Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cloning, Strains, and Vectors
2.2. Cell Culture
2.3. Microscopy
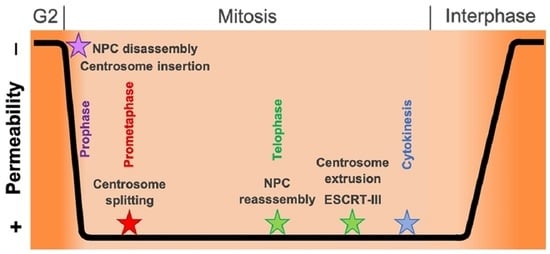
3. Results
3.1. Monitoring Nuclear Envelope Permeabilization versus Spindle Formation
3.2. Monitoring Nuclear Envelope Behavior versus Permeabilization and Centrosome Duplication
3.3. Monitoring NPC Disassembly versus Centrosome Duplication
3.4. Monitoring NPC Disassembly versus Nuclear Envelope Permeabilization
3.5. Monitoring ESCRT Behavior at Nuclear Envelope Fenestrae in Late Mitosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sazer, S.; Lynch, M.; Needleman, D. Deciphering the Evolutionary History of Open and Closed Mitosis. Curr. Biol. 2014, 24, R1099-103. [Google Scholar] [CrossRef]
- Adl, S.M.; Bass, D.; Lane, C.E.; Lukeš, J.; Schoch, C.L.; Smirnov, A.; Agatha, S.; Berney, C.; Brown, M.W.; Burki, F.; et al. Revisions to the Classification, Nomenclature, and Diversity of Eukaryotes. J. Eukaryot. Microbiol. 2019, 66, 4–119. [Google Scholar] [CrossRef] [PubMed]
- Stafstrom, J.P.; Staehelin, L.A. Dynamics of the Nuclear Envelope and of Nuclear Pore Complexes during Mitosis in the Drosophila Embryo. Eur. J. Cell Biol. 1984, 34, 179–189. [Google Scholar] [PubMed]
- De Souza, C.P.; Osmani, A.H.; Hashmi, S.B.; Osmani, S.A. Partial Nuclear Pore Complex Disassembly during Closed Mitosis in Aspergillus Nidulans. Curr. Biol. 2004, 14, 1973–1984. [Google Scholar] [CrossRef] [PubMed]
- Mitic, K.; Grafe, M.; Batsios, P.; Meyer, I. Partial Disassembly of the Nuclear Pore Complex Proteins during Semi-Closed Mitosis in Dictyostelium Discoideum. Cells 2022, 11, 407. [Google Scholar] [CrossRef]
- Gräf, R.; Grafe, M.; Meyer, I.; Mitic, K.; Pitzen, V. The Dictyostelium Centrosome. Cells 2021, 10, 2657. [Google Scholar] [CrossRef] [PubMed]
- McIntosh, J.R.; Roos, U.P.; Neighbors, B.; McDonald, K.L. Architecture of the Microtubule Component of Mitotic Spindles from Dictyostelium Discoideum. J. Cell Sci. 1985, 75, 93–129. [Google Scholar] [CrossRef]
- Schweigel, U.; Batsios, P.; Müller-Taubenberger, A.; Gräf, R.; Grafe, M. Dictyostelium Spastin Is Involved in Nuclear Envelope Dynamics during Semi-Closed Mitosis. Nucleus 2022, 13, 144–154. [Google Scholar] [CrossRef]
- Olmos, Y.; Perdrix-Rosell, A.; Carlton, J.G. Membrane Binding by CHMP7 Coordinates ESCRT-III-Dependent Nuclear Envelope Reformation. Curr. Biol. 2016, 26, 2635–2641. [Google Scholar] [CrossRef]
- Vietri, M.; Schink, K.O.; Campsteijn, C.; Wegner, C.S.; Schultz, S.W.; Christ, L.; Thoresen, S.B.; Brech, A.; Raiborg, C.; Stenmark, H. Spastin and ESCRT-III Coordinate Mitotic Spindle Disassembly and Nuclear Envelope Sealing. Nature 2015, 522, 231–235. [Google Scholar] [CrossRef]
- Gu, M.; LaJoie, D.; Chen, O.S.; von Appen, A.; Ladinsky, M.S.; Redd, M.J.; Nikolova, L.; Bjorkman, P.J.; Sundquist, W.I.; Ullman, K.S.; et al. LEM2 Recruits CHMP7 for ESCRT-Mediated Nuclear Envelope Closure in Fission Yeast and Human Cells. Proc. Natl. Acad. Sci. USA 2017, 114, E2166–E2175. [Google Scholar] [CrossRef] [PubMed]
- von Appen, A.; LaJoie, D.; Johnson, I.E.; Trnka, M.J.; Pick, S.M.; Burlingame, A.L.; Ullman, K.S.; Frost, A. LEM2 Phase Separation Promotes ESCRT-Mediated Nuclear Envelope Reformation. Nature 2020, 582, 115–118. [Google Scholar] [CrossRef]
- Burns, S.; Avena, J.S.; Unruh, J.R.; Yu, Z.; Smith, S.E.; Slaughter, B.D.; Winey, M.; Jaspersen, S.L. Structured Illumination with Particle Averaging Reveals Novel Roles for Yeast Centrosome Components during Duplication. Elife 2015, 4, e08586. [Google Scholar] [CrossRef]
- Ding, D.Q.; Chikashige, Y.; Haraguchi, T.; Hiraoka, Y. Oscillatory Nuclear Movement in Fission Yeast Meiotic Prophase Is Driven by Astral Microtubules, as Revealed by Continuous Observation of Chromosomes and Microtubules in Living Cells. J. Cell Sci. 1998, 6, 701–712. [Google Scholar] [CrossRef] [PubMed]
- Dey, G.; Culley, S.; Curran, S.; Schmidt, U.; Henriques, R.; Kukulski, W.; Baum, B. Closed Mitosis Requires Local Disassembly of the Nuclear Envelope. Nature 2020, 585, 119–123. [Google Scholar] [CrossRef]
- Meseroll, R.A.; Cohen-Fix, O. The Malleable Nature of the Budding Yeast Nuclear Envelope: Flares, Fusion and Fenestrations. J. Cell Physiol. 2016, 231, 2353–2360. [Google Scholar] [CrossRef] [PubMed]
- Jaspersen, S.L.; Ghosh, S. Nuclear Envelope Insertion of Spindle Pole Bodies and Nuclear Pore Complexes. Nucleus 2012, 3, 226–236. [Google Scholar] [CrossRef]
- Jao, L.E.; Akef, A.; Wente, S.R. A Role for Gle1, a Regulator of DEAD-Box RNA Helicases, at Centrosomes and Basal Bodies. Mol. Biol. Cell 2017, 28, 120–127. [Google Scholar] [CrossRef]
- Borlido, J.; D’Angelo, M.A. Nup62: A Novel Regulator of Centrosome Integrity and Function. Cell Cycle 2014, 13, 14. [Google Scholar] [CrossRef]
- Hashizume, C.; Moyori, A.; Kobayashi, A.; Yamakoshi, N.; Endo, A.; Wong, R.W. Nucleoporin Nup62 Maintains Centrosome Homeostasis. Cell Cycle 2013, 12, 3804–3816. [Google Scholar] [CrossRef]
- Bolhy, S.; Bouhlel, I.; Dultz, E.; Nayak, T.; Zuccolo, M.; Gatti, X.; Vallee, R.; Ellenberg, J.; Doye, V. A Nup133-Dependent NPC-Anchored Network Tethers Centrosomes to the Nuclear Envelope in Prophase. J. Cell Biol. 2011, 192, 855–871. [Google Scholar] [CrossRef]
- Itoh, G.; Sugino, S.; Ikeda, M.; Mizuguchi, M.; Kanno, S.; Amin, M.A.; Iemura, K.; Yasui, A.; Hirota, T.; Tanaka, K. Nucleoporin Nup188 Is Required for Chromosome Alignment in Mitosis. Cancer Sci. 2013, 104, 871–879. [Google Scholar] [CrossRef]
- Pitzen, V.; Askarzada, S.; Gräf, R.; Meyer, I. CDK5RAP2 Is an Essential Scaffolding Protein of the Corona of the Dictyostelium Centrosome. Cells 2018, 7, 32. [Google Scholar] [CrossRef]
- Grafe, M.; Hofmann, P.; Batsios, P.; Meyer, I.; Gräf, R. In Vivo Assembly of a Dictyostelium Lamin Mutant Induced by Light, Mechanical Stress, and PH. Cells 2020, 9, 1834. [Google Scholar] [CrossRef] [PubMed]
- Paschke, P.; Knecht, D.A.; Silale, A.; Traynor, D.; Williams, T.D.; Thomason, P.A.; Insall, R.H.; Chubb, J.R.; Kay, R.R.; Veltman, D.M. Rapid and Efficient Genetic Engineering of Both Wild Type and Axenic Strains of Dictyostelium Discoideum. PLoS ONE 2018, 13, e0196809. [Google Scholar] [CrossRef] [PubMed]
- Pitzen, V.; Sander, S.; Baumann, O.; Gräf, R.; Meyer, I. Cep192, a Novel Missing Link between the Centrosomal Core and Corona in Dictyostelium Amoebae. Cells 2021, 10, 2384. [Google Scholar] [CrossRef] [PubMed]
- Bretschneider, T.; Anderson, K.; Ecke, M.; Müller-Taubenberger, A.; Schroth-Diez, B.; Ishikawa-Ankerhold, H.C.; Gerisch, G. The Three-Dimensional Dynamics of Actin Waves, a Model of Cytoskeletal Self-Organization. Biophys. J. 2009, 96, 2888–2900. [Google Scholar] [CrossRef]
- Faix, J.; Kreppel, L.; Shaulsky, G.; Schleicher, M.; Kimmel, A.R. A Rapid and Efficient Method to Generate Multiple Gene Disruptions in Dictyostelium Discoideum Using a Single Selectable Marker and the Cre-LoxP System. Nucleic Acids Res. 2004, 32, e143. [Google Scholar] [CrossRef] [PubMed]
- Shaner, N.C.; Campbell, R.E.; Steinbach, P.A.; Giepmans, B.N.G.; Palmer, A.E.; Tsien, R.Y. Improved Monomeric Red, Orange and Yellow Fluorescent Proteins Derived from Discosoma Sp. Red Fluorescent Protein. Nat. Biotechnol. 2004, 22, 1567–1572. [Google Scholar] [CrossRef]
- Krüger, A.; Batsios, P.; Baumann, O.; Luckert, E.; Schwarz, H.; Stick, R.; Meyer, I.; Gräf, R. Characterization of NE81, the First Lamin-like Nucleoskeleton Protein in a Unicellular Organism. Mol. Biol. Cell. 2012, 23, 360–370. [Google Scholar] [CrossRef]
- Gräf, R.; Daunderer, C.; Schliwa, M. Dictyostelium DdCP224 Is a Microtubule-Associated Protein and a Permanent Centrosomal Resident Involved in Centrosome Duplication. J. Cell Sci. 2000, 113, 1747–1758. [Google Scholar] [CrossRef]
- Faix, J.; Linkner, J.; Nordholz, B.; Platt, J.L.; Liao, X.-H.; Kimmel, A.R. The Application of the Cre-LoxP System for Generating Multiple Knock-out and Knock-in Targeted Loci. Methods Mol. Biol. 2013, 983, 249–267. [Google Scholar] [CrossRef] [PubMed]
- Gräf, R.; Euteneuer, U.; Ho, T.H.; Rehberg, M. Regulated Expression of the Centrosomal Protein DdCP224 Affects Microtubule Dynamics and Reveals Mechanisms for the Control of Supernumerary Centrosome Number. Mol. Biol. Cell 2003, 14, 4067–4074. [Google Scholar] [CrossRef] [PubMed]
- Wehland, J.; Willingham, M.C.; Sandoval, I.V. A Rat Monoclonal Antibody Reacting Specifically with the Tyrosylated Form of Alpha-Tubulin. II. Effects on Cell Movement, Organization of Microtubules, and Intermediate Filaments, and Arrangement of Golgi Elements. J. Cell Biol. 1983, 97, 1476–1490. [Google Scholar] [CrossRef]
- Grafe, M.; Batsios, P.; Meyer, I.; Lisin, D.; Baumann, O.; Goldberg, M.W.; Gräf, R. Supramolecular Structures of the Dictyostelium Lamin NE81. Cells 2019, 8, 162. [Google Scholar] [CrossRef] [PubMed]
- Samereier, M.; Meyer, I.; Koonce, M.P.; Gräf, R. Live Cell-Imaging Techniques for Analyses of Microtubules in Dictyostelium. Methods Cell Biol. 2010, 97, 341–357. [Google Scholar] [CrossRef]
- Schulz, I.; Erle, A.; Gräf, R.; Krüger, A.; Lohmeier, H.; Putzler, S.; Samereier, M.; Weidenthaler, S. Identification and Cell Cycle-Dependent Localization of Nine Novel, Genuine Centrosomal Components in Dictyostelium Discoideum. Cell Motil. Cytoskeleton 2009, 66, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Batsios, P.; Gräf, R.; Koonce, M.P.; Larochelle, D.A.; Meyer, I. Nuclear Envelope Organization in Dictyostelium Discoideum. Int. J. Dev. Biol. 2019, 63, 509–519. [Google Scholar] [CrossRef]
- DuBuc, T.Q.; Dattoli, A.A.; Babonis, L.S.; Salinas-Saavedra, M.; Röttinger, E.; Martindale, M.Q.; Postma, M. In Vivo Imaging of Nematostella Vectensis Embryogenesis and Late Development Using Fluorescent Probes. BMC Cell Biol. 2014, 15, 44. [Google Scholar] [CrossRef] [PubMed]
- Meyer, I.; Peter, T.; Batsios, P.; Kuhnert, O.; Krüger-Genge, A.; Camurça, C.; Gräf, R. CP39, CP75 and CP91 Are Major Structural Components of the Dictyostelium Centrosome’s Core Structure. Eur. J. Cell Biol. 2017, 96, 119–130. [Google Scholar] [CrossRef]
- Weijer, C.J.; Duschl, G.; David, C.N. A Revision of the Dictyostelium Discoideum Cell Cycle. J. Cell Sci. 1984, 70, 111–131. [Google Scholar] [CrossRef] [PubMed]
- Neujahr, R.; Albrecht, R.; Kohler, J.; Matzner, M.; Schwartz, J.M.; Westphal, M.; Gerisch, G. Microtubule-Mediated Centrosome Motility and the Positioning of Cleavage Furrows in Multinucleate Myosin II-Null Cells. J. Cell Sci. 1998, 111, 1227–1240. [Google Scholar] [CrossRef] [PubMed]
- Bindl, J.; Molnar, E.S.; Ecke, M.; Prassler, J.; Müller-Taubenberger, A.; Gerisch, G. Unilateral Cleavage Furrows in Multinucleate Cells. Cells 2020, 9, 1493. [Google Scholar] [CrossRef]
- Dey, G.; Baum, B. Nuclear Envelope Remodelling during Mitosis. Curr. Opin. Cell Biol. 2021, 70, 67–74. [Google Scholar] [CrossRef]
- Ovechkina, Y.; Maddox, P.; Oakley, C.E.; Xiang, X.; Osmani, S.A.; Salmon, E.D.; Oakley, B.R. Spindle Formation in Aspergillus Is Coupled to Tubulin Movement into the Nucleus. Mol. Biol. Cell 2003, 14, 2192–2200. [Google Scholar] [CrossRef]
- Tamm, T.; Grallert, A.; Grossman, E.P.S.; Alvarez-Tabares, I.; Stevens, F.E.; Hagan, I.M. Brr6 Drives the Schizosaccharomyces Pombe Spindle Pole Body Nuclear Envelope Insertion/Extrusion Cycle. J. Cell Biol. 2011, 195, 467–484. [Google Scholar] [CrossRef]
- Cavanaugh, A.M.; Jaspersen, S.L. Big Lessons from Little Yeast: Budding and Fission Yeast Centrosome Structure, Duplication, and Function. Annu. Rev. Genet. 2017, 51, 361–383. [Google Scholar] [CrossRef]
- Rüthnick, D.; Schiebel, E. Duplication and Nuclear Envelope Insertion of the Yeast Microtubule Organizing Centre, the Spindle Pole Body. Cells 2018, 7, 42. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.M.; O’Toole, E.; Jaspersen, S.L. A Mutation in Budding Yeast BRR6 Affecting Nuclear Envelope Insertion of the Spindle Pole Body. MicroPubl. Biol. 2021, 2021. [Google Scholar] [CrossRef]
- Bestul, A.J.; Yu, Z.; Unruh, J.R.; Jaspersen, S.L. Molecular Model of Fission Yeast Centrosome Assembly Determined by Superresolution Imaging. J. Cell Biol. 2017, 216, 2409–2424. [Google Scholar] [CrossRef] [PubMed]
- Schulz, I.; Baumann, O.; Samereier, M.; Zoglmeier, C.; Gräf, R. Dictyostelium Sun1 Is a Dynamic Membrane Protein of Both Nuclear Membranes and Required for Centrosomal Association with Clustered Centromeres. Eur. J. Cell Biol. 2009, 88, 621–638. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitic, K.; Meyer, I.; Gräf, R.; Grafe, M. Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae. Cells 2023, 12, 1380. https://doi.org/10.3390/cells12101380
Mitic K, Meyer I, Gräf R, Grafe M. Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae. Cells. 2023; 12(10):1380. https://doi.org/10.3390/cells12101380
Chicago/Turabian StyleMitic, Kristina, Irene Meyer, Ralph Gräf, and Marianne Grafe. 2023. "Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae" Cells 12, no. 10: 1380. https://doi.org/10.3390/cells12101380
APA StyleMitic, K., Meyer, I., Gräf, R., & Grafe, M. (2023). Temporal Changes in Nuclear Envelope Permeability during Semi-Closed Mitosis in Dictyostelium Amoebae. Cells, 12(10), 1380. https://doi.org/10.3390/cells12101380