Interleukin-21 Influences Glioblastoma Course: Biological Mechanisms and Therapeutic Potential
Abstract
:1. Introduction
2. Primary Brain Tumors Classification and Features: Focus on GB
3. Role of Immune System in GB: Players and Involved Pathways
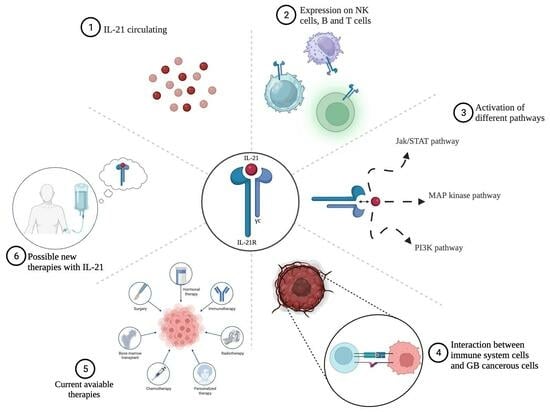
4. Role of IL-21 in GB: Significance and Effectiveness of Immunotherapy
5. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mattiuzzi, C.; Lippi, G. Current cancer epidemiology. J. Epidemiol. Glob. Health 2019, 9, 217. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, A.; Calabrese, G.; Campolo, M.; Filippone, A.; Giuffrida, D.; Esposito, F.; Colarossi, C.; Cuzzocrea, S.; Esposito, E.; Paterniti, I. Role of miRNA-19a in Cancer Diagnosis and Poor Prognosis. Int. J. Mol. Sci. 2021, 22, 4697. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastomaGBM epidemiology and biomarkers. Cancer Epidemiol. Biomark. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Gittleman, H.; Fulop, J.; Liu, M.; Blanda, R.; Kromer, C.; Wolinsky, Y.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS statistical report: Primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro-Oncology 2015, 17, iv1–iv62. [Google Scholar] [CrossRef]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Ardizzone, A.; Basilotta, R.; Filippone, A.; Crupi, L.; Lanza, M.; Lombardo, S.P.; Colarossi, C.; Sciacca, D.; Cuzzocrea, S.; Esposito, E.; et al. Recent Emerging Immunological Treatments for Primary Brain Tumors: Focus on Chemokine-Targeting Immunotherapies. Cells 2023, 12, 841. [Google Scholar] [CrossRef]
- Sampson, J.H.; Gunn, M.D.; Fecci, P.E.; Ashley, D.M. Brain immunology and immunotherapy in brain tumours. Nat. Rev. Cancer 2020, 20, 12–25. [Google Scholar] [CrossRef]
- Lim, M.; Xia, Y.; Bettegowda, C.; Weller, M. Current state of immunotherapy for glioblastoma. Nat. Rev. Clin. Oncol. 2018, 15, 422–442. [Google Scholar] [CrossRef]
- Doulabi, H.; Masoumi, E.; Rastin, M.; Azarnaminy, A.F.; Esmaeili, S.-A.; Mahmoudi, M. The role of Th22 cells, from tissue repair to cancer progression. Cytokine 2022, 149, 155749. [Google Scholar] [CrossRef]
- Pulliam, S.R.; Uzhachenko, R.V.; Adunyah, S.E.; Shanker, A. Common gamma chain cytokines in combinatorial immune strategies against cancer. Immunol. Lett. 2016, 169, 61–72. [Google Scholar] [CrossRef]
- Santegoets, S.J.; Turksma, A.W.; Powell, D.J., Jr.; Hooijberg, E.; de Gruijl, T.D. IL-21 in cancer immunotherapy: At the right place at the right time. Oncoimmunology 2013, 2, e24522. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Yi, X.; Hajavi, J. New and old adjuvants in allergen-specific immunotherapy: With a focus on nanoparticles. J. Cell. Physiol. 2021, 236, 863–876. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Spolski, R.; Casas, E.; Zhu, W.; Levy, D.E.; Leonard, W.J. The molecular basis of IL-21–mediated proliferation. Blood 2007, 109, 4135–4142. [Google Scholar] [CrossRef] [PubMed]
- Pène, J.; Gauchat, J.-F.; Lécart, S.; Drouet, E.; Guglielmi, P.; Boulay, V.; Delwail, A.; Foster, D.; Lecron, J.-C.; Yssel, H. Cutting edge: IL-21 is a switch factor for the production of IgG1 and IgG3 by human B cells. J. Immunol. 2004, 172, 5154–5157. [Google Scholar] [CrossRef] [PubMed]
- Eivary, S.H.A.; Kheder, R.K.; Najmaldin, S.K.; Kheradmand, N.; Esmaeili, S.A.; Hajavi, J. Implications of IL-21 in solid tumor therapy. Med. Oncol. 2023, 40, 191. [Google Scholar] [CrossRef] [PubMed]
- Davis, I.D.; Brady, B.; Kefford, R.F.; Millward, M.; Cebon, J.; Skrumsager, B.K.; Mouritzen, U.; Hansen, L.T.; Skak, K.; Lundsgaard, D. Clinical and biological efficacy of recombinant human interleukin-21 in patients with stage IV malignant melanoma without prior treatment: A phase IIa trial. Clin. Cancer Res. 2009, 15, 2123–2129. [Google Scholar] [CrossRef]
- Kanderi, T.; Gupta, V. Glioblastoma multiforme. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- McFaline-Figueroa, J.R.; Lee, E.Q. Brain tumors. Am. J. Med. 2018, 131, 874–882. [Google Scholar] [CrossRef]
- Louis, D.N.; Perry, A.; Wesseling, P.; Brat, D.J.; Cree, I.A.; Figarella-Branger, D.; Hawkins, C.; Ng, H.K.; Pfister, S.M.; Reifenberger, G.; et al. The 2021 WHO Classification of Tumors of the Central Nervous System: A summary. Neuro Oncol. 2021, 23, 1231–1251. [Google Scholar] [CrossRef]
- Alentorn, A.; Hoang-Xuan, K.; Mikkelsen, T. Presenting signs and symptoms in brain tumors. Handb. Clin. Neurol. 2016, 134, 19–26. [Google Scholar] [CrossRef]
- Lapointe, S.; Perry, A.; Butowski, N.A. Primary brain tumours in adults. Lancet 2018, 392, 432–446. [Google Scholar] [CrossRef]
- Armstrong, T.S.; Vera-Bolanos, E.; Acquaye, A.A.; Gilbert, M.R.; Ladha, H.; Mendoza, T. The symptom burden of primary brain tumors: Evidence for a core set of tumor- and treatment-related symptoms. Neuro Oncol. 2016, 18, 252–260. [Google Scholar] [CrossRef]
- Butowski, N.A. Epidemiology and diagnosis of brain tumors. Continuum 2015, 21, 301–313. [Google Scholar] [CrossRef]
- Lubic, L.G.; Marotta, J.T. Brain tumor and lumbar puncture. AMA Arch. Neurol. Psychiatry 1954, 72, 568–572. [Google Scholar] [CrossRef]
- Choi, D.-J.; Armstrong, G.; Lozzi, B.; Vijayaraghavan, P.; Plon, S.E.; Wong, T.C.; Boerwinkle, E.; Muzny, D.M.; Chen, H.-C.; Gibbs, R.A. The genomic landscape of familial glioma. Sci. Adv. 2023, 9, eade2675. [Google Scholar] [CrossRef] [PubMed]
- Pace, A.; Tanzilli, A.; Benincasa, D. Prognostication in brain tumors. Handb. Clin. Neurol. 2022, 190, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Rivera, M.; Norman, S.; Sehgal, R.; Juthani, R. Updates on Surgical Management and Advances for Brain Tumors. Curr. Oncol. Rep. 2021, 23, 35. [Google Scholar] [CrossRef] [PubMed]
- Scaringi, C.; Agolli, L.; Minniti, G. Technical Advances in Radiation Therapy for Brain Tumors. Anticancer Res. 2018, 38, 6041–6045. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.A.; Cloughesy, T.F. Brain tumor treatment: Chemotherapy and other new developments. Semin. Oncol. Nurs. 2004, 20, 260–272. [Google Scholar] [CrossRef]
- Kitamura, Y.; Toda, M. Medical Treatments for Malignant Brain Tumor. Brain Nerve 2023, 75, 561–566. [Google Scholar] [CrossRef]
- National Institute of Diabetes and Digestive and Kidney Diseases (US). LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012. [Google Scholar]
- Alfonso-Triguero, P.; Lorenzo, J.; Candiota, A.P.; Arus, C.; Ruiz-Molina, D.; Novio, F. Platinum-Based Nanoformulations for Glioblastoma Treatment: The Resurgence of Platinum Drugs? Nanomaterials 2023, 13, 1619. [Google Scholar] [CrossRef]
- Ha, H.; Lim, J.H. Managing Side Effects of Cytotoxic Chemotherapy in Patients with High Grade Gliomas. Brain Tumor Res. Treat. 2022, 10, 158–163. [Google Scholar] [CrossRef]
- Perkins, A.; Liu, G. Primary Brain Tumors in Adults: Diagnosis and Treatment. Am. Fam. Physician 2016, 93, 211–217. [Google Scholar]
- Ohgaki, H.; Kleihues, P. Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J. Neuropathol. Exp. Neurol. 2005, 64, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Holland, E.C. Glioblastoma multiforme: The terminator. Proc. Natl. Acad. Sci. USA 2000, 97, 6242–6244. [Google Scholar] [CrossRef] [PubMed]
- Ou, A.; Yung, W.K.A.; Majd, N. Molecular Mechanisms of Treatment Resistance in Glioblastoma. Int. J. Mol. Sci. 2020, 22, 351. [Google Scholar] [CrossRef] [PubMed]
- Campos, B.; Olsen, L.R.; Urup, T.; Poulsen, H.S. A comprehensive profile of recurrent glioblastoma. Oncogene 2016, 35, 5819–5825. [Google Scholar] [CrossRef]
- Valdes-Rives, S.A.; Casique-Aguirre, D.; German-Castelan, L.; Velasco-Velazquez, M.A.; Gonzalez-Arenas, A. Apoptotic Signaling Pathways in Glioblastoma and Therapeutic Implications. Biomed. Res. Int. 2017, 2017, 7403747. [Google Scholar] [CrossRef]
- Daniele, S.; Costa, B.; Zappelli, E.; Da Pozzo, E.; Sestito, S.; Nesi, G.; Campiglia, P.; Marinelli, L.; Novellino, E.; Rapposelli, S.; et al. Combined inhibition of AKT/mTOR and MDM2 enhances Glioblastoma Multiforme cell apoptosis and differentiation of cancer stem cells. Sci. Rep. 2015, 5, 9956. [Google Scholar] [CrossRef]
- Datta, S.R.; Dudek, H.; Tao, X.; Masters, S.; Fu, H.; Gotoh, Y.; Greenberg, M.E. Akt phosphorylation of BAD couples survival signals to the cell-intrinsic death machinery. Cell 1997, 91, 231–241. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Hou, C.; Chen, H.; Zong, X.; Zong, P. Genetics and epigenetics of glioblastoma: Applications and overall incidence of IDH1 mutation. Front. Oncol. 2016, 6, 16. [Google Scholar] [CrossRef] [PubMed]
- Rathinaswamy, M.K.; Dalwadi, U.; Fleming, K.D.; Adams, C.; Stariha, J.T.B.; Pardon, E.; Baek, M.; Vadas, O.; DiMaio, F.; Steyaert, J.; et al. Structure of the phosphoinositide 3-kinase (PI3K) p110gamma-p101 complex reveals molecular mechanism of GPCR activation. Sci. Adv. 2021, 7, eabj4282. [Google Scholar] [CrossRef]
- Huang, C.H.; Mandelker, D.; Schmidt-Kittler, O.; Samuels, Y.; Velculescu, V.E.; Kinzler, K.W.; Vogelstein, B.; Gabelli, S.B.; Amzel, L.M. The structure of a human p110alpha/p85alpha complex elucidates the effects of oncogenic PI3Kalpha mutations. Science 2007, 318, 1744–1748. [Google Scholar] [CrossRef]
- Pietrak, B.; Zhao, H.; Qi, H.; Quinn, C.; Gao, E.; Boyer, J.G.; Concha, N.; Brown, K.; Duraiswami, C.; Wooster, R. A tale of two subunits: How the neomorphic R132H IDH1 mutation enhances production of αHG. Biochemistry 2011, 50, 4804–4812. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Li, W.; Xu, H.; Liu, J.; Ren, L.; Yang, Y.; Li, S.; Wang, J.; Ji, T.; Du, G. Sinomenine ester derivative inhibits glioblastoma by inducing mitochondria-dependent apoptosis and autophagy by PI3K/AKT/mTOR and AMPK/mTOR pathway. Acta Pharm. Sin. B 2021, 11, 3465–3480. [Google Scholar] [CrossRef]
- Halatsch, M.E.; Schmidt, U.; Unterberg, A.; Vougioukas, V.I. Uniform MDM2 overexpression in a panel of glioblastoma multiforme cell lines with divergent EGFR and p53 expression status. Anticancer Res. 2006, 26, 4191–4194. [Google Scholar]
- Duzgun, Z.; Eroglu, Z.; Biray Avci, C. Role of mTOR in glioblastoma. Gene 2016, 575, 187–190. [Google Scholar] [CrossRef]
- Gawdi, R.; Emmady, P.D. Physiology, Blood Brain Barrier; StatPearls Publishing: Treasure Island, FL, USA, 2020. [Google Scholar]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure, regulation, and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar] [CrossRef]
- Louveau, A.; Harris, T.H.; Kipnis, J. Revisiting the Mechanisms of CNS Immune Privilege. Trends Immunol. 2015, 36, 569–577. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef]
- Harry, G.J. Microglia during development and aging. Pharmacol. Ther. 2013, 139, 313–326. [Google Scholar] [CrossRef]
- Lin, C.; Wang, N.; Xu, C. Glioma-associated microglia/macrophages (GAMs) in glioblastoma: Immune function in the tumor microenvironment and implications for immunotherapy. Front. Immunol. 2023, 14, 1123853. [Google Scholar] [PubMed]
- Prionisti, I.; Buhler, L.H.; Walker, P.R.; Jolivet, R.B. Harnessing Microglia and Macrophages for the Treatment of Glioblastoma. Front. Pharmacol. 2019, 10, 506. [Google Scholar] [CrossRef] [PubMed]
- Guadagno, E.; Presta, I.; Maisano, D.; Donato, A.; Pirrone, C.K.; Cardillo, G.; Corrado, S.D.; Mignogna, C.; Mancuso, T.; Donato, G.; et al. Role of Macrophages in Brain Tumor Growth and Progression. Int. J. Mol. Sci. 2018, 19, 1005. [Google Scholar] [CrossRef] [PubMed]
- Justiz Vaillant, A.; Qurie, A. Interleukin. [Updated 22 August 2022]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Krause, K.; Metz, M.; Makris, M.; Zuberbier, T.; Maurer, M. The role of interleukin-1 in allergy-related disorders. Curr. Opin. Allergy Clin. Immunol. 2012, 12, 477–484. [Google Scholar] [CrossRef]
- Commins, S.P.; Borish, L.; Steinke, J.W. Immunologic messenger molecules: Cytokines, interferons, and chemokines. J. Allergy Clin. Immunol. 2010, 125, S53–S72. [Google Scholar] [PubMed]
- Attur, M.G.; Dave, M.; Cipolletta, C.; Kang, P.; Goldring, M.B.; Patel, I.R.; Abramson, S.B.; Amin, A.R. Reversal of autocrine and paracrine effects of interleukin 1 (IL-1) in human arthritis by type II IL-1 decoy receptor. Potential for pharmacological intervention. J. Biol. Chem. 2000, 275, 40307–40315. [Google Scholar] [CrossRef] [PubMed]
- Yeung, Y.; McDonald, K.; Grewal, T.; Munoz, L. Interleukins in glioblastoma pathophysiology: Implications for therapy. Br. J. Pharmacol. 2013, 168, 591–606. [Google Scholar] [PubMed]
- Lu, T.; Tian, L.; Han, Y.; Vogelbaum, M.; Stark, G.R. Dose-dependent cross-talk between the transforming growth factor-β and interleukin-1 signaling pathways. Proc. Natl. Acad. Sci. USA 2007, 104, 4365–4370. [Google Scholar] [CrossRef]
- Sasaki, A.; Tamura, M.; Hasegawa, M.; Ishiuchi, S.; Hirato, J.; Nakazato, Y. Expression of interleukin-1beta mRNA and protein in human gliomas assessed by RT-PCR and immunohistochemistry. J. Neuropathol. Exp. Neurol. 1998, 57, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Yoshino, Y.; Aoyagi, M.; Tamaki, M.; Duan, L.; Morimoto, T.; Ohno, K. Activation of p38 MAPK and/or JNK contributes to increased levels of VEGF secretion in human malignant glioma cells. Int. J. Oncol. 2006, 29, 981–987. [Google Scholar] [CrossRef] [PubMed]
- Spooren, A.; Mestdagh, P.; Rondou, P.; Kolmus, K.; Haegeman, G.; Gerlo, S. IL-1β potently stabilizes IL-6 mRNA in human astrocytes. Biochem. Pharmacol. 2011, 81, 1004–1015. [Google Scholar] [CrossRef] [PubMed]
- Menna, G.; Mattogno, P.P.; Donzelli, C.M.; Lisi, L.; Olivi, A.; Della Pepa, G.M. Glioma-Associated Microglia Characterization in the Glioblastoma Microenvironment through a ‘Seed-and Soil’Approach: A Systematic Review. Brain Sci. 2022, 12, 718. [Google Scholar] [CrossRef]
- Dapash, M.; Hou, D.; Castro, B.; Lee-Chang, C.; Lesniak, M.S. The interplay between glioblastoma and its microenvironment. Cells 2021, 10, 2257. [Google Scholar] [CrossRef]
- Chaudhary, R.; Morris, R.J.; Steinson, E. The multifactorial roles of microglia and macrophages in the maintenance and progression of glioblastoma. J. Neuroimmunol. 2021, 357, 577633. [Google Scholar] [CrossRef]
- Han, J.; Alvarez-Breckenridge, C.A.; Wang, Q.E.; Yu, J. TGF-beta signaling and its targeting for glioma treatment. Am. J. Cancer Res. 2015, 5, 945–955. [Google Scholar]
- Maxwell, M.; Galanopoulos, T.; Neville-Golden, J.; Antoniades, H.N. Effect of the expression of transforming growth factor-beta 2 in primary human glioblastomas on immunosuppression and loss of immune surveillance. J. Neurosurg. 1992, 76, 799–804. [Google Scholar] [CrossRef]
- Rolle, C.E.; Sengupta, S.; Lesniak, M.S. Mechanisms of immune evasion by gliomas. Adv. Exp. Med. Biol. 2012, 746, 53–76. [Google Scholar] [CrossRef]
- Wiendl, H.; Mitsdoerffer, M.; Hofmeister, V.; Wischhusen, J.R.; Bornemann, A.; Meyermann, R.; Weiss, E.H.; Melms, A.; Weller, M. A functional role of HLA-G expression in human gliomas: An alternative strategy of immune escape. J. Immunol. 2002, 168, 4772–4780. [Google Scholar] [CrossRef]
- Kren, L.; Slaby, O.; Muckova, K.; Lzicarova, E.; Sova, M.; Vybihal, V.; Svoboda, T.; Fadrus, P.; Lakomy, R.; Vanhara, P. Expression of immune-modulatory molecules HLA-G and HLA-E by tumor cells in glioblastomas: An unexpected prognostic significance? Neuropathology 2011, 31, 129–134. [Google Scholar] [CrossRef]
- Jiang, J.; Qiu, J.; Li, Q.; Shi, Z. Prostaglandin E2 Signaling: Alternative Target for Glioblastoma? Trends Cancer 2017, 3, 75–78. [Google Scholar] [CrossRef]
- Payner, T.; Leaver, H.A.; Knapp, B.; Whittle, I.R.; Trifan, O.C.; Miller, S.; Rizzo, M.T. Microsomal prostaglandin E synthase-1 regulates human glioma cell growth via prostaglandin E(2)-dependent activation of type II protein kinase A. Mol. Cancer Ther. 2006, 5, 1817–1826. [Google Scholar] [CrossRef] [PubMed]
- Du, R.; Lu, K.V.; Petritsch, C.; Liu, P.; Ganss, R.; Passegue, E.; Song, H.; Vandenberg, S.; Johnson, R.S.; Werb, Z.; et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 2008, 13, 206–220. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, Z.; Shaw, G.; Sharma, V.P.; Yang, C.; McGowan, E.; Dickson, D.W. Actin-binding proteins coronin-1a and IBA-1 are effective microglial markers for immunohistochemistry. J. Histochem. Cytochem. 2007, 55, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Suto, A.; Nakajima, H.; Hirose, K.; Suzuki, K.; Kagami, S.; Seto, Y.; Hoshimoto, A.; Saito, Y.; Foster, D.C.; Iwamoto, I. Interleukin 21 prevents antigen-induced IgE production by inhibiting germ line C(epsilon) transcription of IL-4-stimulated B cells. Blood 2002, 100, 4565–4573. [Google Scholar] [CrossRef]
- Dinarello, C.A. Role of pro- and anti-inflammatory cytokines during inflammation: Experimental and clinical findings. J. Biol. Regul. Homeost. Agents 1997, 11, 91–103. [Google Scholar]
- Hart, P.H.; Vitti, G.F.; Burgess, D.R.; Whitty, G.A.; Piccoli, D.S.; Hamilton, J.A. Potential antiinflammatory effects of interleukin 4: Suppression of human monocyte tumor necrosis factor alpha, interleukin 1, and prostaglandin E2. Proc. Natl. Acad. Sci. USA 1989, 86, 3803–3807. [Google Scholar] [CrossRef]
- Guven-Maiorov, E.; Acuner-Ozbabacan, S.E.; Keskin, O.; Gursoy, A.; Nussinov, R. Structural pathways of cytokines may illuminate their roles in regulation of cancer development and immunotherapy. Cancers 2014, 6, 663–683. [Google Scholar] [CrossRef]
- Lobo-Silva, D.; Carriche, G.M.; Castro, A.G.; Roque, S.; Saraiva, M. Balancing the immune response in the brain: IL-10 and its regulation. J. Neuroinflamm. 2016, 13, 297. [Google Scholar]
- Tilg, H.; Trehu, E.; Atkins, M.B.; Dinarello, C.A.; Mier, J.W. Interleukin-6 (IL-6) as an anti-inflammatory cytokine: Induction of circulating IL-1 receptor antagonist and soluble tumor necrosis factor receptor p55. Blood 1994, 83, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Anton, K.; Banerjee, D.; Glod, J. Macrophage-associated mesenchymal stem cells assume an activated, migratory, pro-inflammatory phenotype with increased IL-6 and CXCL10 secretion. PLoS ONE 2012, 7, e35036. [Google Scholar]
- Vaillant, A.A.J.; Ahmad, F. Leukocyte adhesion deficiency. In Stat Pearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Asao, H.; Okuyama, C.; Kumaki, S.; Ishii, N.; Tsuchiya, S.; Foster, D.; Sugamura, K. Cutting edge: The common γ-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 2001, 167, 1–5. [Google Scholar] [PubMed]
- Jin, H.; Carrio, R.; Yu, A.; Malek, T.R. Distinct activation signals determine whether IL-21 induces B cell costimulation, growth arrest, or Bim-dependent apoptosis. J. Immunol. 2004, 173, 657–665. [Google Scholar] [CrossRef]
- Rochman, Y.; Spolski, R.; Leonard, W.J. New insights into the regulation of T cells by γc family cytokines. Nat. Rev. Immunol. 2009, 9, 480–490. [Google Scholar]
- Brandt, K.; Singh, P.B.; Bulfone-Paus, S.; Ruckert, R. Interleukin-21: A new modulator of immunity, infection, and cancer. Cytokine Growth Factor Rev. 2007, 18, 223–232. [Google Scholar] [CrossRef]
- Ozaki, K.; Kikly, K.; Michalovich, D.; Young, P.R.; Leonard, W.J. Cloning of a type I cytokine receptor most related to the IL-2 receptor beta chain. Proc. Natl. Acad. Sci. USA 2000, 97, 11439–11444. [Google Scholar] [CrossRef]
- Parrish-Novak, J.; Dillon, S.R.; Nelson, A.; Hammond, A.; Sprecher, C.; Gross, J.A.; Johnston, J.; Madden, K.; Xu, W.; West, J.; et al. Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000, 408, 57–63. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu. Rev. Immunol. 2008, 26, 57–79. [Google Scholar] [CrossRef]
- Gowda, A.; Roda, J.; Hussain, S.-R.A.; Ramanunni, A.; Joshi, T.; Schmidt, S.; Zhang, X.; Lehman, A.; Jarjoura, D.; Carson, W.E. IL-21 mediates apoptosis through up-regulation of the BH3 family member BIM and enhances both direct and antibody-dependent cellular cytotoxicity in primary chronic lymphocytic leukemia cells in vitro. Blood J. Am. Soc. Hematol. 2008, 111, 4723–4730. [Google Scholar]
- Ettinger, R.; Sims, G.P.; Fairhurst, A.-M.; Robbins, R.; da Silva, Y.S.; Spolski, R.; Leonard, W.J.; Lipsky, P.E. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005, 175, 7867–7879. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, K.; Spolski, R.; Feng, C.G.; Qi, C.F.; Cheng, J.; Sher, A.; Morse, H.C., 3rd; Liu, C.; Schwartzberg, P.L.; Leonard, W.J. A critical role for IL-21 in regulating immunoglobulin production. Science 2002, 298, 1630–1634. [Google Scholar] [CrossRef] [PubMed]
- Spolski, R.; Kashyap, M.; Robinson, C.; Yu, Z.; Leonard, W.J. IL-21 signaling is critical for the development of type I diabetes in the NOD mouse. Proc. Natl. Acad. Sci. USA 2008, 105, 14028–14033. [Google Scholar] [CrossRef]
- Bubier, J.A.; Sproule, T.J.; Foreman, O.; Spolski, R.; Shaffer, D.J.; Morse, H.C., 3rd; Leonard, W.J.; Roopenian, D.C. A critical role for IL-21 receptor signaling in the pathogenesis of systemic lupus erythematosus in BXSB-Yaa mice. Proc. Natl. Acad. Sci. USA 2009, 106, 1518–1523. [Google Scholar] [CrossRef]
- Nakou, M.; Papadimitraki, E.D.; Fanouriakis, A.; Bertsias, G.K.; Choulaki, C.; Goulidaki, N.; Sidiropoulos, P.; Boumpas, D.T. Interleukin-21 is increased in active systemic lupus erythematosus patients and contributes to the generation of plasma B cells. Clin. Exp. Rheumatol. 2013, 31, 172–179. [Google Scholar] [PubMed]
- Rasmussen, T.K.; Andersen, T.; Hvid, M.; Hetland, M.L.; Hørslev-Petersen, K.; Stengaard-Pedersen, K.; Holm, C.K.; Deleuran, B. Increased interleukin 21 (IL-21) and IL-23 are associated with increased disease activity and with radiographic status in patients with early rheumatoid arthritis. J. Rheumatol. 2010, 37, 2014–2020. [Google Scholar] [CrossRef]
- Kasaian, M.T.; Whitters, M.J.; Carter, L.L.; Lowe, L.D.; Jussif, J.M.; Deng, B.; Johnson, K.A.; Witek, J.S.; Senices, M.; Konz, R.F.; et al. IL-21 limits NK cell responses and promotes antigen-specific T cell activation: A mediator of the transition from innate to adaptive immunity. Immunity 2002, 16, 559–569. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Finkelstein, S.E.; Oh, S.; Kovanen, P.E.; Hinrichs, C.S.; Pise-Masison, C.A.; Radonovich, M.F.; Brady, J.N.; Restifo, N.P. Synergy of IL-21 and IL-15 in regulating CD8+ T cell expansion and function. J. Exp. Med. 2005, 201, 139–148. [Google Scholar] [CrossRef]
- Elsaesser, H.; Sauer, K.; Brooks, D.G. IL-21 is required to control chronic viral infection. Science 2009, 324, 1569–1572. [Google Scholar] [CrossRef]
- Fröhlich, A.; Kisielow, J.; Schmitz, I.; Freigang, S.; Shamshiev, A.T.; Weber, J.; Marsland, B.J.; Oxenius, A.; Kopf, M. IL-21R on T cells is critical for sustained functionality and control of chronic viral infection. Science 2009, 324, 1576–1580. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Z.; Zhang, C.; Zhang, N.; Wang, P.; Chu, Y.; Chard Dunmall, L.S.; Lemoine, N.R.; Wang, Y. An effective therapeutic regime for treatment of glioma using oncolytic vaccinia virus expressing IL-21 in combination with immune checkpoint inhibition. Mol. Ther. Oncolytics 2022, 26, 105–119. [Google Scholar] [CrossRef]
- Tran, T.A.; Kim, Y.H.; Duong, T.H.; Thangaraj, J.; Chu, T.H.; Jung, S.; Kim, I.Y.; Moon, K.S.; Kim, Y.J.; Lee, T.K.; et al. Natural killer cell therapy potentially enhances the antitumor effects of bevacizumab plus irinotecan in a glioblastoma mouse model. Front. Immunol. 2022, 13, 1009484. [Google Scholar] [CrossRef]
- Bender, D.E.; Schaettler, M.O.; Sheehan, K.C.; Johanns, T.M.; Dunn, G.P. Cytokine Profiling in Plasma from Patients with Brain Tumors versus Healthy Individuals using 2 Different Multiplex Immunoassay Platforms. Biomark. Insights 2021, 16, 11772719211006666. [Google Scholar] [CrossRef]
- Liu, Z.; Rao, M.; Poiret, T.; Nava, S.; Meng, Q.; von Landenberg, A.; Bartek, J., Jr.; Xie, S.; Sinclair, G.; Peredo, I. Mesothelin as a novel biomarker and immunotherapeutic target in human glioblastoma. Oncotarget 2017, 8, 80208. [Google Scholar] [CrossRef]
- Liu, Z.; Poiret, T.; Persson, O.; Meng, Q.; Rane, L.; Bartek, J.; Karbach, J.; Altmannsberger, H.-M.; Illies, C.; Luo, X. NY-ESO-1-and survivin-specific T-cell responses in the peripheral blood from patients with glioma. Cancer Immunol. Immunother. 2018, 67, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Moyes, K.W.; Lieberman, N.A.; Kreuser, S.A.; Chinn, H.; Winter, C.; Deutsch, G.; Hoglund, V.; Watson, R.; Crane, C.A. Genetically Engineered Macrophages: A Potential Platform for Cancer Immunotherapy. Hum. Gene Ther. 2017, 28, 200–215. [Google Scholar] [CrossRef] [PubMed]
- Wolfl, M.; Merker, K.; Morbach, H.; Van Gool, S.W.; Eyrich, M.; Greenberg, P.D.; Schlegel, P.G. Primed tumor-reactive multifunctional CD62L+ human CD8+ T cells for immunotherapy. Cancer Immunol. Immunother. 2011, 60, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Joalland, N.; Chauvin, C.; Oliver, L.; Vallette, F.M.; Pecqueur, C.; Jarry, U.; Scotet, E. IL-21 increases the reactivity of allogeneic human Vγ9Vδ2 T cells against primary glioblastoma tumors. J. Immunother. 2018, 41, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Daga, A.; Orengo, A.M.; Gangemi, R.M.; Marubbi, D.; Perera, M.; Comes, A.; Ferrini, S.; Corte, G. Glioma immunotherapy by IL-21 gene-modified cells or by recombinant IL-21 involves antibody responses. Int. J. Cancer 2007, 121, 1756–1763. [Google Scholar] [CrossRef]
- Zhenjiang, L.; Rao, M.; Luo, X.; Valentini, D.; von Landenberg, A.; Meng, Q.; Sinclair, G.; Hoffmann, N.; Karbach, J.; Altmannsberger, H.M.; et al. Cytokine Networks and Survivin Peptide-Specific Cellular Immune Responses Predict Improved Survival in Patients With Glioblastoma Multiforme. EBioMedicine 2018, 33, 49–56. [Google Scholar] [CrossRef]

| Gliomas, Glioneuronal Tumors and Neuronal Tumors | Choroid Plexus Tumors | Embryonal Tumors | Pineal Tumors | Mesenchymal, Non-Meningothelial Tumors | Tumors of the Sellar Region |
|---|---|---|---|---|---|
| Adult-type diffuse gliomas | Medulloblastoma | Uncertain differentiation | |||
| Pediatric type diffuses low-grade gliomas | Other Central Nervoys System embryonal tumors | ||||
| Pediatric type diffuses high-grade gliomas | |||||
| Circumscribed astrocytic gliomas | |||||
| Glioneuronal and neuronal tumors | |||||
| Ependymal tumors |
| Cancer Type | Therapeutic Target | Conclusions | Reference |
|---|---|---|---|
| Glioblastoma | Use of a vaccinia virus to treat a mouse model of GBM by releasing IL-21. | Treatment in combination with checkpoint inhibitors has significantly improved the condition of the GB. | [106] |
| Glioblastoma | NK expansion and increase with K562 cells expressing the ligand OX40 and IL-18 and IL-21. | It shows the limited therapeutic potential of therapy with irinotecan and bevacizumab to treat GB. | [107] |
| Glioblastoma (also other types) | Evaluation with two different methods to evaluate circulating cytokines in patients with GB. | Double analysis of serum levels of different ILs via LMX and MSD. | [108] |
| Glioblastoma | Enhance the immunological activity of mesothelin through the following gamma chain cytokines: IL-2, IL-15, and IL-21. | The immunological importance of mesothelin via IL-2, IL-15, and IL-21. | [109] |
| Glioblastoma | T cell stimulation via a cocktail of ILs: IL-2, IL-15, and IL-21. | The presence of NY-ESO-1 or survivin in GB is a valuable target for cancer-directed T cells. | [110] |
| Glioblastoma | Genetically engineered macrophages capable of promoting the activation of other cells via IL-21. | Genetically engineered macrophages are an ideal cell for remodeling the tumor microenvironment and enhancing antitumor immunity. | [111] |
| Glioblastoma | CD8+ T cell stimulation with three different ILs, including IL-21. | Use in immunotherapy of multifunctional T cells to counteract different malignancies. | [112] |
| Glioblastoma | A particular type of T cells, Vγ9Vδ2, stimulated with IL-21. | T cells Vγ9Vδ2 stimulated with IL-21 significantly eliminated GB cells. | [113] |
| Glioblastoma (also other types) | Exploit the modified IL-21 gene or recombined IL-21 for immunotherapy. | Local treatment of IL-21 may be appropriate to treat GB. | [114] |
| Glioblastoma | The study of the patterns of some cytokines with the survivin. | The relationship between some serum cytokines and lymphocytes with survivin97-111 is deepened to predict the survival of patients with GB. | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Repici, A.; Ardizzone, A.; Filippone, A.; Colarossi, C.; Mare, M.; Raciti, G.; Mannino, D.; Cuzzocrea, S.; Paterniti, I.; Esposito, E. Interleukin-21 Influences Glioblastoma Course: Biological Mechanisms and Therapeutic Potential. Cells 2023, 12, 2284. https://doi.org/10.3390/cells12182284
Repici A, Ardizzone A, Filippone A, Colarossi C, Mare M, Raciti G, Mannino D, Cuzzocrea S, Paterniti I, Esposito E. Interleukin-21 Influences Glioblastoma Course: Biological Mechanisms and Therapeutic Potential. Cells. 2023; 12(18):2284. https://doi.org/10.3390/cells12182284
Chicago/Turabian StyleRepici, Alberto, Alessio Ardizzone, Alessia Filippone, Cristina Colarossi, Marzia Mare, Gabriele Raciti, Deborah Mannino, Salvatore Cuzzocrea, Irene Paterniti, and Emanuela Esposito. 2023. "Interleukin-21 Influences Glioblastoma Course: Biological Mechanisms and Therapeutic Potential" Cells 12, no. 18: 2284. https://doi.org/10.3390/cells12182284
APA StyleRepici, A., Ardizzone, A., Filippone, A., Colarossi, C., Mare, M., Raciti, G., Mannino, D., Cuzzocrea, S., Paterniti, I., & Esposito, E. (2023). Interleukin-21 Influences Glioblastoma Course: Biological Mechanisms and Therapeutic Potential. Cells, 12(18), 2284. https://doi.org/10.3390/cells12182284