Teng, Y.; Yang, X. Ezh2 acts as a tumor suppressor in kras-driven lung adenocarcinoma. Int. J. Biol. Sci. 2017, 13, 652–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, L.; Yang, Y.; Li, Q.; Feng, Y.; Liu, T.; Guo, W. Epigenetic regulation of cancer progression by ezh2: From biological insights to therapeutic potential. Biomark Res. 2018, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Hu, P.; Donskov, F.; Wang, G.; Liu, Q.; Du, J. Tumor-associated neutrophils as a new prognostic factor in cancer: A systematic review and meta-analysis. PLoS ONE 2014, 9, e98259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, G.R.; Kurita, T.; Cao, M.; Shen, J.; Robboy, S.; Baskin, L. Molecular mechanisms of development of the human fetal female reproductive tract. Differentiation 2017, 97, 54–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCaffrey, L.M.; Macara, I.G. Epithelial organization, cell polarity and tumorigenesis. Trends Cell Biol. 2011, 21, 727–735. [Google Scholar] [CrossRef]
- Filant, J.; DeMayo, F.J.; Pru, J.K.; Lydon, J.P.; Spencer, T.E. Fibroblast growth factor receptor two (fgfr2) regulates uterine epithelial integrity and fertility in mice. Biol. Reprod. 2014, 90, 7. [Google Scholar] [CrossRef]
- Rodriguez, A.C.; Blanchard, Z.; Maurer, K.A.; Gertz, J. Estrogen signaling in endometrial cancer: A key oncogenic pathway with several open questions. Horm. Cancer 2019, 10, 51–63. [Google Scholar] [CrossRef] [Green Version]
- Lindberg, M.E.; Stodden, G.R.; King, M.L.; MacLean, J.A., 2nd; Mann, J.L.; DeMayo, F.J.; Lydon, J.P.; Hayashi, K. Loss of cdh1 and pten accelerates cellular invasiveness and angiogenesis in the mouse uterus. Biol. Reprod. 2013, 89, 8. [Google Scholar] [CrossRef] [Green Version]
- Fedorko, A.M.; Kim, T.H.; Broaddus, R.; Schmandt, R.; Chandramouli, G.V.R.; Kim, H.I.; Jeong, J.W.; Risinger, J.I. An immune competent orthotopic model of endometrial cancer with metastasis. Heliyon 2020, 6, e04075. [Google Scholar] [CrossRef]
- Wang, X.; Wendel, J.R.H.; Emerson, R.E.; Broaddus, R.R.; Creighton, C.J.; Rusch, D.B.; Buechlein, A.; DeMayo, F.J.; Lydon, J.P.; Hawkins, S.M. Pten and dicer1 loss in the mouse uterus causes poorly differentiated endometrial adenocarcinoma. Oncogene 2020, 39, 6286–6299. [Google Scholar] [CrossRef] [PubMed]
- Koppens, M.A.; Tanger, E.; Nacerddine, K.; Westerman, B.; Song, J.Y.; van Lohuizen, M. A new transgenic mouse model for conditional overexpression of the polycomb group protein ezh2. Transgenic Res. 2017, 26, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Ideker, T.; Galitski, T.; Hood, L. A new approach to decoding life: Systems biology. Annu. Rev. Genom. Hum. Genet. 2001, 2, 343–372. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Chen, K.; Zhong, C.; Zhu, S.; Ma, X. Network-based protein-protein interaction prediction method maps perturbations of cancer interactome. PLoS Genet. 2021, 17, e1009869. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Park, J.; Bouhaddou, M.; Kim, K.; Rojc, A.; Modak, M.; Soucheray, M.; McGregor, M.J.; O’Leary, P.; Wolf, D.; et al. A protein interaction landscape of breast cancer. Science 2021, 374, eabf3066. [Google Scholar] [CrossRef]
- Pilot-Storck, F.; Chopin, E.; Rual, J.F.; Baudot, A.; Dobrokhotov, P.; Robinson-Rechavi, M.; Brun, C.; Cusick, M.E.; Hill, D.E.; Schaeffer, L.; et al. Interactome mapping of the phosphatidylinositol 3-kinase-mammalian target of rapamycin pathway identifies deformed epidermal autoregulatory factor-1 as a new glycogen synthase kinase-3 interactor. Mol. Cell Proteom. 2010, 9, 1578–1593. [Google Scholar] [CrossRef] [Green Version]
- Pane, K.; Affinito, O.; Zanfardino, M.; Castaldo, R.; Incoronato, M.; Salvatore, M.; Franzese, M. An integrative computational approach based on expression similarity signatures to identify protein-protein interaction networks in female-specific cancers. Front. Genet. 2020, 11, 612521. [Google Scholar] [CrossRef]
- Yap, T.A.; Winter, J.N.; Giulino-Roth, L.; Longley, J.; Lopez, J.; Michot, J.M.; Leonard, J.P.; Ribrag, V.; McCabe, M.T.; Creasy, C.L.; et al. Phase i study of the novel enhancer of zeste homolog 2 (ezh2) inhibitor gsk2816126 in patients with advanced hematologic and solid tumors. Clin. Cancer Res. 2019, 25, 7331–7339. [Google Scholar] [CrossRef] [Green Version]
- Italiano, A.; Soria, J.C.; Toulmonde, M.; Michot, J.M.; Lucchesi, C.; Varga, A.; Coindre, J.M.; Blakemore, S.J.; Clawson, A.; Suttle, B.; et al. Tazemetostat, an ezh2 inhibitor, in relapsed or refractory b-cell non-hodgkin lymphoma and advanced solid tumours: A first-in-human, open-label, phase 1 study. Lancet Oncol. 2018, 19, 649–659. [Google Scholar] [CrossRef]








| Name | Manufacturer | Cat. # | Species | IHC/IF | WB |
|---|---|---|---|---|---|
| EZH2 | Cell Signaling (Danvers, MA, USA) | 5246 | Rabbit | 1:400 | 1:1000 |
| pAKT | Cell Signaling | 4060 | Rabbit | 1:50 | 1:2000 |
| Ki67 | Cell Signaling | 12202 | Rabbit | 1:500 | |
| ECAD | Cell Signaling | 3195 | Rabbit | 1:400 | |
| KRT14 | Thermo Fisher Scientific | PA5-16722 | Rabbit | 1:400 | |
| ΔNp63 | BioLegend (San Diego, CA, USA) | 619001 | Rabbit | 1:200 | |
| F4/80 | Cell Signaling | 70076 | Rabbit | 1:250 | |
| LY6G | BioLegend | 127601 | Rat | 1:500 | |
| PTEN | Cell Signaling | 9188 | Rabbit | 1:1000 | |
| AKT | Cell Signaling | 4691 | Rabbit | 1:1000 | |
| H3K27me3 | Cell Signaling | 9733 | Rabbit | 1:1000 | |
| Histone H3 | Cell Signaling | 4499 | Rabbit | 1:5000 | |
| ACTB | MilliporeSigma | A3854 | Mouse | 1:50,000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Ni, N.; Wang, X.; Tian, Y.; Ivanov, I.; Rijnkels, M.; Bayless, K.J.; Lydon, J.P.; Li, Q. EZH2 and Endometrial Cancer Development: Insights from a Mouse Model. Cells 2022, 11, 909. https://doi.org/10.3390/cells11050909
Fang X, Ni N, Wang X, Tian Y, Ivanov I, Rijnkels M, Bayless KJ, Lydon JP, Li Q. EZH2 and Endometrial Cancer Development: Insights from a Mouse Model. Cells. 2022; 11(5):909. https://doi.org/10.3390/cells11050909
Chicago/Turabian StyleFang, Xin, Nan Ni, Xiaofang Wang, Yanan Tian, Ivan Ivanov, Monique Rijnkels, Kayla J. Bayless, John P. Lydon, and Qinglei Li. 2022. "EZH2 and Endometrial Cancer Development: Insights from a Mouse Model" Cells 11, no. 5: 909. https://doi.org/10.3390/cells11050909
APA StyleFang, X., Ni, N., Wang, X., Tian, Y., Ivanov, I., Rijnkels, M., Bayless, K. J., Lydon, J. P., & Li, Q. (2022). EZH2 and Endometrial Cancer Development: Insights from a Mouse Model. Cells, 11(5), 909. https://doi.org/10.3390/cells11050909