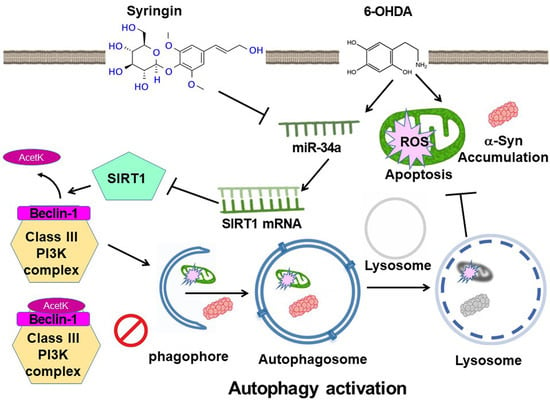
Syringin Prevents 6-Hydroxydopamine Neurotoxicity by Mediating the MiR-34a/SIRT1/Beclin-1 Pathway and Activating Autophagy in SH-SY5Y Cells and the Caenorhabditis elegans Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals, Handling, and Manipulation of SH-SY5Y Cells
2.2. Viability Assay of SH-SY5Y Cells
2.3. Detection of Mitochondrial Membrane Potential in SH-SY5Y Cells
2.4. A Measure of DNA Fragmentation via TUNEL Assay in SH-SY5Y Cells
2.5. Apoptosis Analysis Using Flow Cytometry of FITC-Annexin-V/PI on SH-SY5Y Cells
2.6. Protein Expressing Analysis Using Western Blot on SH-SY5Y Cells
2.7. Measuring ROS in SH-SY5Y Cells
2.8. Staining of Acridine Orange in SH-SY5Y Cells
2.9. Measuring Autophagic Activity in SH-SY5Y Cells
2.10. Immunoprecipitation Assay of Acetylation of Beclin-1 in SH-SY5Y Cells
2.11. Measurement of Human MiR-34a Expression via RT-qPCR in SH-SY5Y Cells
2.12. Transient Transfection of Human MiR-34a Inhibitors and Mimics in SH-SY5Y Cells
2.13. C. elegans Strains and Maintenance/Synchronization of Worms
2.14. Determining the Optimal Treatment Concentration of SRG for Worms via Food Clearance Assay
2.15. Pretreatment of SRG and Exposure to 6-OHDA in Worms
2.16. Analysis of Degeneration of 6-OHDA-Induced DA Neurons in BZ555 Worms
2.17. Assessing DA Neuron Function via Food Sensitivity Behavioral Test in N2 Worms
2.18. The Analysis of Lifespans in N2 Worms
2.19. Measuring of α-Synuclein Accumulation in Muscle Cells of NL5901 Worms
2.20. The Analysis of α-Synuclein Protein Expression in NL5901 Worms
2.21. Measuring Autophagic Activity Using DA2123 Worms
2.22. Detecting the ROS Level in N2 Worms
2.23. Extraction of Total RNA and RT-qPCR in Worms
2.24. Statistical Analysis in this Study
3. Results
3.1. Syringin (SRG) Improves Apoptosis and Exhibits Neuroprotective Activity in SH-SY5Y Cells Exposed to 6-OHDA
3.2. SRG Reduces ROS Production Induced via 6-OHDA in SH-SY5Y Cells via Activating Autophagy
3.3. The Ability of SRG to Reverse the Autophagy Dysfunction and Apoptosis Caused by 6-OHDA Was Abolished via Wortmannin and Bafilomycin A1 Treatment
3.4. SRG Restores 6-OHDA-Induced Downregulation of SIRT1 Protein Expression and Promotes Deacetylation of Beclin-1
3.5. The 6-OHDA-Induced Upregulation of Endogenous MiR-34a Targeting SIRT1 Was Reversed via SRG Pretreatment
3.6. The 6-OHDA-Induced Degeneration of DA Neurons in Caenorhabditis Elegans Can Be Reversed via SRG Pretreatment
3.7. The Loss of Dopamine-Mediated Food-Sensitive Behavior in Worms Induced via 6-OHDA Exposure Can Be Reversed via SRG Pretreatment
3.8. The Shortened Lifespan of Worms Due to 6-OHDA Toxicity Can Be Restored via SRG Pretreatment
3.9. The α-Synuclein Accumulating in Muscle Cells of Worms Can Be Alleviated via SRG-Induced Autophagic Activity
3.10. SRG May Protect against 6-OHDA Toxicity to DA Neurons of Worms via Strengthening the Sir-2.1 Pathway
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Skidmore, S.; Barker, R.A. Challenges in the clinical advancement of cell therapies for Parkinson’s disease. Nat. Biomed. Eng. 2023, 7, 370–386. [Google Scholar] [CrossRef] [PubMed]
- Ball, N.; Teo, W.P.; Chandra, S.; Chapman, J. Parkinson’s Disease and the Environment. Front. Neurol. 2019, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Bargues-Carot, A.; Riaz, Z.; Wickham, H.; Zenitsky, G.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Impact of Environmental Risk Factors on Mitochondrial Dysfunction, Neuroinflammation, Protein Misfolding, and Oxidative Stress in the Etiopathogenesis of Parkinson’s Disease. Int. J. Mol. Sci. 2022, 23, 10808. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.; McKernan, D.P. The Effect of Aggregated Alpha Synuclein on Synaptic and Axonal Proteins in Parkinson’s Disease-A Systematic Review. Biomolecules 2022, 12, 1199. [Google Scholar] [CrossRef]
- Sahoo, S.; Padhy, A.A.; Kumari, V.; Mishra, P. Role of Ubiquitin-Proteasome and Autophagy-Lysosome Pathways in alpha-Synuclein Aggregate Clearance. Mol. Neurobiol. 2022, 59, 5379–5407. [Google Scholar] [CrossRef]
- Sanchez-Mirasierra, I.; Ghimire, S.; Hernandez-Diaz, S.; Soukup, S.F. Targeting Macroautophagy as a Therapeutic Opportunity to Treat Parkinson’s Disease. Front. Cell Dev. Biol. 2022, 10, 921314. [Google Scholar] [CrossRef]
- Zhang, S.; Yu, Z.; Xia, J.; Zhang, X.; Liu, K.; Sik, A.; Jin, M. Anti-Parkinson’s disease activity of phenolic acids from Eucommia ulmoides Oliver leaf extracts and their autophagy activation mechanism. Food Funct. 2020, 11, 1425–1440. [Google Scholar] [CrossRef]
- Ning, B.; Zhang, Q.; Deng, M.; Wang, N.; Fang, Y. Endoplasmic reticulum stress induced autophagy in 6-OHDA-induced Parkinsonian rats. Brain Res. Bull. 2019, 146, 224–227. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, Y.N.; Zhang, J.Y.; Liu, J.; Li, Y.B.; Su, H.; Qu, Q.M. SIRT3 Protects Rotenone-induced Injury in SH-SY5Y Cells by Promoting Autophagy through the LKB1-AMPK-mTOR Pathway. Aging Dis. 2018, 9, 273–286. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Xie, W.; Huang, L.; Zhong, C.; Chen, Z. Beclin1 and HMGB1 ameliorate the alpha-synuclein-mediated autophagy inhibition in PC12 cells. Diagn. Pathol. 2016, 11, 15. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Chiba, T.; Murata, S.; Iwata, J.; Tanida, I.; Ueno, T.; Koike, M.; Uchiyama, Y.; Kominami, E.; et al. Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 2006, 441, 880–884. [Google Scholar] [CrossRef] [PubMed]
- Pupyshev, A.B.; Tikhonova, M.A.; Akopyan, A.A.; Tenditnik, M.V.; Dubrovina, N.I.; Korolenko, T.A. Therapeutic activation of autophagy by combined treatment with rapamycin and trehalose in a mouse MPTP-induced model of Parkinson’s disease. Pharmacol. Biochem. Behav. 2019, 177, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Lu, P.; Zeng, X.; Jin, S.; Chen, X. Study on the Mechanism for SIRT1 during the Process of Exercise Improving Depression. Brain Sci. 2023, 13, 719. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.; Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Elekhnawy, E. SIRT1 pathway in Parkinson’s disease: A faraway snapshot but so close. Inflammopharmacology 2023, 31, 37–56. [Google Scholar] [CrossRef]
- Li, X.; Feng, Y.; Wang, X.X.; Truong, D.; Wu, Y.C. The Critical Role of SIRT1 in Parkinson’s Disease: Mechanism and Therapeutic Considerations. Aging Dis. 2020, 11, 1608–1622. [Google Scholar] [CrossRef]
- Sun, T.; Li, X.; Zhang, P.; Chen, W.D.; Zhang, H.L.; Li, D.D.; Deng, R.; Qian, X.J.; Jiao, L.; Ji, J.; et al. Acetylation of Beclin 1 inhibits autophagosome maturation and promotes tumour growth. Nat. Commun. 2015, 6, 7215. [Google Scholar] [CrossRef]
- Deng, Z.; Sun, M.; Wu, J.; Fang, H.; Cai, S.; An, S.; Huang, Q.; Chen, Z.; Wu, C.; Zhou, Z.; et al. SIRT1 attenuates sepsis-induced acute kidney injury via Beclin1 deacetylation-mediated autophagy activation. Cell Death Dis. 2021, 12, 217. [Google Scholar] [CrossRef]
- Majeed, Y.; Halabi, N.; Madani, A.Y.; Engelke, R.; Bhagwat, A.M.; Abdesselem, H.; Agha, M.V.; Vakayil, M.; Courjaret, R.; Goswami, N.; et al. SIRT1 promotes lipid metabolism and mitochondrial biogenesis in adipocytes and coordinates adipogenesis by targeting key enzymatic pathways. Sci. Rep. 2021, 11, 8177. [Google Scholar] [CrossRef]
- Liang, D.; Zhuo, Y.; Guo, Z.; He, L.; Wang, X.; He, Y.; Li, L.; Dai, H. SIRT1/PGC-1 pathway activation triggers autophagy/mitophagy and attenuates oxidative damage in intestinal epithelial cells. Biochimie 2020, 170, 10–20. [Google Scholar] [CrossRef]
- Lee, T.K.; Ashok Kumar, K.; Huang, C.Y.; Liao, P.H.; Ho, T.J.; Kuo, W.W.; Liao, S.C.; Hsieh, D.J.; Chiu, P.L.; Chang, Y.M.; et al. Garcinol protects SH-SY5Y cells against MPP+-induced cell death by activating DJ-1/SIRT1 and PGC-1alpha mediated antioxidant pathway in sequential stimulation of p-AMPK mediated autophagy. Environ. Toxicol. 2023, 38, 857–866. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, J.; Qiu, J.; Wang, L.; Zhuo, J.; Wang, B.; Sun, D.; Yu, S.; Lou, H. Urolithin A protects dopaminergic neurons in experimental models of Parkinson’s disease by promoting mitochondrial biogenesis through the SIRT1/PGC-1alpha signaling pathway. Food Funct. 2022, 13, 375–385. [Google Scholar] [CrossRef]
- Mone, P.; de Donato, A.; Varzideh, F.; Kansakar, U.; Jankauskas, S.S.; Pansini, A.; Santulli, G. Functional role of miR-34a in diabetes and frailty. Front. Aging 2022, 3, 949924. [Google Scholar] [CrossRef]
- Wang, J.; He, P.; Tian, Q.; Luo, Y.; He, Y.; Liu, C.; Gong, P.; Guo, Y.; Ye, Q.; Li, M. Genetic modification of miR-34a enhances efficacy of transplanted human dental pulp stem cells after ischemic stroke. Neural Regen. Res. 2023, 18, 2029–2036. [Google Scholar] [PubMed]
- Guo, Y.; Li, P.; Gao, L.; Zhang, J.; Yang, Z.; Bledsoe, G.; Chang, E.; Chao, L.; Chao, J. Kallistatin reduces vascular senescence and aging by regulating microRNA-34a-SIRT1 pathway. Aging Cell 2017, 16, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Yamakuchi, M.; Ferlito, M.; Lowenstein, C.J. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. USA 2008, 105, 13421–13426. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Ahmed, S.; Gul, S.; Khan, A.; Al-Harrasi, A. Search for safer and potent natural inhibitors of Parkinson’s disease. Neurochem. Int. 2021, 149, 105135. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, K.; Wang, J.; Shao, H. Syringin exerts anti-inflammatory and antioxidant effects by regulating SIRT1 signaling in rat and cell models of acute myocardial infarction. Immun. Inflamm. Dis. 2023, 11, e775. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, S.I.; Ku, H.Y.; Lee, D.S.; Lee, J.W.; Kim, Y.S.; Seong, Y.H.; Song, K.S. Syringin from stem bark of Fraxinus rhynchophylla protects Abeta(25-35)-induced toxicity in neuronal cells. Arch. Pharm. Res. 2010, 33, 531–538. [Google Scholar] [CrossRef]
- Sun, S.; Zhang, Y.; Xu, W.; Wang, B.; Chen, J. Mitochondrial dysfunction is involved in the cellular activity inhibition by eleutheroside B in SMMC-7721 and HeLa cells. Hum. Exp. Toxicol. 2022, 41, 9603271221089006. [Google Scholar] [CrossRef]
- Li, F.; Zhang, N.; Wu, Q.; Yuan, Y.; Yang, Z.; Zhou, M.; Zhu, J.; Tang, Q. Syringin prevents cardiac hypertrophy induced by pressure overload through the attenuation of autophagy. Int. J. Mol. Med. 2017, 39, 199–207. [Google Scholar] [CrossRef]
- Lin, C.Y.; Huang, Y.N.; Fu, R.H.; Liao, Y.H.; Kuo, T.Y.; Tsai, C.W. Promotion of mitochondrial biogenesis via the regulation of PARIS and PGC-1alpha by parkin as a mechanism of neuroprotection by carnosic acid. Phytomedicine 2021, 80, 153369. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.M.; Liu, S.P.; Lin, H.L.; Chan, M.C.; Chen, Y.C.; Huang, Y.L.; Tsai, M.C.; Fu, R.H. Irisflorentin improves alpha-synuclein accumulation and attenuates 6-OHDA-induced dopaminergic neuron degeneration, implication for Parkinson’s disease therapy. Biomedicine 2015, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Kostrzewa, R.M. Neonatal 6-hydroxydopamine lesioning of rats and dopaminergic neurotoxicity: Proposed animal model of Parkinson’s disease. J. Neural Transm. 2022, 129, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Sivakumar, P.; Nagashanmugam, K.B.; Priyatharshni, S.; Lavanya, R.; Prabhu, N.; Ponnusamy, S. Review on the interactions between dopamine metabolites and alpha-Synuclein in causing Parkinson’s disease. Neurochem. Int. 2023, 162, 105461. [Google Scholar] [CrossRef]
- Fu, R.H.; Tsai, C.W.; Liu, S.P.; Chiu, S.C.; Chen, Y.C.; Chiang, Y.T.; Kuo, Y.H.; Shyu, W.C.; Lin, S.Z. Neuroprotective Capability of Narcissoside in 6-OHDA-Exposed Parkinson’s Disease Models through Enhancing the MiR200a/Nrf-2/GSH Axis and Mediating MAPK/Akt Associated Signaling Pathway. Antioxidants 2022, 11, 2089. [Google Scholar] [CrossRef]
- Fu, R.H.; Huang, L.C.; Lin, C.Y.; Tsai, C.W. Modulation of ARTS and XIAP by Parkin Is Associated with Carnosic Acid Protects SH-SY5Y Cells against 6-Hydroxydopamine-Induced Apoptosis. Mol. Neurobiol. 2018, 55, 1786–1794. [Google Scholar] [CrossRef]
- Lin, C.Y.; Tsai, C.W. Carnosic Acid Attenuates 6-Hydroxydopamine-Induced Neurotoxicity in SH-SY5Y Cells by Inducing Autophagy Through an Enhanced Interaction of Parkin and Beclin1. Mol. Neurobiol. 2017, 54, 2813–2822. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Hung, H.S.; Tsai, C.W.; Liu, S.P.; Chiang, Y.T.; Kuo, Y.H.; Shyu, W.C.; Lin, S.Z.; Fu, R.H. Peiminine Reduces ARTS-Mediated Degradation of XIAP by Modulating the PINK1/Parkin Pathway to Ameliorate 6-Hydroxydopamine Toxicity and alpha-Synuclein Accumulation in Parkinson’s Disease Models In Vivo and In Vitro. Int. J. Mol. Sci. 2021, 22, 10240. [Google Scholar] [CrossRef]
- Pantic, I.; Cumic, J.; Skodric, S.R.; Dugalic, S.; Brodski, C. Oxidopamine and oxidative stress: Recent advances in experimental physiology and pharmacology. Chem. Biol. Interact. 2021, 336, 109380. [Google Scholar] [CrossRef]
- Szabo, I.; Szewczyk, A. Mitochondrial Ion Channels. Annu. Rev. Biophys. 2023, 52, 229–254. [Google Scholar] [CrossRef]
- Luo, Y.; Sakamoto, K. Ethyl pyruvate protects SHSY5Y cells against 6-hydroxydopamine-induced neurotoxicity by upregulating autophagy. PLoS ONE 2023, 18, e0281957. [Google Scholar] [CrossRef] [PubMed]
- Themistokleous, C.; Bagnoli, E.; Parulekar, R.; Muqit, M.M.K. Role of Autophagy Pathway in Parkinson’s Disease and Related Genetic Neurological Disorders. J. Mol. Biol. 2023, 435, 168144. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.F.; Van Raamsdonk, J.M. Modeling Parkinson’s Disease in C. elegans. J. Parkinsons Dis. 2018, 8, 17–32. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, R.; Ding, F.; Wang, H.; Han, W.; Ma, F.; Hu, M.; Ma, C.W.; Huang, Z. Astragalus Polysaccharide Suppresses 6-Hydroxydopamine-Induced Neurotoxicity in Caenorhabditis elegans. Oxid. Med. Cell Longev. 2016, 2016, 4856761. [Google Scholar] [CrossRef]
- Kinet, R.; Dehay, B. Pathogenic Aspects and Therapeutic Avenues of Autophagy in Parkinson’s Disease. Cells 2023, 12, 621. [Google Scholar] [CrossRef]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef]
- Gassen, N.C.; Niemeyer, D.; Muth, D.; Corman, V.M.; Martinelli, S.; Gassen, A.; Hafner, K.; Papies, J.; Mosbauer, K.; Zellner, A.; et al. SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-Coronavirus infection. Nat. Commun. 2019, 10, 5770. [Google Scholar] [CrossRef]
- Liu, K.; Guo, C.; Lao, Y.; Yang, J.; Chen, F.; Zhao, Y.; Yang, Y.; Yang, J.; Yi, J. A fine-tuning mechanism underlying self-control for autophagy: deSUMOylation of BECN1 by SENP3. Autophagy 2020, 16, 975–990. [Google Scholar] [CrossRef]
- Esteves, A.R.; Filipe, F.; Magalhaes, J.D.; Silva, D.F.; Cardoso, S.M. The Role of Beclin-1 Acetylation on Autophagic Flux in Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 5654–5670. [Google Scholar] [CrossRef]
- Selvi, R.B.; Swaminathan, A.; Chatterjee, S.; Shanmugam, M.K.; Li, F.; Ramakrishnan, G.B.; Siveen, K.S.; Chinnathambi, A.; Zayed, M.E.; Alharbi, S.A.; et al. Inhibition of p300 lysine acetyltransferase activity by luteolin reduces tumor growth in head and neck squamous cell carcinoma (HNSCC) xenograft mouse model. Oncotarget 2015, 6, 43806–43818. [Google Scholar] [CrossRef]
- Sethi, G.; Chatterjee, S.; Rajendran, P.; Li, F.; Shanmugam, M.K.; Wong, K.F.; Kumar, A.P.; Senapati, P.; Behera, A.K.; Hui, K.M.; et al. Inhibition of STAT3 dimerization and acetylation by garcinol suppresses the growth of human hepatocellular carcinoma in vitro and in vivo. Mol. Cancer 2014, 13, 66. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shentu, T.P.; Wen, L.; Johnson, D.A.; Shyy, J.Y. Regulation of SIRT1 by oxidative stress-responsive miRNAs and a systematic approach to identify its role in the endothelium. Antioxid. Redox Signal 2013, 19, 1522–1538. [Google Scholar] [CrossRef] [PubMed]
- Jiao, F.; Gong, Z. The Beneficial Roles of SIRT1 in Neuroinflammation-Related Diseases. Oxid. Med. Cell Longev. 2020, 2020, 6782872. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.; Wang, H.; Gao, T.; Peng, J.; Meng, P.; Zhang, X.; Guo, D.; Liu, G.; Shi, J.; Peng, Q. The protective effect of luteolin on the depression-related dry eye disorder through Sirt1/NF-kappaB/NLRP3 pathway. Aging 2023, 15, 261–275. [Google Scholar] [CrossRef]
- Donmez, G.; Arun, A.; Chung, C.Y.; McLean, P.J.; Lindquist, S.; Guarente, L. SIRT1 protects against alpha-synuclein aggregation by activating molecular chaperones. J. Neurosci. 2012, 32, 124–132. [Google Scholar] [CrossRef]
- Manjula, R.; Anuja, K.; Alcain, F.J. SIRT1 and SIRT2 Activity Control in Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 585821. [Google Scholar] [CrossRef]
- Maszlag-Torok, R.; Boros, F.A.; Vecsei, L.; Klivenyi, P. Gene variants and expression changes of SIRT1 and SIRT6 in peripheral blood are associated with Parkinson’s disease. Sci. Rep. 2021, 11, 10677. [Google Scholar] [CrossRef]
- Li, Q.; Zhang, Q. miR-34a and endothelial biology. Life Sci. 2023, 330, 121976. [Google Scholar] [CrossRef]
- Choi, S.E.; Fu, T.; Seok, S.; Kim, D.H.; Yu, E.; Lee, K.W.; Kang, Y.; Li, X.; Kemper, B.; Kemper, J.K. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell 2013, 12, 1062–1072. [Google Scholar] [CrossRef]
- Li, J.; Wang, K.; Chen, X.; Meng, H.; Song, M.; Wang, Y.; Xu, X.; Bai, Y. Transcriptional activation of microRNA-34a by NF-kappa B in human esophageal cancer cells. BMC Mol. Biol. 2012, 13, 4. [Google Scholar] [CrossRef]
- Ma, X.; Yin, B.; Guo, S.; Umar, T.; Liu, J.; Wu, Z.; Zhou, Q.; Zahoor, A.; Deng, G. Enhanced Expression of miR-34a Enhances Escherichia coli Lipopolysaccharide-Mediated Endometritis by Targeting LGR4 to Activate the NF-kappaB Pathway. Oxid. Med. Cell Longev. 2021, 2021, 1744754. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wei, X.; He, J.; Cao, Q.; Du, D.; Zhan, X.; Zeng, Y.; Yuan, S.; Sun, L. The comprehensive landscape of miR-34a in cancer research. Cancer Metastasis Rev. 2021, 40, 925–948. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, H.U.; Kaleta, C.; Cossais, F.; Schaeffer, E.; Berndt, H.; Best, L.; Dost, T.; Glusing, S.; Groussin, M.; Poyet, M.; et al. Microbiome and Metabolome Insights into the Role of the Gastrointestinal-Brain Axis in Parkinson’s and Alzheimer’s Disease: Unveiling Potential Therapeutic Targets. Metabolites 2022, 12, 1222. [Google Scholar] [CrossRef] [PubMed]
- Xxxx, S.; Ahmad, M.H.; Rani, L.; Mondal, A.C. Convergent Molecular Pathways in Type 2 Diabetes Mellitus and Parkinson’s Disease: Insights into Mechanisms and Pathological Consequences. Mol. Neurobiol. 2022, 59, 4466–4487. [Google Scholar] [CrossRef]
- Liu, D.X.; Zhao, C.S.; Wei, X.N.; Ma, Y.P.; Wu, J.K. Semaglutide Protects against 6-OHDA Toxicity by Enhancing Autophagy and Inhibiting Oxidative Stress. Parkinsons Dis. 2022, 2022, 6813017. [Google Scholar] [CrossRef]
- Long, T.; Wu, Q.; Wei, J.; Tang, Y.; He, Y.N.; He, C.L.; Chen, X.; Yu, L.; Yu, C.L.; Law, B.Y.; et al. Ferulic Acid Exerts Neuroprotective Effects via Autophagy Induction in C. elegans and Cellular Models of Parkinson’s Disease. Oxid. Med. Cell Longev. 2022, 2022, 3723567. [Google Scholar] [CrossRef]
- Li, R.; Lu, Y.; Zhang, Q.; Liu, W.; Yang, R.; Jiao, J.; Liu, J.; Gao, G.; Yang, H. Piperine promotes autophagy flux by P2RX4 activation in SNCA/alpha-synuclein-induced Parkinson disease model. Autophagy 2022, 18, 559–575. [Google Scholar] [CrossRef]
- Deng, Y.N.; Shi, J.; Liu, J.; Qu, Q.M. Celastrol protects human neuroblastoma SH-SY5Y cells from rotenone-induced injury through induction of autophagy. Neurochem. Int. 2013, 63, 1–9. [Google Scholar] [CrossRef]












Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, R.-H.; Hong, S.-Y.; Chen, H.-J. Syringin Prevents 6-Hydroxydopamine Neurotoxicity by Mediating the MiR-34a/SIRT1/Beclin-1 Pathway and Activating Autophagy in SH-SY5Y Cells and the Caenorhabditis elegans Model. Cells 2023, 12, 2310. https://doi.org/10.3390/cells12182310
Fu R-H, Hong S-Y, Chen H-J. Syringin Prevents 6-Hydroxydopamine Neurotoxicity by Mediating the MiR-34a/SIRT1/Beclin-1 Pathway and Activating Autophagy in SH-SY5Y Cells and the Caenorhabditis elegans Model. Cells. 2023; 12(18):2310. https://doi.org/10.3390/cells12182310
Chicago/Turabian StyleFu, Ru-Huei, Syuan-Yu Hong, and Hui-Jye Chen. 2023. "Syringin Prevents 6-Hydroxydopamine Neurotoxicity by Mediating the MiR-34a/SIRT1/Beclin-1 Pathway and Activating Autophagy in SH-SY5Y Cells and the Caenorhabditis elegans Model" Cells 12, no. 18: 2310. https://doi.org/10.3390/cells12182310
APA StyleFu, R. -H., Hong, S. -Y., & Chen, H. -J. (2023). Syringin Prevents 6-Hydroxydopamine Neurotoxicity by Mediating the MiR-34a/SIRT1/Beclin-1 Pathway and Activating Autophagy in SH-SY5Y Cells and the Caenorhabditis elegans Model. Cells, 12(18), 2310. https://doi.org/10.3390/cells12182310