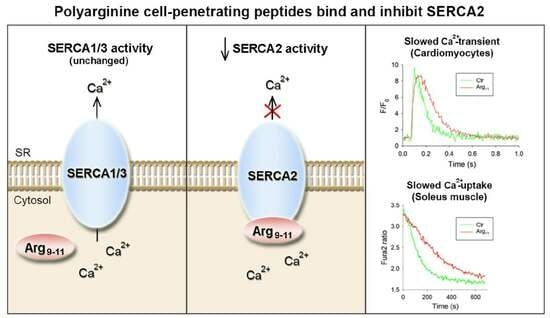
Polyarginine Cell-Penetrating Peptides Bind and Inhibit SERCA2
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Peptides and Recombinant Protein
- TAT: RKKRRQRRR
- Arg9: RRRRRRRRR
- Arg11: RRRRRRRRRRR
- Cargo: IEKELAQQYQNADAITLE, a scrambled control sequence of RIAD (LEQYNQLADQIIKEATEK) which is a high-affinity disruptor peptide for protein kinase A (PKA) type I [9].
- Cargo1–9: IEKELAQQY
- Cargo8–18: QYQNADAITLE
- Cargo1–11: IEKELAQQYQN
- Recombinant 12xHis-SERCA2111–253, containing the actuator domain, also known as the A-domain, of SERCA2 [10], and His-CaMKIIδ1–165 were generated by Genscript.
2.3. Isolated Cardiomyocyte Experiments
2.4. Ca2+ Homeostasis in Ventricular and Muscle Homogenates
2.5. Cell Culture and Transfection of HEK293 Cells
2.6. ELISA Analyses
2.7. Surface Plasmon Resonance
2.8. Statistics
3. Results
3.1. Arg11 Slows Ca2+ Transient Decline in Cardiomyocytes
3.2. Polyarginine CPPs Inhibit SR Ca2+ Uptake in Ventricular Vesicles, in the Presence and Absence of Attached Cargo
3.3. SERCA Inhibition by Polyarginine CPPs Is Not Dependent on Phospholamban
3.4. Polyarginine CPPs Inhibit Ca2+ Uptake in SR Vesicles from Rat Soleus, but Not EDL
3.5. Polyarginine CPPs Inhibit SERCA2 Isoforms, but Not SERCA1 or SERCA3
3.6. Analysis of CPP Binding to SERCA2
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Desale, K.; Kuche, K.; Jain, S. Cell-penetrating peptides (CPPs): An overview of applications for improving the potential of nanotherapeutics. Biomater. Sci. 2021, 9, 1153–1188. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Bi, Y.; Zhang, H.; Dong, S.; Teng, L.; Lee, R.J.; Yang, Z. Cell-Penetrating Peptides in Diagnosis and Treatment of Human Diseases: From Preclinical Research to Clinical Application. Front. Pharmacol. 2020, 11, 697. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef] [PubMed]
- Ruseska, I.; Zimmer, A. Internalization mechanisms of cell-penetrating peptides. Beilstein J. Nanotechnol. 2020, 11, 101–123. [Google Scholar] [CrossRef] [PubMed]
- Herce, H.D.; Garcia, A.E.; Litt, J.; Kane, R.S.; Martin, P.; Enrique, N.; Rebolledo, A.; Milesi, V. Arginine-rich peptides destabilize the plasma membrane, consistent with a pore formation translocation mechanism of cell-penetrating peptides. Biophys. J. 2009, 97, 1917–1925. [Google Scholar] [CrossRef]
- Herce, H.D.; Garcia, A.E. Molecular dynamics simulations suggest a mechanism for translocation of the HIV-1 TAT peptide across lipid membranes. Proc. Natl. Acad. Sci. USA 2007, 104, 20805–20810. [Google Scholar] [CrossRef]
- Wender, P.A.; Mitchell, D.J.; Pattabiraman, K.; Pelkey, E.T.; Steinman, L.; Rothbard, J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA 2000, 97, 13003–13008. [Google Scholar] [CrossRef]
- Luo, W.; Grupp, I.L.; Harrer, J.; Ponniah, S.; Grupp, G.; Duffy, J.J.; Doetschman, T.; Kranias, E.G. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ. Res. 1994, 75, 401–409. [Google Scholar] [CrossRef]
- Carlson, C.R.; Lygren, B.; Berge, T.; Hoshi, N.; Wong, W.; Tasken, K.; Scott, J.D. Delineation of type I protein kinase A-selective signaling events using an RI anchoring disruptor. J. Biol. Chem. 2006, 281, 21535–21545. [Google Scholar] [CrossRef]
- Inoue, M.; Sakuta, N.; Watanabe, S.; Zhang, Y.; Yoshikaie, K.; Tanaka, Y.; Ushioda, R.; Kato, Y.; Takagi, J.; Tsukazaki, T.; et al. Structural Basis of Sarco/Endoplasmic Reticulum Ca2+-ATPase 2b Regulation via Transmembrane Helix Interplay. Cell Rep. 2019, 27, 1221–1230.e3. [Google Scholar] [CrossRef]
- Gattoni, S.; Roe, A.T.; Frisk, M.; Louch, W.E.; Niederer, S.A.; Smith, N.P. The calcium-frequency response in the rat ventricular myocyte: An experimental and modelling study. J. Physiol. 2016, 594, 4193–4224. [Google Scholar] [CrossRef]
- Roe, A.T.; Ruud, M.; Espe, E.K.; Manfra, O.; Longobardi, S.; Aronsen, J.M.; Norden, E.S.; Husebye, T.; Kolstad, T.R.S.; Cataliotti, A.; et al. Regional diastolic dysfunction in post-infarction heart failure: Role of local mechanical load and SERCA expression. Cardiovasc. Res. 2019, 115, 752–764. [Google Scholar] [CrossRef]
- Ottesen, A.H.; Carlson, C.R.; Eken, O.S.; Sadredini, M.; Myhre, P.L.; Shen, X.; Dalhus, B.; Laver, D.R.; Lunde, P.K.; Kurola, J.; et al. Secretoneurin Is an Endogenous Calcium/Calmodulin-Dependent Protein Kinase II Inhibitor That Attenuates Ca2+-Dependent Arrhythmia. Circ. Arrhythmia Electrophysiol. 2019, 12, e007045. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Calcium sequestration by isolated sarcoplasmic reticulum: Real-time monitoring using ratiometric dual-emission spectrofluorometry and the fluorescent calcium-binding dye indo-1. Mol. Cell. Biochem. 1990, 94, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Li, J.L.; Wang, X.N.; Fraser, S.F.; Carey, M.F.; Wrigley, T.V.; McKenna, M.J. Effects of fatigue and training on sarcoplasmic reticulum Ca2+ regulation in human skeletal muscle. J. Appl. Physiol. 2002, 92, 912–922. [Google Scholar] [CrossRef] [PubMed]
- Kolstad, T.R.; van den Brink, J.; Macquaide, N.; Lunde, P.K.; Frisk, M.; Aronsen, J.M.; Norden, E.S.; Cataliotti, A.; Sjaastad, I.; Sejersted, O.M.; et al. Ryanodine receptor dispersion disrupts Ca2+ release in failing cardiac myocytes. eLife 2018, 7, e39427. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, J.; Woo, J.G.; Garcia, A.M.; Alsaied, T.; Li, J.; Lunde, P.K.; Moore, R.A.; Laasmaa, M.; Sammons, A.; Mays, W.A.; et al. Probenecid Improves Cardiac Function in Subjects with a Fontan Circulation and Augments Cardiomyocyte Calcium Homeostasis. Pediatr. Cardiol. 2020, 41, 1675–1688. [Google Scholar] [CrossRef]
- Li, L.; Louch, W.E.; Niederer, S.A.; Aronsen, J.M.; Christensen, G.; Sejersted, O.M.; Smith, N.P. Sodium accumulation in SERCA KO induced HF. Biophys. J. 2012, 102, 2039–2049. [Google Scholar] [CrossRef]
- Louch, W.E.; Hougen, K.; Mork, H.K.; Swift, F.; Aronsen, J.M.; Sjaastad, I.; Reims, H.M.; Roald, B.; Andersson, K.B.; Christensen, G.; et al. Sodium accumulation promotes diastolic dysfunction in end-stage heart failure following Serca2 knockout. J. Physiol. 2010, 588, 465–478. [Google Scholar] [CrossRef]
- Wanichawan, P.; Louch, W.E.; Hortemo, K.H.; Austbo, B.; Lunde, P.K.; Scott, J.D.; Sejersted, O.M.; Carlson, C.R. Full-length cardiac Na+/Ca2+ exchanger 1 protein is not phosphorylated by protein kinase A. Am. J. Physiol. Cell Physiol. 2011, 300, C989–C997. [Google Scholar] [CrossRef]
- Skogestad, J.; Albert, I.; Hougen, K.; Lothe, G.B.; Lunde, M.; Eken, O.S.; Veras, I.; Huynh, N.T.T.; Borstad, M.; Marshall, S.; et al. Disruption of Phosphodiesterase 3A Binding to SERCA2 Increases SERCA2 Activity and Reduces Mortality in Mice With Chronic Heart Failure. Circulation 2023, 147, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Louch, W.E.; Stokke, M.K.; Sjaastad, I.; Christensen, G.; Sejersted, O.M. No rest for the weary: Diastolic calcium homeostasis in the normal and failing myocardium. Physiology 2012, 27, 308–323. [Google Scholar] [CrossRef] [PubMed]
- Lunde, P.K.; Verburg, E.; Eriksen, M.; Sejersted, O.M. Contractile properties of in situ perfused skeletal muscles from rats with congestive heart failure. J. Physiol. 2002, 540, 571–580. [Google Scholar] [CrossRef]
- Periasamy, M.; Kalyanasundaram, A. SERCA pump isoforms: Their role in calcium transport and disease. Muscle Nerve 2007, 35, 430–442. [Google Scholar] [CrossRef] [PubMed]
- Gallivan, J.P.; Dougherty, D.A. Cation-pi interactions in structural biology. Proc. Natl. Acad. Sci. USA 1999, 96, 9459–9464. [Google Scholar] [CrossRef]
- Aguayo-Ortiz, R.; Espinoza-Fonseca, L.M. Linking Biochemical and Structural States of SERCA: Achievements, Challenges, and New Opportunities. Int. J. Mol. Sci. 2020, 21, 4146. [Google Scholar] [CrossRef]
- Suresh, B.; Ramakrishna, S.; Kim, H. Cell-Penetrating Peptide-Mediated Delivery of Cas9 Protein and Guide RNA for Genome Editing. Methods Mol. Biol. 2017, 1507, 81–94. [Google Scholar] [CrossRef]
- Komuro, I.; Kurabayashi, M.; Shibazaki, Y.; Takaku, F.; Yazaki, Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J. Clin. Investig. 1989, 83, 1102–1108. [Google Scholar] [CrossRef]
- Nagai, R.; Zarain-Herzberg, A.; Brandl, C.J.; Fujii, J.; Tada, M.; MacLennan, D.H.; Alpert, N.R.; Periasamy, M. Regulation of myocardial Ca2+-ATPase and phospholamban mRNA expression in response to pressure overload and thyroid hormone. Proc. Natl. Acad. Sci. USA 1989, 86, 2966–2970. [Google Scholar] [CrossRef]
- Frisk, M.; Le, C.; Shen, X.; Røe, A.T.; Hou, Y.; Manfra, O.; Silva, G.; van Hout, I.; Norden, E.; Aronsen, J.M.; et al. Etiology-Dependent Impairment of Diastolic Cardiomyocyte Calcium Homeostasis in Heart Failure With Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2021, 77, 405–419. [Google Scholar] [CrossRef]
- Roe, A.T.; Frisk, M.; Louch, W.E. Targeting cardiomyocyte Ca2+ homeostasis in heart failure. Curr. Pharm. Des. 2015, 21, 431–448. [Google Scholar] [CrossRef] [PubMed]
- Westerblad, H.; Lannergren, J.; Allen, D.G. Slowed relaxation in fatigued skeletal muscle fibers of Xenopus and Mouse. Contribution of [Ca2+]i and cross-bridges. J. Gen. Physiol. 1997, 109, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Simonini, A.; Chang, K.; Yue, P.; Long, C.S.; Massie, B.M. Expression of skeletal muscle sarcoplasmic reticulum calcium-ATPase is reduced in rats with postinfarction heart failure. Heart 1999, 81, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Dode, L.; Andersen, J.P.; Leslie, N.; Dhitavat, J.; Vilsen, B.; Hovnanian, A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J. Biol. Chem. 2003, 278, 47877–47889. [Google Scholar] [CrossRef]
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A Gatekeeper of Neuronal Calcium Homeostasis in the Brain. Cell. Mol. Neurobiol. 2018, 38, 981–994. [Google Scholar] [CrossRef]
- Kiessling, M.; Djalinac, N.; Voglhuber, J.; Ljubojevic-Holzer, S. Nuclear Calcium in Cardiac (Patho)Physiology: Small Compartment, Big Impact. Biomedicines 2023, 11, 960. [Google Scholar] [CrossRef]
- Belke, D.D.; Swanson, E.; Suarez, J.; Scott, B.T.; Stenbit, A.E.; Dillmann, W.H. Increased expression of SERCA in the hearts of transgenic mice results in increased oxidation of glucose. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1755–H1763. [Google Scholar] [CrossRef]
- Goodman, J.B.; Qin, F.; Morgan, R.J.; Chambers, J.M.; Croteau, D.; Siwik, D.A.; Hobai, I.; Panagia, M.; Luptak, I.; Bachschmid, M.; et al. Redox-Resistant SERCA [Sarco(endo)plasmic Reticulum Calcium ATPase] Attenuates Oxidant-Stimulated Mitochondrial Calcium and Apoptosis in Cardiac Myocytes and Pressure Overload-Induced Myocardial Failure in Mice. Circulation 2020, 142, 2459–2469. [Google Scholar] [CrossRef]
- Carlson, C.R.; Aronsen, J.M.; Bergan-Dahl, A.; Moutty, M.C.; Lunde, M.; Lunde, P.K.; Jarstadmarken, H.; Wanichawan, P.; Pereira, L.; Kolstad, T.R.S.; et al. AKAP18delta Anchors and Regulates CaMKII Activity at Phospholamban-SERCA2 and RYR. Circ. Res. 2022, 130, 27–44. [Google Scholar] [CrossRef]
- Mi, Z.; Mai, J.; Lu, X.; Robbins, P.D. Characterization of a class of cationic peptides able to facilitate efficient protein transduction in vitro and in vivo. Mol. Ther. 2000, 2, 339–347. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef] [PubMed]
- Madani, F.; Lindberg, S.; Langel, U.; Futaki, S.; Graslund, A. Mechanisms of cellular uptake of cell-penetrating peptides. J. Biophys. 2011, 2011, 414729. [Google Scholar] [CrossRef] [PubMed]
- Fei, L.; Yap, L.P.; Conti, P.S.; Shen, W.C.; Zaro, J.L. Tumor targeting of a cell penetrating peptide by fusing with a pH-sensitive histidine-glutamate co-oligopeptide. Biomaterials 2014, 35, 4082–4087. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.E.; Zahid, M. Cell Penetrating Peptides, Novel Vectors for Gene Therapy. Pharmaceutics 2020, 12, 225. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lunde, P.K.; Manfra, O.; Støle, T.P.; Lunde, M.; Martinsen, M.; Carlson, C.R.; Louch, W.E. Polyarginine Cell-Penetrating Peptides Bind and Inhibit SERCA2. Cells 2023, 12, 2358. https://doi.org/10.3390/cells12192358
Lunde PK, Manfra O, Støle TP, Lunde M, Martinsen M, Carlson CR, Louch WE. Polyarginine Cell-Penetrating Peptides Bind and Inhibit SERCA2. Cells. 2023; 12(19):2358. https://doi.org/10.3390/cells12192358
Chicago/Turabian StyleLunde, Per Kristian, Ornella Manfra, Thea Parsberg Støle, Marianne Lunde, Marita Martinsen, Cathrine Rein Carlson, and William E. Louch. 2023. "Polyarginine Cell-Penetrating Peptides Bind and Inhibit SERCA2" Cells 12, no. 19: 2358. https://doi.org/10.3390/cells12192358
APA StyleLunde, P. K., Manfra, O., Støle, T. P., Lunde, M., Martinsen, M., Carlson, C. R., & Louch, W. E. (2023). Polyarginine Cell-Penetrating Peptides Bind and Inhibit SERCA2. Cells, 12(19), 2358. https://doi.org/10.3390/cells12192358