Allogeneic Serum and Macromolecular Crowding Maintain Native Equine Tenocyte Function in Culture
Abstract
:1. Introduction
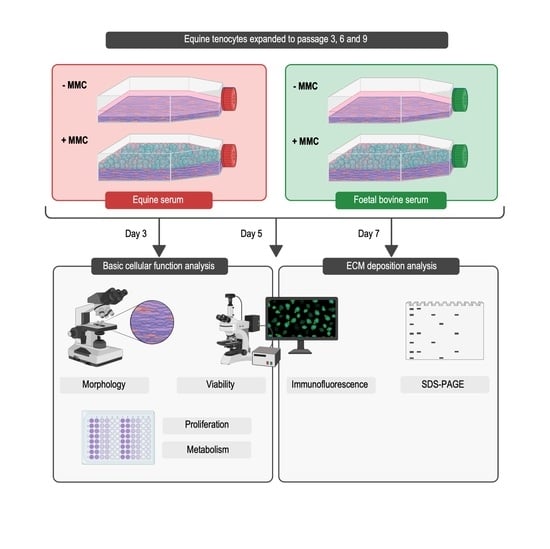
2. Materials and Methods
2.1. Materials
2.2. Cell Isolation and Culture
2.3. Cell Morphology
2.4. Cell Proliferation Analysis
2.5. Cell Metabolic Activity Analysis
2.6. Cell Viability Analysis
2.7. Gel Electrophoresis Analysis
2.8. Immunofluorescence Analysis
2.9. Statistical Analysis
3. Results
3.1. Cell Morphology and Growth Surface Coverage Analyses
3.2. Cell Proliferation, Metabolic Activity and Viability Analyses
3.3. Gel Electrophoresis Analysis
3.4. Immunofluorescence Analysis
3.5. Comparison to Passage 3, in FBS, without MMC at Day 3
4. Discussion
4.1. Basic Cell Function Analysis
4.2. SDS-PAGE and Immunofluorescence Analyses
4.3. Comparison to Passage 3, in FBS, without MMC at Day 3
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cigognini, D.; Lomas, A.; Kumar, P.; Satyam, A.; English, A.; Azeem, A.; Pandit, A.; Zeugolis, D. Engineering in vitro microenvironments for cell based therapies and drug discovery. Drug Discov. Today 2013, 18, 1099–1108. [Google Scholar] [CrossRef] [PubMed]
- Turinetto, V.; Vitale, E.; Giachino, C. Senescence in human mesenchymal stem cells: Functional changes and implications in stem cell-based therapy. Int. J. Mol. Sci. 2016, 17, 1164. [Google Scholar] [CrossRef] [PubMed]
- McKee, C.; Chaudhry, G.R. Advances and challenges in stem cell culture. Colloids Surf. B Biointerfaces 2017, 159, 62–77. [Google Scholar] [CrossRef] [PubMed]
- De Pieri, A.; Rochev, Y.; Zeugolis, D.I. Scaffold-free cell-based tissue engineering therapies: Advances, shortfalls and forecast. npj Regen. Med. 2021, 6, 18. [Google Scholar] [CrossRef]
- Yang, Y.K.; Ogando, C.R.; Wang See, C.; Chang, T.Y.; Barabino, G.A. Changes in phenotype and differentiation potential of human mesenchymal stem cells aging in vitro. Stem Cell Res. Ther. 2018, 9, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stute, N.; Holtz, K.; Bubenheim, M.; Lange, C.; Blake, F.; Zander, A.R. Autologous serum for isolation and expansion of human mesenchymal stem cells for clinical use. Exp. Hematol. 2004, 32, 1212–1225. [Google Scholar] [CrossRef]
- Satyam, A.; Tsokos, M.G.; Tresback, J.S.; Zeugolis, D.I.; Tsokos, G.C. Cell-derived extracellular matrix-rich biomimetic substrate supports podocyte proliferation, differentiation, and maintenance of native phenotype. Adv. Funct. Mater. 2020, 30, 1908752. [Google Scholar] [CrossRef]
- Peng, Y.; Bocker, M.T.; Holm, J.; Toh, W.S.; Hughes, C.S.; Kidwai, F.; Lajoie, G.A.; Cao, T.; Lyko, F.; Raghunath, M. Human fibroblast matrices bio-assembled under macromolecular crowding support stable propagation of human embryonic stem cells. J. Tissue Eng. Regen. Med. 2012, 6, e74–e86. [Google Scholar] [CrossRef]
- Lozito, T.P.; Kuo, C.K.; Taboas, J.M.; Tuan, R.S. Human mesenchymal stem cells express vascular cell phenotypes upon interaction with endothelial cell matrix. J. Cell Biochem. 2009, 107, 714–722. [Google Scholar] [CrossRef]
- Boudreau, N.; Bissell, M.J. Extracellular matrix signaling: Integration of form and function in normal and malignant cells. Curr. Opin. Cell Biol. 1998, 10, 640–646. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, D.; Spanoudes, K.; Holladay, C.; Pandit, A.; Zeugolis, D. Progress in cell-based therapies for tendon repair. Adv. Drug Deliv. Rev. 2015, 84, 240–256. [Google Scholar] [CrossRef] [PubMed]
- English, A.; Azeem, A.; Spanoudes, K.; Jones, E.; Tripathi, B.; Basu, N.; McNamara, K.; Tofail, S.; Rooney, N.; Riley, G.; et al. Substrate topography: A valuable in vitro tool, but a clinical red herring for in vivo tenogenesis. Acta Biomater. 2015, 27, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermeulen, S.; Vasilevich, A.; Tsiapalis, D.; Roumans, N.; Vroemen, P.; Beijer, N.; Dede Eren, A.; Zeugolis, D.; de Boer, J. Identification of topographical architectures supporting the phenotype of rat tenocytes. Acta Biomater. 2019, 83, 277–290. [Google Scholar] [CrossRef]
- Huisman, E.; Lu, A.; McCormack, R.; Scott, A. Enhanced collagen type I synthesis by human tenocytes subjected to periodic in vitro mechanical stimulation. BMC Musculoskelet. Disord. 2014, 15, 386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atkinson, F.; Evans, R.; Guest, J.; Bavin, E.; Cacador, D.; Holland, C.; Guest, D. Cyclical strain improves artificial equine tendon constructs in vitro. J. Tissue Eng. Regen. Med. 2020, 14, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wang, X.; Zhang, Y.; Carr, A.; Zhu, L.; Xia, Z.; Sabokbar, A. Development of a refined tenocyte expansion culture technique for tendon tissue engineering. J. Tissue Eng. Regen. Med. 2014, 8, 955–962. [Google Scholar] [CrossRef]
- Pauly, S.; Klatte-Schulz, F.; Stahnke, K.; Scheibel, M.; Wildemann, B. The effect of autologous platelet rich plasma on tenocytes of the human rotator cuff. BMC Musculoskelet. Disord. 2018, 19, 422. [Google Scholar] [CrossRef]
- Ryan, C.N.M.; Zeugolis, D.I. Engineering the tenogenic niche in vitro with microenvironmental tools. Adv. Ther. Ger. 2020, 3, 1900072. [Google Scholar] [CrossRef]
- Spanoudes, K.; Gaspar, D.; Pandit, A.; Zeugolis, D.I. The biophysical, biochemical, and biological toolbox for tenogenic phenotype maintenance in vitro. Trends Biotechnol. 2014, 32, 474–482. [Google Scholar] [CrossRef]
- Yao, L.; Bestwick, C.S.; Bestwick, L.A.; Maffulli, N.; Aspden, R.M. Phenotypic drift in human tenocyte culture. Tissue Eng. 2006, 12, 1843–1849. [Google Scholar] [CrossRef]
- Brink, H.E.; Miller, G.J.; Beredjiklian, P.K.; Nicoll, S.B. Serum-dependent effects on adult and fetal tendon fibroblast migration and collagen expression. Wound Repair Regen. 2006, 14, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Riley, C.B.; Archer, F.J.; Bailey, J.V. Comparison of media and sera used for in vitro culture of equine superficial digital flexor tendon explants. Am. J. Vet. Res. 1996, 57, 1118–1123. [Google Scholar] [PubMed]
- Lohan, A.; Kohl, B.; Meier, C.; Schulze-Tanzil, G. Tenogenesis of decellularized porcine Achilles tendon matrix reseeded with human tenocytes in the nude mice xenograft model. Int. J. Mol. Sci. 2018, 19, 2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lohan, A.; Stoll, C.; Albrecht, M.; Denner, A.; John, T.; Kruger, K.; Ertel, W.; Schulze-Tanzil, G. Human hamstring tenocytes survive when seeded into a decellularized porcine Achilles tendon extracellular matrix. Connect. Tissue Res. 2013, 54, 305–312. [Google Scholar] [CrossRef]
- Qin, T.; Sun, Y.; Thoreson, A.; Steinmann, S.; Amadio, P.; An, K.; Zhao, C. Effect of mechanical stimulation on bone marrow stromal cell-seeded tendon slice constructs: A potential engineered tendon patch for rotator cuff repair. Biomaterials 2015, 51, 43–50. [Google Scholar] [CrossRef] [Green Version]
- Tong, W.; Shen, W.; Yeung, C.; Zhao, Y.; Cheng, S.; Chu, P.; Chan, D.; Chan, G.; Cheung, K.; Yeung, K.; et al. Functional replication of the tendon tissue microenvironment by a bioimprinted substrate and the support of tenocytic differentiation of mesenchymal stem cells. Biomaterials 2012, 33, 7686–7698. [Google Scholar] [CrossRef]
- Yelin, E.; Weinstein, S.; King, T. The burden of musculoskeletal diseases in the United States. Semin. Arthritis Rheum. 2016, 46, 259–260. [Google Scholar] [CrossRef]
- Kaux, J.; Forthomme, B.; Goff, C.; Crielaard, J.; Croisier, J. Current opinions on tendinopathy. J. Sports Sci. Med. 2011, 10, 238–253. [Google Scholar]
- Sleeswijk Visser, T.; van der Vlist, A.; van Oosterom, R.; van Veldhoven, P.; Verhaar, J.; de Vos, R. Impact of chronic Achilles tendinopathy on health-related quality of life, work performance, healthcare utilisation and costs. BMJ Open Sport Exerc. Med. 2021, 7, e001023. [Google Scholar] [CrossRef]
- Thorpe, C.; Clegg, P.; Birch, H. A review of tendon injury: Why is the equine superficial digital flexor tendon most at risk? Equine Vet. J. 2010, 42, 174–180. [Google Scholar] [CrossRef]
- Crawford, K.; Finnane, A.; Phillips, C.; Greer, R.; Woldeyohannes, S.; Perkins, N.; Kidd, L.; Ahern, B. The risk factors for musculoskeletal injuries in thoroughbred racehorses in Queensland, Australia: How these vary for two-year-old and older horses and with type of injury. Animals 2021, 11, 270. [Google Scholar] [CrossRef] [PubMed]
- Ellis, R.J.; Minton, A.P. Cell biology: Join the crowd. Nature 2003, 425, 27–28. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, S.; Trach, S. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J. Mol. Biol. 1991, 222, 599–620. [Google Scholar] [CrossRef] [Green Version]
- Minton, A.P.; Wilf, J. Effect of macromolecular crowding upon the structure and function of an enzyme: Glyceraldehyde-3-phosphate dehydrogenase. Biochemistry 1981, 20, 4821–4826. [Google Scholar] [CrossRef] [PubMed]
- Weiss, M. Crowding, diffusion, and biochemical reactions. In International Review of Cell and Molecular Biology; Hancock, R., Jeon, K.W., Eds.; Academic Press: Cambridge, MA, USA, 2014; Volume 307, pp. 383–417. [Google Scholar]
- Tabaka, M.; Kalwarczyk, T.; Szymanski, J.; Hou, S.; Holyst, R. The effect of macromolecular crowding on mobility of biomolecules, association kinetics, and gene expression in living cells. Front. Phys. 2014, 2, 54. [Google Scholar] [CrossRef] [Green Version]
- Dix, J.A.; Verkman, A.S. Crowding effects on diffusion in solutions and cells. Annu. Rev. Biophys. 2008, 37, 247–263. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Zeugolis, D.I. It is time to crowd your cell culture media—Physicochemical considerations with biological consequences. Biomaterials 2021, 275, 120943. [Google Scholar] [CrossRef]
- Raghunath, M.; Zeugolis, D.I. Transforming eukaryotic cell culture with macromolecular crowding. Trends Biochem. Sci. 2021, 46, 805–811. [Google Scholar] [CrossRef]
- Zeugolis, D.I. Bioinspired in vitro microenvironments to control cell fate: Focus on macromolecular crowding. Am. J. Physiol. Cell Physiol. 2021, 320, C842–C849. [Google Scholar] [CrossRef]
- Tsiapalis, D.; Kearns, S.; Kelly, J.; Zeugolis, D. Growth factor and macromolecular crowding supplementation in human tenocyte culture. Biomater. Biosyst. 2021, 1, 100009. [Google Scholar] [CrossRef]
- Capella-Monsonís, H.; Coentro, J.Q.; Graceffa, V.; Wu, Z.; Zeugolis, D.I. An experimental toolbox for characterization of mammalian collagen type I in biological specimens. Nat. Protoc. 2018, 13, 507–529. [Google Scholar] [CrossRef] [PubMed]
- Roberts, S.; Owen, H.; Tam, W.; Solie, L.; Van Cromphaut, S.; Van den Berghe, G.; Luyten, F. Humanized culture of periosteal progenitors in allogeneic serum enhances osteogenic differentiation and in vivo bone formation. Stem Cells Transl. Med. 2014, 3, 218–228. [Google Scholar] [CrossRef] [PubMed]
- Onuma, K.; Urabe, K.; Naruse, K.; Uchida, K.; Itoman, M. Allogenic serum improves cold preservation of osteochondral allografts. Clin. Orthop. Relat. Res. 2012, 470, 2905–2914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, Y.; Ringe, J.; Liang, Z.; Loch, A.; Chen, L.; Sittinger, M. Osteogenic potential of human periosteum-derived progenitor cells in PLGA scaffold using allogeneic serum. J. Zhejiang Univ. Sci. B 2006, 7, 817–824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shafaei, H.; Esmaeili, A.; Mardani, M.; Razavi, S.; Hashemibeni, B.; Nasr-Esfahani, M.; Shiran, M.; Esfandiari, E. Effects of human placental serum on proliferation and morphology of human adipose tissue-derived stem cells. Bone Marrow Transplant. 2011, 46, 1464–1471. [Google Scholar] [CrossRef]
- Omae, H.; Zhao, C.; Sun, Y.; An, K.; Amadio, P. Multilayer tendon slices seeded with bone marrow stromal cells: A novel composite for tendon engineering. J. Orthop. Res. 2009, 27, 937–942. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.; Chen, X.; Zhu, T.; Hu, J.; Song, H.; Shen, W.; Jiang, L.; Heng, B.; Ji, J.; Ouyang, H. The effect of decellularized matrices on human tendon stem/progenitor cell differentiation and tendon repair. Acta Biomater. 2013, 9, 9317–9329. [Google Scholar] [CrossRef]
- Franke, J.; Abs, V.; Zizzadoro, C.; Abraham, G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet. Res. 2014, 10, 119. [Google Scholar] [CrossRef] [Green Version]
- Fedoroff, S.; Hall, C. Effect of horse serum on neural cell differentiation in tissue culture. In Vitro 1979, 15, 641–648. [Google Scholar] [CrossRef]
- Ziegler, A.; Everett, H.; Hamza, E.; Garbani, M.; Gerber, V.; Marti, E.; Steinbach, F. Equine dendritic cells generated with horse serum have enhanced functionality in comparison to dendritic cells generated with fetal bovine serum. BMC Vet. Res. 2016, 12, 254. [Google Scholar] [CrossRef] [Green Version]
- Tateishi, K.; Ando, W.; Higuchi, C.; Hart, D.; Hashimoto, J.; Nakata, K.; Yoshikawa, H.; Nakamura, N. Comparison of human serum with fetal bovine serum for expansion and differentiation of human synovial MSC: Potential feasibility for clinical applications. Cell Transplant. 2008, 17, 549–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwon, D.; Kim, J.; Cha, B.; Park, K.; Han, I.; Park, K.; Bae, H.; Han, M.; Kim, K.; Lee, S. The effect of fetal bovine serum (FBS) on efficacy of cellular reprogramming for induced pluripotent stem cell (iPSC) generation. Cell Transplant. 2016, 25, 1025–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, Y.; Tatarczuch, L.; Pagel, C.; Davies, H.; Mirams, M.; Mackie, E. Hypertrophy and physiological death of equine chondrocytes in vitro. Equine Vet. J. 2007, 39, 546–552. [Google Scholar] [CrossRef] [PubMed]
- Gstraunthaler, G.; Lindl, T.; van der Valk, J. A plea to reduce or replace fetal bovine serum in cell culture media. Cytotechnology 2013, 65, 791–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.E.; Kim, M.; Pokharel, S.; Kim, J.; Choi, I.; Choe, N.-H.; Nahm, S.-S. Potential use of adult bovine serum obtained during the slaughtering process as a biological reagent. Anim. Cells Syst. 2013, 17, 106–112. [Google Scholar] [CrossRef] [Green Version]
- Von Seefried, A.; MacMorine, H. The use of foetal, calf and adult bovine sera for the growth of serially subcultivated diploid cells. Dev. Biol. Stand. 1976, 37, 83–89. [Google Scholar]
- Zamansky, G.; Arundel, C.; Nagasawa, H.; Little, J. Adaptation of human diploid fibroblasts in vitro to serum from different sources. J. Cell Sci. 1983, 61, 289–297. [Google Scholar] [CrossRef]
- Pezzanite, L.; Chow, L.; Griffenhagen, G.; Dow, S.; Goodrich, L. Impact of three different serum sources on functional properties of equine mesenchymal stromal cells. Front. Vet. Sci. 2021, 8, 634064. [Google Scholar] [CrossRef]
- Satyam, A.; Kumar, P.; Fan, X.; Gorelov, A.; Rochev, Y.; Joshi, L.; Peinado, H.; Lyden, D.; Thomas, B.; Rodriguez, B.; et al. Macromolecular crowding meets tissue engineering by self-assembly: A paradigm shift in regenerative medicine. Adv. Mater. 2014, 26, 3024–3034. [Google Scholar] [CrossRef] [Green Version]
- De Pieri, A.; Rana, S.; Korntner, S.; Zeugolis, D.I. Seaweed polysaccharides as macromolecular crowding agents. Int. J. Biol. Macromol. 2020, 164, 434–446. [Google Scholar] [CrossRef]
- Garnica-Galvez, S.; Korntner, S.; Skoufos, I.; Tzora, A.; Diakakis, N.; Prassinos, N.; Zeugolis, D. Hyaluronic acid as macromolecular crowder in equine adipose-derived stem cell cultures. Cells 2021, 10, 859. [Google Scholar] [CrossRef] [PubMed]
- Lomas, A.; Ryan, C.; Sorushanova, A.; Shologu, N.; Sideri, A.; Tsioli, V.; Fthenakis, G.; Tzora, A.; Skoufos, I.; Quinlan, L.; et al. The past, present and future in scaffold-based tendon treatments. Adv. Drug Deliv. Rev. 2015, 84, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Sorushanova, A.; Delgado, L.; Wu, Z.; Shologu, N.; Kshirsagar, A.; Raghunath, R.; Mullen, A.; Bayon, Y.; Pandit, A.; Raghunath, M.; et al. The collagen suprafamily: From biosynthesis to advanced biomaterial development. Adv. Mater. 2019, 31, e1801651. [Google Scholar] [CrossRef] [Green Version]
- Spang, C.; Chen, J.; Backman, L. The tenocyte phenotype of human primary tendon cells in vitro is reduced by glucocorticoids. BMC Musculoskelet. Disord. 2016, 17, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiu, Y.; Wang, X.; Zhang, Y.; Rout, R.; Carr, A.; Zhu, L.; Xia, Z.; Sabokbar, A. Development of a refined tenocyte differentiation culture technique for tendon tissue engineering. Cells Tissues Organs 2013, 197, 27–36. [Google Scholar] [CrossRef]
- Lareu, R.; Zeugolis, D.; Abu-Rub, M.; Pandit, A.; Raghunath, M. Essential modification of the Sircol Collagen Assay for the accurate quantification of collagen content in complex protein solutions. Acta Biomater. 2010, 6, 3146–3151. [Google Scholar] [CrossRef]
- Giusti, I.; D’Ascenzo, S.; Mancò, A.; Di Stefano, G.; Di Francesco, M.; Rughetti, A.; Dal Mas, A.; Properzi, G.; Calvisi, V.; Dolo, V. Platelet concentration in platelet-rich plasma affects tenocyte behavior in vitro. BioMed Res. Int. 2014, 2014, 630870. [Google Scholar] [CrossRef] [Green Version]
- Turlo, A.; Ashraf Kharaz, Y.; Clegg, P.; Anderson, J.; Peffers, M. Donor age affects proteome composition of tenocyte-derived engineered tendon. BMC Biotechnol. 2018, 18, 2. [Google Scholar] [CrossRef] [Green Version]
- Gaspar, D.; Fuller, K.P.; Zeugolis, D.I. Polydispersity and negative charge are key modulators of extracellular matrix deposition under macromolecular crowding conditions. Acta Biomater. 2019, 88, 197–210. [Google Scholar] [CrossRef]
- Kumar, P.; Satyam, A.; Fan, X.; Rochev, Y.; Rodriguez, B.; Gorelov, A.; Joshi, L.; Raghunath, M.; Pandit, A.; Zeugolis, D. Accelerated development of supramolecular corneal stromal-like assemblies from corneal fibroblasts in the presence of macromolecular crowders. Tissue Eng. Part C Methods 2015, 21, 660–670. [Google Scholar] [CrossRef]
- Cigognini, D.; Gaspar, D.; Kumar, P.; Satyam, A.; Alagesan, S.; Sanz-Nogués, C.; Griffin, M.; O’Brien, T.; Pandit, A.; Zeugolis, D. Macromolecular crowding meets oxygen tension in human mesenchymal stem cell culture—A step closer to physiologically relevant in vitro organogenesis. Sci. Rep. 2016, 6, 30746. [Google Scholar] [CrossRef] [PubMed]
- Tsiapalis, D.; De Pieri, A.; Spanoudes, K.; Sallent, I.; Kearns, S.; Kelly, J.L.; Raghunath, M.; Zeugolis, D.I. The synergistic effect of low oxygen tension and macromolecular crowding in the development of extracellular matrix-rich tendon equivalents. Biofabrication 2020, 12, 025018. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, D.; Ryan, C.; Zeugolis, D. Multifactorial bottom-up bioengineering approaches for the development of living tissue substitutes. FASEB J. 2019, 33, 5741–5754. [Google Scholar] [CrossRef] [PubMed]
- Ryan, C.; Pugliese, E.; Shologu, N.; Gaspar, D.; Rooney, P.; Islam, M.; O’Riordan, A.; Biggs, M.; Griffin, M.; Zeugolis, D. A combined physicochemical approach towards human tenocyte phenotype maintenance. Mater. Today Biol. 2021, 12, 100130. [Google Scholar] [CrossRef]
- Djalali-Cuevas, A.; Garnica-Galvez, S.; Rampin, A.; Gaspar, D.; Skoufos, I.; Tzora, A.; Prassinos, N.; Diakakis, N.; Zeugolis, D.I. Preparation and characterization of tissue surrogates rich in extracellular matrix using the principles of macromolecular crowding. Methods Mol. Biol. 2019, 1952, 245–259. [Google Scholar] [CrossRef]
- Birk, D.E.; Mayne, R. Localization of collagen types I, III and V during tendon development. Changes in collagen types I and III are correlated with changes in fibril diameter. Eur. J. Cell Biol. 1997, 72, 352–361. [Google Scholar]
- Sodersten, F.; Hultenby, K.; Heinegard, D.; Johnston, C.; Ekman, S. Immunolocalization of collagens (I and III) and cartilage oligomeric matrix protein in the normal and injured equine superficial digital flexor tendon. Connect. Tissue Res. 2013, 54, 62–69. [Google Scholar] [CrossRef] [Green Version]
- Hance, A.; Crystal, R. Rigid control of synthesis of collagen types I and III by cells in culture. Nature 1977, 268, 152–154. [Google Scholar] [CrossRef]
- Jessen, H.; Hoyer, N.; Prior, T.; Frederiksen, P.; Karsdal, M.; Leeming, D.; Bendstrup, E.; Sand, J.; Shaker, S. Turnover of type I and III collagen predicts progression of idiopathic pulmonary fibrosis. Respir. Res. 2021, 22, 205. [Google Scholar] [CrossRef]
- Cheng, W.; Yan-hua, R.; Fang-gang, N.; Guo-an, Z. The content and ratio of type I and III collagen in skin differ with age and injury. Afr. J. Biotechnol. 2011, 10, 2524–2529. [Google Scholar]
- Xue, M.; Jackson, C. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care 2015, 4, 119–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasaki, K.; Nakashima, A.; Tamura, R.; Ishiuchi, N.; Honda, K.; Ueno, T.; Doi, S.; Kato, Y.; Masaki, T. Mesenchymal stem cells cultured in serum-free medium ameliorate experimental peritoneal fibrosis. Stem Cell Res. Ther. 2021, 12, 203. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, K.; Nakashima, A.; Doi, S.; Ueno, T.; Okubo, T.; Kawano, K.; Kanawa, M.; Kato, Y.; Higashi, Y.; Masaki, T. Serum-free medium enhances the immunosuppressive and antifibrotic abilities of mesenchymal stem cells utilized in experimental renal fibrosis. Stem Cells Transl. Med. 2018, 7, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Park, C. Allogeneic ADSCs induce CD8 T cell-mediated cytotoxicity and faster cell death after exposure to xenogeneic serum or proinflammatory cytokines. Exp. Mol. Med. 2019, 51, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Naskou, M.; Norton, N.; Copland, I.; Galipeau, J.; Peroni, J. Innate immune responses of equine monocytes cultured in equine platelet lysate. Vet. Immunol. Immunopathol. 2018, 195, 65–71. [Google Scholar] [CrossRef]
- Taylor, S.; Al-Youha, S.; Van Agtmael, T.; Lu, Y.; Wong, J.; McGrouther, D.; Kadler, K. Tendon is covered by a basement membrane epithelium that is required for cell retention and the prevention of adhesion formation. PLoS ONE 2011, 6, e16337. [Google Scholar] [CrossRef] [Green Version]
- Sun, M.; Connizzo, B.K.; Adams, S.M.; Freedman, B.R.; Wenstrup, R.J.; Soslowsky, L.J.; Birk, D.E. Targeted deletion of collagen V in tendons and ligaments results in a classic Ehlers-Danlos syndrome joint phenotype. Am. J. Pathol. 2015, 185, 1436–1447. [Google Scholar] [CrossRef] [Green Version]
- Knuppel, L.; Heinzelmann, K.; Lindner, M.; Hatz, R.; Behr, J.; Eickelberg, O.; Staab-Weijnitz, C.A. FK506-binding protein 10 (FKBP10) regulates lung fibroblast migration via collagen VI synthesis. Respir. Res. 2018, 19, 67. [Google Scholar] [CrossRef]
- Cescon, M.; Chen, P.; Castagnaro, S.; Gregorio, I.; Bonaldo, P. Lack of collagen VI promotes neurodegeneration by impairing autophagy and inducing apoptosis during aging. Aging 2016, 8, 1083–1101. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Pan, W. The biological role of the collagen alpha-3 (VI) chain and its cleaved C5 domain fragment endotrophin in cancer. Onco Targets Ther. 2020, 13, 5779–5793. [Google Scholar] [CrossRef]
- McDonald, J.; Kelley, D.; Broekelmann, T. Role of fibronectin in collagen deposition: Fab’ to the gelatin-binding domain of fibronectin inhibits both fibronectin and collagen organization in fibroblast extracellular matrix. J. Cell Biol. 1982, 92, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Sottile, J.; Shi, F.; Rublyevska, I.; Chiang, H.; Lust, J.; Chandler, J. Fibronectin-dependent collagen I deposition modulates the cell response to fibronectin. Am. J. Physiol. Cell Physiol. 2007, 293, C1934–C1946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siani, A.; Khaw, R.; Manley, O.; Tirella, A.; Cellesi, F.; Donno, R.; Tirelli, N. Fibronectin localization and fibrillization are affected by the presence of serum in culture media. Sci. Rep. 2015, 5, 9278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Knox, P. Kinetics of cell spreading in the presence of different concentrations of serum or fibronectin-depleted serum. J. Cell Sci. 1984, 71, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Hayman, E.; Ruoslahti, E. Distribution of fetal bovine serum fibronectin and endogenous rat cell fibronectin in extracellular matrix. J. Cell Biol. 1979, 83, 255–259. [Google Scholar] [CrossRef] [PubMed]
- Grinnell, F.; Feld, M. Initial adhesion of human fibroblasts in serum-free medium: Possible role of secreted fibronectin. Cell 1979, 17, 117–129. [Google Scholar] [CrossRef]
- Reed, C.C.; Iozzo, R.V. The role of decorin in collagen fibrillogenesis and skin homeostasis. Glycoconj. J. 2002, 19, 249–255. [Google Scholar] [CrossRef]
- Maeda, E.; Ye, S.; Wang, W.; Bader, D.; Knight, M.; Lee, D. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol. 2012, 11, 439–447. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Schwartz, A.; Civitelli, R.; Thomopoulos, S. Connexin 43 is necessary for murine tendon enthesis formation and response to loading. J. Bone Miner. Res. 2020, 35, 1494–1503. [Google Scholar] [CrossRef]
- Maeda, E.; Pian, H.; Ohashi, T. Temporal regulation of gap junctional communication between tenocytes subjected to static tensile strain with physiological and non-physiological amplitudes. Biochem. Biophys. Res. Commun. 2017, 482, 1170–1175. [Google Scholar] [CrossRef]
- Young, N.; Becker, D.; Fleck, R.; Goodship, A.; Patterson-Kane, J. Maturational alterations in gap junction expression and associated collagen synthesis in response to tendon function. Matrix Biol. 2009, 28, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Maeda, E.; Ohashi, T. Mechano-regulation of gap junction communications between tendon cells is dependent on the magnitude of tensile strain. Biochem. Biophys. Res. Commun. 2015, 465, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Egerbacher, M.; Gardner, K.; Caballero, O.; Hlavaty, J.; Schlosser, S.; Arnoczky, S.; Lavagnino, M. Stress-deprivation induces an up-regulation of versican and connexin-43 mRNA and protein synthesis and increased ADAMTS-1 production in tendon cells in situ. Connect. Tissue Res. 2022, 63, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Tekkatte, C.; Gunasingh, G.P.; Cherian, K.M.; Sankaranarayanan, K. “Humanized” stem cell culture techniques: The animal serum controversy. Stem Cells Int. 2011, 2011, 504723. [Google Scholar] [CrossRef] [Green Version]
- Van der Valk, J. Fetal bovine serum—A cell culture dilemma. Science 2022, 375, 143–144. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rampin, A.; Skoufos, I.; Raghunath, M.; Tzora, A.; Diakakis, N.; Prassinos, N.; Zeugolis, D.I. Allogeneic Serum and Macromolecular Crowding Maintain Native Equine Tenocyte Function in Culture. Cells 2022, 11, 1562. https://doi.org/10.3390/cells11091562
Rampin A, Skoufos I, Raghunath M, Tzora A, Diakakis N, Prassinos N, Zeugolis DI. Allogeneic Serum and Macromolecular Crowding Maintain Native Equine Tenocyte Function in Culture. Cells. 2022; 11(9):1562. https://doi.org/10.3390/cells11091562
Chicago/Turabian StyleRampin, Andrea, Ioannis Skoufos, Michael Raghunath, Athina Tzora, Nikolaos Diakakis, Nikitas Prassinos, and Dimitrios I. Zeugolis. 2022. "Allogeneic Serum and Macromolecular Crowding Maintain Native Equine Tenocyte Function in Culture" Cells 11, no. 9: 1562. https://doi.org/10.3390/cells11091562
APA StyleRampin, A., Skoufos, I., Raghunath, M., Tzora, A., Diakakis, N., Prassinos, N., & Zeugolis, D. I. (2022). Allogeneic Serum and Macromolecular Crowding Maintain Native Equine Tenocyte Function in Culture. Cells, 11(9), 1562. https://doi.org/10.3390/cells11091562