The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine
Abstract
:1. Reconstructive Ladder
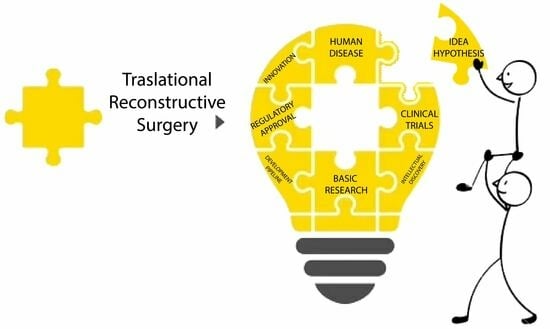
2. Translational Reconstructive Ladder
2.1. Autologous Adipose Tissue Grafting and Adipose Stem Cells (ASCs)
2.2. ECM Scaffold and Dermal Regeneration Template (DRT)
2.3. D Bioprinting Applications
2.4. Exosomes
3. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamai, S. History of Microsurgery-from the beginning until the end of the 1970s. Microsurgery 1993, 14, 6–13. [Google Scholar] [CrossRef]
- Simman, R. Wound closure and the reconstructive ladder in plastic surgery. J. Am. Coll. Certif. Wound Spec. 2009, 1, 6–11. [Google Scholar] [CrossRef]
- Scott Levin, L. The reconstructive ladder. An orthoplastic approach. Orthop. Clin. 1993, 24, 393–409. [Google Scholar]
- Mathes, S.J.; Nahai, F. Clinical Applications for Muscle and Musculocutaneous Flaps, 2nd ed.; C.V. Mosby Company: St. Louis, MO, USA, 2010. [Google Scholar]
- Mardini, S.; Wei, F.C.; Salgado, C.J.; Chen, H.C. Reconstruction of the reconstructive ladder. Plast. Reconstr. Surg. 2005, 115, 2174. [Google Scholar] [CrossRef]
- Gottlieb, L.J.; Krieger, L.M. From the reconstructive ladder to the reconstructive elevator. Plast. Reconstr. Surg. 1994, 93, 1503–1504. [Google Scholar] [CrossRef]
- Tamai, S. History of microsurgery. Plast. Reconstr. Surg. 2009, 124, e282–e294. [Google Scholar] [CrossRef]
- Erba, P.; Ogawa, R.; Vyas, R.; Orgill, D.P. The reconstructive matrix: A new paradigm in reconstructive plastic surgery. Plast. Reconstr. Surg. 2010, 126, 492–498. [Google Scholar] [CrossRef]
- Knobloch, K.; Vogt, P.M. The reconstructive clockwork of the twenty-first century: An extension of the concept of the reconstructive ladder and reconstructive elevator. Plast. Reconstr. Surg. 2010, 126, 220e–222e. [Google Scholar] [CrossRef]
- Sandhir, R.K. Learn to climb the simple reconstructive ladder properly for optimum results. Indian J. Plast. Surg. 2018, 51, 331–332. [Google Scholar] [CrossRef]
- Carrel, T. The relationship between surgeon and basic scientist. Transpl. Immunol. 2002, 9, 331–337. [Google Scholar] [CrossRef]
- Tetteh, E.S.; Bajaj, S.; Ghodadra, N.S. Basic science and surgical treatment options for articular cartilage injuries of the knee. J. Orthop. Sports Phys. Ther. 2012, 42, 243–253. [Google Scholar] [CrossRef]
- Keswani, S.G.; Moles, C.M.; Morowitz, M.; Zeh, H.; Kuo, J.S.; Levine, M.H.; Cheng, L.S.; Hackam, D.J.; Ahuja, N.; Goldstein, A.M. Basic Science Committee of the Society of University Surgeons. The future of basic science in academic surgery: Identifying barriers to success for surgeon-scientists. Ann. Surg. 2017, 265, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Friebel, T.; Nikkhah, D. Decision-Making in Flap Surgery: Reconstructive Ladder versus Elevator. In Core Techniques in Flap Reconstructive Microsurgery; Nikkhah, D., Rawlins, J., Pafitanis, G., Eds.; Springer: Cham, Switzerland, 2023. [Google Scholar]
- Shahzad, A.; Gilden, D.; Cohrs, R.J. Translational medicine and varicella zoster visrus: Need for disease modeling. New Horiz. Transl. Med. 2015, 2, 89–91. [Google Scholar] [PubMed]
- Gohar, F.; Gohar, A.; Hulskamp, G.; Debus, O. The translational medicine professional: A bridge between bench and bedside? Front. Med. 2018, 5, 294. [Google Scholar] [CrossRef] [PubMed]
- Cohrs, R.J.; Ghahramani, P.; Bidaut, L.; Higgins, P.J.; Shahzad, A. Translational medicine definition by the European Society for Translational Medicine. New Horiz. Transl. Med. 2015, 2, 86–88. [Google Scholar] [CrossRef]
- Goh, D.; Yang, Y.; Lee, E.H.; Hui, J.H.P.; Yang, Z. Managing the heterogeneity of mesenchymal stem cells for cartilage regenerative therapy: A review. Bioengineering 2023, 10, 355. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Shi, Y.; Lin, G.; Tang, B.; Li, X.; Zhang, J.; Ding, X.; Zhou, G. Advances in mechanical properties of hydrogels for cartilage tissue defect repair. Macromol. Biosci. 2023, 23, e2200539. [Google Scholar] [CrossRef]
- Klabukov, I.; Tenchurin, T.; Shepelev, A.; Baranovskii, D.; Mamagulashvili, V.; Dyuzheva, T.; Krasilnikova, O.; Balyasin, M.; Lyundup, A.; Krasheninnikov, M.; et al. Biomechanical behaviors and degradation properties of multilayered polymer scaffolds: The phase space method for bile duct design and bioengineering. Biomedicine 2023, 11, 745. [Google Scholar] [CrossRef]
- Abbott, R.D.; Kaplan, D.L. Strategies for improving the physiological relevance of human engineered tissues. Trends Biotechnol. 2015, 33, 401–407. [Google Scholar] [CrossRef]
- Waldman, S.A.; Terzic, A. Translational medicine in the era of health care reform. Clin. Transl. Sci. 2009, 2, 96–97. [Google Scholar] [CrossRef]
- Hamburg, M.A.; Collins, F.S. The path of personalized medicine. N. Engl. J. Med. 2010, 363, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Krasilnikova, O.A.; Baranovskii, D.S.; Yakimova, A.O.; Arguchinskaya, N.; Kisel, A.; Sosin, D.; Sulina, Y.; Ivanov, S.A.; Shegay, P.V.; Kaprin, A.D.; et al. Intraoperative creation of tissue-engineered grafts with minimally manipulated cells: New concept of bone tissue engineering in situ. Bioengineering 2022, 9, 704. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, G.; Gentile, P.; Marcarelli, M.; Balli, M.; Ronzoni, F.L.; Benedetti, L.; Cusella De Angelis, M.G. In vitro and in vivo studies of alar-nasal cartilage using autologous micro-grafts: The use of the rigenera protocol on the treatment of an osteochondral lesion of the nose. Pharmaceuticals 2017, 10, 53. [Google Scholar] [CrossRef]
- Palumbo Piccionello, A.; Riccio, V.; Senesi, L.; Volta, A.; Pennasilico, L.; Botto, R.; Rossi, G.; Tambella, A.M.; Galosi, L.; Marini, C.; et al. Adipose micro-grafts enhance tendinopathy healing in ovine model: An in vivo experimental perspective study. Stem Cells Transl. Med. 2021, 10, 1544–1560. [Google Scholar] [CrossRef] [PubMed]
- Sandberg, L.J. The plastic surgery compass: Navigating the reconstructive ladder in the personalized health care era. Plast. Reconstr. Surg. Glob. Open 2016, 4, e1035. [Google Scholar] [CrossRef]
- Borrelli, M.R. What is the role of plastic surgery in global health? A review. World J. Plast. Surg. 2018, 7, 275–282. [Google Scholar] [CrossRef]
- Giesen, T.; Politikou, O.; Tami, I.; Calcagni, M. Retrograde free venous flaps for extremity reconstruction: A roadmap. Medicina 2022, 58, 1065. [Google Scholar] [CrossRef]
- Marchesini, A.; Senesi, L.; De Francesco, F.; Pangrazi, P.P.; Campodonico, A.; Politano, R.; Riccio, M. Efficacy of the arteriovenous loop for free flap reconstruction in patients complex limb trauma: Case series and literature review. Medicina 2020, 56, 632. [Google Scholar] [CrossRef]
- Anolik, R.A.; Sacks, J.M. Advances and innovations in Breast Microsurgery. Mo. Med. 2021, 118, 153–155. [Google Scholar]
- Gravina, P.; De Francesco, F.; Pangrazi, P.P.; Marchesini, A.; Neuendorf, A.D.; Campodonico, A.; Gigante, A.; Riccio, M. A case report of upper limb loss of substance: Use of functional gracilis free flap, brachioradialis transposition and bioglass for bone regeneration. Trauma Case Rep. 2022, 38, 100609. [Google Scholar] [CrossRef]
- Augustin, A.; Pulzl, P.; Morandi, E.M.; Winkelmann, S.; Schoberleitner, I.; Brunner, C.; Ritter, M.; Bauer, T.; Wachter, T.; Wolfram, D. Donor-site morbidity and quality of life after autologous breast reconstruction with PAP versus TMG flap. Curr. Oncol. 2022, 29, 5682–5697. [Google Scholar] [CrossRef] [PubMed]
- Rotatori, R.M.; Starr, B.; Peake, M.; Fowler, L.; James, L.; Nelson, J.; Dale, E.L. Prevalence and risk factor for hypertrophic scarring of split thickness autograft donor sites in a pediatric burn population. Burns 2019, 45, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Al-Himdani, S.; Jessop, Z.M.; Al-Sabah, A.; Combellack, E.; Ibrahim, A.; Doak, S.H.; Hart, A.M.; Archer, C.W.; Thornton, C.A.; Whitaker, I.S. Tissue-Engineered solutions in plastic and reconstructive surgery: Principles and practice. Front. Surg. 2017, 4, 4. [Google Scholar] [CrossRef]
- Shores, J.T.; Brandacher, G.; Lee, W.P.A. Hand and upper extremity transplantation: An update of outcomes in the worldwide experience. Plast. Reconstr. Surg. 2015, 135, 351e–360e. [Google Scholar] [CrossRef]
- Matar, A.J.; Crepeau, R.L.; Mundinger, G.S.; Cetrulo Jr, C.L.; Ttorabi, R. Large animal models of vascularized composite allotransplantation: A review of immune strategies to improve allograft outcomes. Front. Immunol. 2020, 12, 664577. [Google Scholar] [CrossRef]
- Tan, A.; Chawla, r.; Natasha, G.; Mahdibeiraghdar, S.; Jeyaraj, R.; Rajadas, J.; Hamblin, M.R.; Seifalian, A.M. Nanotechnology and regenerative therapeutics in plastic surgery: The next frontier. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 1–13. [Google Scholar] [CrossRef]
- Peng, W.; Peng, Z.; Tang, P.; Sun, H.; Lei, H.; Li, Z.; Hui, D.; Du, C.; Zhou, C.; Wang, Y. Review of plastic surgery biomaterials and current progress in their 3D manufacturing technology. Materials 2020, 13, 4108. [Google Scholar] [CrossRef] [PubMed]
- Lynn, A.Q.; Pflibsen, L.R.; Smith, A.A.; Rebecca, A.M.; Teven, C.M. Three-dimensional printing in plastic surgery: Current applications, future directions, and ethical implications. Plast. Reconstr. Surg. Glob. Open 2021, 9, e3465. [Google Scholar] [CrossRef] [PubMed]
- Webber, M.J.; Khan, O.F.; Sydlik, S.A.; Tang, B.C.; Langer, R. A perspective on the clinical translation of scaffolds for tissue engineering. Ann. Biomed. Eng. 2015, 43, 641–656. [Google Scholar] [CrossRef]
- Graf, T.; Stadtfeld, M. heterogeneity of embryonic and adult stem cells. Cell Stem Cell 2008, 3, 480–483. [Google Scholar] [CrossRef]
- Baksh, D.; Song, L.; Tuan, R.S. Adult mesenchymal stem cells: Characterization, differentiation, and application in cell and gene therapy. J. Cell Mol. Med. 2004, 8, 301–316. [Google Scholar] [CrossRef]
- Bianco, P.; Robey, P.G. Marrow stromal stem cells. J. Clin. Investig. 2000, 105, 1663–1668. [Google Scholar] [CrossRef] [PubMed]
- Mendez-Ferrer, S.; Michurina, T.V.; Ferraro, F.; Mazloom, A.R.; Macarthur, B.D.; Lira, S.A.; Scadden, D.T.; Ma’ayan, A.; Enikolopov, G.N.; Frenette, P.S. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 2010, 466, 829–834. [Google Scholar] [CrossRef]
- Ferraces-Riegas, P.; Galbraith, A.C.; Doupè, D.P. Epithelial Stem Cells: Making, shaping and creaking the niche. Adv. Exp. Med. Biol. 2022, 1387, 1–12. [Google Scholar] [PubMed]
- Shaikh, M.S.; Shahzad, Z.; Tash, E.A.; Janjua, O.S.; Khan, M.I.; Zafar, M.S. Human Umbelical cord mesenchymal stem cells: Current literature and role in periodontal regeneration. Cells 2022, 11, 1168. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Lyu, H.Z.; Lee, J.R.; Han, S.H.; Lee, J.H.; Kim, B.S. Umbelical cord mesenchymal stem cell-derived nanovesicles potentiate the bone-formation efficacy of bone morphogenetic protein 2. Int. J. Mol. Sci. 2020, 21, 6425. [Google Scholar] [CrossRef] [PubMed]
- Wankhade, U.D.; Shen, M.; Kolhe, R.; Fulzele, S. Advances in adipose-derived stem cells isolation, characterization, and application in regenerative tissue engineering. Stem Cells Int. 2016, 2016, 3206807. [Google Scholar] [CrossRef]
- Ferraro, G.A.; De Francesco, F.; Nicoletti, G.; Paino, F.; Desiderio, V.; Tirino, V.; D’Andrea, F. Human adipose Cd34+ CD90+ stem cells and collagen scaffold constructs grafted in vivo fabricate loose connective and adipose tissues. J. Cell Biochem. 2013, 114, 1039–1049. [Google Scholar] [CrossRef]
- De Francesco, F.; Ricci, G.; D’Andrea, F.; Nicoletti, G.F.; Ferraro, G.A. Human adipose stem cells: From bench to bedside. Tissue Eng. Part B Rev. 2015, 21, 572–584. [Google Scholar] [CrossRef]
- Ferroni, L.; De Francesco, F.; Pinton, P.; Gardin, C.; Zavan, B. Methods to isolate adipose tissue-derived stem cells. Methods Cell Biol. 2022, 171, 215–228. [Google Scholar]
- Bellini, E.; Grieco, M.P.; Raposio, E. The science behind autologous fat grafting. Ann. Med. Surg. 2017, 24, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Gir, P.; Brown, S.A.; Oni, G.; Kashefi, N.; Mojallal, A.; Rohrich, R.J. Fat grafting: Evidence-based review on autologous fat harvesting, processing, reinjection, and storage. Plast. Reconstr. Surg. 2012, 130, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Xue, E.Y.; Narvaez, L.; Chu, C.K.; Hanson, S.E. Fat processing techniques. Semin. Plast. Surg. 2020, 34, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Guo, J.; Banyard, D.A.; Fadavi, D.; Toranto, J.D.; Wirth, G.A.; Paydar, K.Z.; Evans, G.R.D.; Widgerow, A.D. Stromal vascular fraction: A regenerative reality? Part 1: Current concepts and review of the literature. J. Plast. Reconstr. Aesthet. Surg. 2016, 69, 170–179. [Google Scholar] [CrossRef]
- Han, S.; Sun, H.M.; Hwang, K.C.; Kim, S.W. Adipose-derived stromal vascular fraction cells: Update on clinical utility and efficacy. Crit. Rev. Eukaryot. Gene Expr. 2015, 25, 145–152. [Google Scholar] [CrossRef]
- Shridhar, A.; Amsden, B.G.; Gillies, E.R.; Flynn, L.E. Investigating the effects of tissue-specific extracellular matrix on the adipogenic and osteogenic differentiation of human adipose-derived stromal cells within composite hydrogel scaffolds. Front. Bioeng. Biotechnol. 2019, 7, 402. [Google Scholar] [CrossRef]
- Nicoletti, G.F.; De Francesco, F.; D’Andrea, F.; Ferraro, G.A. Methods and procedures in adipose stem cells: State of the art and perspective for translation medicine. J. Cell Physiol. 2015, 230, 489–495. [Google Scholar] [CrossRef]
- Romano, I.R.; D’Angeli, F.; Vicario, N.; Russo, C.; Genovese, C.; Lo Furno, D.; Mannino, G.; Tamburino, S.; Parenti, R.; Giuffrida, R. Adipose-derived mesenchymal stromal cells: A tool for bone and cartilage repair. Biomedicines 2023, 11, 1781. [Google Scholar] [CrossRef]
- Silva, K.R.; Baptista, L.S. Adipose-derived stromal/stem cells from different adipose depots in obesity development. World J. Stem Cells 2019, 11, 147–166. [Google Scholar] [CrossRef]
- Pawitan, J.A.; Bui, T.A.; Mubarok, W.; Antarianto, R.D.; Nurhayati, R.W.; Dilogo, I.H.; Oceandy, D. Enhancement of the therapeutic capacity of mesenchymal stem cells by genetic modification: A systematic review. Front. Cell Dev. Biol. 2020, 8, 587776. [Google Scholar] [CrossRef]
- Melief, S.M.; Zwaginga, J.J.; Fibbe, W.E.; Roelofs, H. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Transl. Med. 2013, 2, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Long, C.; Wang, J.; Gan, W.; Qin, X.; Yang, R.; Chen, X. Therapeutic potential of exosomes from adipose-derived stem cells in chronic wound healing. Front. Surg. 2022, 9, 1030288. [Google Scholar] [CrossRef] [PubMed]
- Quesenberry, P.; Goldberg, L.R. A new stem cell biology: Transplantation and baseline, cell cycles and exosomes. Adv. Exp. Med. Biol. 2018, 1056, 3–9. [Google Scholar] [PubMed]
- Munes, G.; Sipos, F. Mesenchymal stem cell-derived secretome: A potential therapeutic option for autoiummune and immune-medianted inflammatory diseases. Cells 2022, 11, 2300. [Google Scholar]
- Xiong, M.; Zhang, Q.; Hu, W.; Zhao, C.; Lv, W.; Yi, Y.; Wu, Y.; Wu, M. Exosomes from adipose-derived stem cells: The emerging roles and applications in tissue regeneration of plastic and cosmetic surgery. Front. Cell Dev. Biol. 2020, 8, 574223. [Google Scholar] [CrossRef]
- Chen, H.; Hou, K.; Wu, Y.; Liu, Z. Use of adipose stem cells against hypertrophic scarring or keloid. Front. Cell Dev. Biol. 2022, 9, 823694. [Google Scholar] [CrossRef]
- Surowiecka, A.; Struzyma, J. Adipose-derived stem cells for facial rejuvenation. J. Pers. Med. 2022, 12, 117. [Google Scholar] [CrossRef]
- Chen, S.; He, Z.; Xu, J. Application of adipose-derived stem cells in photoaging: Basic science and literature review. Stem Cell Res. Ther. 2020, 11, 491. [Google Scholar] [CrossRef]
- Brembilla, N.C.; Vuagnat, H.; Boehncke, W.H.; Krause, K.H.; Preynat-Seauve, O. Adipose-derived stromal cells for chronic wounds: Scientific evidence and roadmap toward clinical practice. Stem Cells Transl. Med. 2023, 12, 17–25. [Google Scholar] [CrossRef]
- Hao, Z.; Qi, W.; Sun, J.; Zhou, M.; Guo, N. Review: Research progress of adipose-derived stem cells in the treatment of chronic wounds. Front. Chem. 2023, 11, 1094693. [Google Scholar] [CrossRef]
- Karagergou, E.; Ligomenou, T.; Chalidis, B.; Kitridis, D.; Papadopoulou, S.; Givissis, P. Evaluation of adipose cell-based therapies for the treatment of thumb carpometacarpal joinjtg osteoarthritis. Biomolecules 2022, 12, 473. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Lv, S.; Tong, P.; Yan, L.; Chen, Z.; Zhou, L.; Yuan, Q.; Guo, L.; Shan, L. Intra-articular injection of adipose-derived stem cells ameliorates pain and cartilage anabolism/catabolism in osteoarthritis: Preclinical and clinical evidences. Front. Pharmacol. 2022, 13, 854025. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Gravina, P.; Busato, A.; Farinelli, L.; Soranzo, C.; Vidal, L.; Zingaretti, N.; Zavan, B.; Sbarbati, A.; Riccio, M.; et al. Stem Cells in autologous microfragmented adipose tissue: Current perspectives in osteoarthritis disease. Int. J. Mol. Sci. 2021, 22, 10197. [Google Scholar] [CrossRef] [PubMed]
- Senesi, L.; De Francesco, F.; Marchesini, A.; Pangrazi, P.P.; Bertolini, M.; Riccio, V.; Riccio, M. Efficacy of adipose-derived mesenchymal stem cells and stromal vascular fraction alone and combined to biomaterials in tendinopathy or tendon injury: Systematic review of current concepts. Medicina 2023, 59, 273. [Google Scholar] [CrossRef] [PubMed]
- Kokubu, S.; Inaki, R.; Hoshi, K.; Hikita, A. Adipose-derived stem cells improve tendon repair and prevent ectopic ossification in tendinopathy by inhibiting inflammation and inducing neovascularization in the early stage of tendon healing. Regen. Ther. 2020, 14, 103–110. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, X.; Cao, X.; Qin, A.; Zhao, J. Current applications of adipose-derived mesenchymal stem cells in bone repair and regeneration: A review of cell experiments, animal models, and clinical trials. Front. Bioeng. Biotechnol. 2022, 10, 942128. [Google Scholar] [CrossRef]
- Storti, G.; Scioli, M.G.; Kim, B.S.; Orlandi, A.; Cervelli, V. Adipose-derived stem cells in bone tissue engineering: Useful tools with new application. Stem Cells Int. 2019, 2019, 3673857. [Google Scholar] [CrossRef]
- Chan, B.P.; Leong, K.W. Scaffolding in tissue engineering: General approaches and tissue-specific considerations. Eur. Spine J. 2008, 17, 467–479. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Babu, P.J. Design of 3D smart scaffolds using natural, synthetic and hybrid derived polymers for skin regenerative applications. Smart Mater. Med. 2023, 4, 243–256. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Noth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef] [PubMed]
- Cottone, G.; Amendola, F.; Strada, C.; Bagnato, M.C.; Brambilla, R.; De Francesco, F.; Vaienti, L. Comparison of efficacy among three dermal substitutes in the management of critical lower-limb wounds: The largest biases-reduced single-center retrospective cohort study in literature. Medicina 2021, 57, 1367. [Google Scholar] [CrossRef] [PubMed]
- De Francesco, F.; Busato, A.; Mannucci, S.; Zingaretti, N.; Cottone, G.; Amendola, F.; De Francesco, M.; Merigo, F.; Riccio, V.; Vaienti, L.; et al. Artificial dermal substitutes for tissue regeneration: Comparison of the clinical outcomes and histological findings of two templates. J. Int. Med. Res. 2020, 48, 3000605520945508. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Wang, J.; Yang, M.; Zhang, W.; Wu, P.; You, C.; Han, C.; Wang, X. Nanomaterials for the delivery of bioactive factors to enhance angiogenesis of dermal substitutes during wound healing. Burns Trauma 2022, 10, tkab049. [Google Scholar] [CrossRef] [PubMed]
- Gainza, G.; Villullas, S.; Pedraz, J.L.; Hernandez, R.M.; Igartua, M. Advances in drug delivery systems (DDSs) to release growth factors for wound healing and skin regeneration. Nanomedicine 2015, 11, 1551–1573. [Google Scholar] [CrossRef]
- Anitua, E.; Tejero, R.; Alkhraisat, M.H.; Orive, G. Platelet-Rich plasma to improve the bio-functionality of biomaterials. Biodrugs 2013, 27, 97–111. [Google Scholar] [CrossRef]
- Cavani, F.; Trifirò, F.; Vaccari, A. Hydrotalcite.type anionic clays: Preparation, properties and applications. Catal. Today 1991, 11, 173–301. [Google Scholar] [CrossRef]
- Ray, S.S.; Mosangi, D.; Pillai, S. Layered double hydroxide-based functional nanohybrids as controlled release carriers of pharmaceutically active ingredients. Chem. Rec. 2018, 18, 913–927. [Google Scholar] [CrossRef]
- Munhoz, D.R.; Bernardo, M.P.; Malafatti, J.O.; Moreira, F.K.V.; Mattoso, L.H.C. Alginate films functionalized with silver sulfadiazine-loaded [Mg-Al] layered double hydroxide as antimicrobial wound dressing. Int. J. Biol. Macromol. 2019, 141, 504–510. [Google Scholar] [CrossRef]
- Meek, C.P. Successful microdermagrafting using the Meel-Wall microdermatome. Am. J. Surg. 1958, 96, 557–558. [Google Scholar] [CrossRef]
- Astarita, C.; Arora, C.L.; Trovato, L. Tissue regeneration: An overview from stem cells to micrografts. J. Int. Med. Res. 2020, 48, 300060520914794. [Google Scholar] [CrossRef] [PubMed]
- Purpura, V.; Bondioli, E.; Graziano, A.; Trovato, L.; Melandri, D.; Ghetti, M.; Marchesini, A.; Cusella De Angelis, M.G.; Benedetti, L.; Ceccarelli, G.; et al. Tissue characterization after a new disaggregation method for skin micro-grafts generation. J. Vis. Exp. 2016, 109, e53579. [Google Scholar]
- Riccio, M.; Bondioli, E.; Senesi, L.; Zingaretti, N.; Gargiulo, P.; De Francesco, F.; Parodi, P.C.; Zavan, B. Fragmented dermo-epidermal units (FdeU) as an emerging strategy to improve wound healing process: An in vitro evaluation and a pilot clinical study. J. Clin. Med. 2023, 12, 6165. [Google Scholar] [CrossRef] [PubMed]
- Riccio, M.; Marchesini, A.; Zingaretti, N.; Carella, S.; Senesi, L.; Onesti, M.G.; Parodi, P.C.; Ribuffo, D.; Vaienti, L.; De Francesco, F. A multicentre study: The use of micrografts in the reconstruction of full-thickness posttraumatic skin defects of the limbs-a whole innovative concept in regenerativer surgery. Stem Cells Int. 2019, 2019, 5043518. [Google Scholar] [CrossRef]
- Davidson, D.J.; Spratt, D.; Liddle, A.D. Implant materials and prosthetic joint infection: The battle with the biofilm. EFORT Open Rev. 2019, 4, 633–639. [Google Scholar] [CrossRef]
- Murphy, S.V.; Atala, A. 3D bioprinting of tissues and organs. Nat. Biotechnol. 2014, 32, 773–785. [Google Scholar] [CrossRef]
- Yan, W.C.; Davoodi, P.; Vijayavenkataraman, S.; Tian, Y.; Ng, W.C.; Fuh, J.Y.H.; Robinson, K.S.; Wang, C.H. 3D bioprinting of skin tissue: From pre-processing to final product evaluation. Adv. Drug Deliv. Rev. 2018, 132, 270–295. [Google Scholar] [CrossRef]
- Irvine, S.A.; Venkatraman, S.S. Bioprinting and differentiation of stem cells. Molecules 2016, 21, 1188. [Google Scholar] [CrossRef]
- Xie, Z.; Gao, M.; Lobo, A.O.; Webster, T.J. 3D bioprinting in tissue engineering for medical applications: The classic and the hybrid. Polymers 2020, 12, 1717. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jungst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A definition of bioinks and their distinction from biomaterial inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Bianchi, E.; Vigani, B.; Viseras, C.; Ferrari, F.; Rossi, S.; Sandri, G. Inorganic nanomaterials in tissue engineering. Pharmaceutics 2022, 14, 1127. [Google Scholar] [CrossRef] [PubMed]
- Vaiani, L.; Boccaccio, A.; Uva, A.E.; Palimbo, G.; Piccinini, A.; Guglielmi, P.; Cantore, S.; Santacroce, L.; Charitos, I.A.; Ballini, A. Ceramic materials for biomedical applications: An overview on properties and fabrication processes. J. Funct. Biomater. 2023, 14, 146. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Wang, X. Synthetic polymers for organ 3D printing. Polymers 2020, 12, 1765. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Sun, L.; Xu, W.; Wang, Q.; Yu, S.; Sun, J. Current advances and future perspectives of 3D printing natural-derived biopolymers. Carbohydr. Polym. 2019, 1, 207–316. [Google Scholar] [CrossRef]
- Petta, D.; D’Amora, U.; Ambrosio, L.; Grijpma, D.W.; Eglin, D.; D’Este, M. Hyaluronic acid as a bioink for extrusion-based 3D printing. Biofabrication 2020, 12, 032001. [Google Scholar] [CrossRef]
- Fang, H.; Xu, J.; Ma, H.; Liu, J.; Xing, E.; Cheng, Y.Y.; Wang, H.; Nie, Y.; Pan, B.; Song, K. Functional materials of 3D bioprinting for wound dressings and skin tissue engineering applications: A review. Int. J. Bioprint. 2023, 9, 757. [Google Scholar] [CrossRef]
- Theus, A.S.; Ning, L.; Jin, l.; Roeder, R.K.; Zhang, J.; Serpooshan, V. Nanomaterials for bioprinting: Functionalization of tissue-specific bioinks. Essays Biochem. 2021, 65, 429–439. [Google Scholar]
- Roshangar, L.; Rad, J.S.; Kheirjou, R.; Khosroshahi, A.F. Using 3D-bioprinting scaffold loaded with adipose-derived stem cells to burns wound healing. J. Tissue Eng. Regen. Med. 2021, 15, 546–555. [Google Scholar] [CrossRef]
- Luo, Y.; Xu, X.; Ye, Z.; Xu, Q.; Li, J.; Liu, N.; Du, Y. 3D bioprinted mesenchymal stromal cells in skin wound repair. Front. Surg. 2022, 9, 988843. [Google Scholar] [CrossRef]
- Yasti, A.C.; Akgun, A.E.; Surel, A.A.; Kim, J.; Akin, M. Graft of 3D bioprinted autologous minimally manipulated homologous adipose tissue for the treatment of diabetic foot ulcer. Wounds 2023, 35, E22–E28. [Google Scholar] [CrossRef]
- Turner, P.R.; McConnell, M.; Young, S.L.; Cabral, J.D. 3D living dressing improves healing and modulates immune response in a thermal injury model. Tissue Eng. Part C Methods 2022, 28, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Ju, Y.; Hu, Y.; Xie, X.; Fang, B.; Lei, L. Emerging 3D bioprinting applications in plastic surgery. Biomater. Res. 2023, 27, 1. [Google Scholar] [CrossRef] [PubMed]
- Adel, I.M.; ElMeligy, M.F.; Elkasabgy, N.A. Conventional and Recent trends of scaffolds fabrication: A superior mode for tissue engineering. Pharmaceutics 2022, 14, 306. [Google Scholar] [CrossRef] [PubMed]
- Municoy, S.; Echazù, M.I.A.; Antezana, P.E.; Galdoporpora, J.M.; Olivetti, C.; Mebert, A.M.; Foglia, M.L.; Tuttolomondo, M.V.; Alvarez, G.S.; Hardy, J.G.; et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020, 21, 4724. [Google Scholar] [CrossRef] [PubMed]
- Jessop, Z.M.; Al-Sabah, A.; Gardiner, M.D.; Combellack, E.; Hawkins, K.; Whitaker, I.S. 3D bioprinting for reconstructive surgery: Principles, applications and challenges. J. Plast. Reconstr. Aesth. Surg. 2017, 70, 1155–1170. [Google Scholar] [CrossRef]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Vlassov, A.V.; Magdaleno, S.; Setterquist, R.; Conrad, R. Exosomes: Current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim. Biophys. Acta 2012, 1820, 940–948. [Google Scholar] [CrossRef]
- Boukouris, S.; Mathivanan, S. Exosomes in body fluids are a highly stable resource of disease biomarkers. Proteom. Clin. Appl. 2015, 9, 358–367. [Google Scholar] [CrossRef] [PubMed]
- D’Agneli, S.; Gerra, M.C.; Bignami, E.; Arendt-Nielsen, L. Exosomes as a new pain biomarker opportunity. Mol. Pain. 2020, 16, 1744806920957800. [Google Scholar] [CrossRef]
- Suh, J.H.; Joo, H.S.; Hong, E.B.; Lee, H.J.; Lee, J.M. Therapeutic application of exosomes in inflammatory diseases. Int. J. Mol. Sci. 2021, 22, 1144. [Google Scholar] [CrossRef]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem Cell Res. Ther. 2023, 14, 254. [Google Scholar] [CrossRef] [PubMed]
- Costigan, M.; Scholz, J.; Woolf, C.J. Neuropathic pain: A maladaptive response of the nervous system to damage. Annu. Rev. Neurosci. 2009, 32, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.R.; Campana, W.M.; Shubayev, V.I. The role of neuroinflammation in neuropathic pain: Mechanisms and therapeutic targets. Drug Discov. Today 2006, 11, 8–20. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 26–37. [Google Scholar] [CrossRef]
- Yu, T.; Xu, Y.; Ahmad, M.A.; Javed, R.; Hagiwara, H.; Tian, X. Exosomes as a promising therapeutic strategy for peripheral nerve injury. Curr. Neuropharmacol. 2021, 19, 2141–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Li, P.; Jia, Y.; Liu, M.; Jiang, J. Concise review: Current understanding of extracellular vesicles to treat neuropathic pain. Front. Aging Neurosci. 2023, 15, 1131536. [Google Scholar] [CrossRef]
- Thakur, M.; Dickenson, A.H.; Baron, R. Osteoarthritis pain: Nociceptive or neuropathic? Nat. Rev. Rheumatol. 2014, 10, 374–380. [Google Scholar] [CrossRef]
- Wu, C.; He, Y.; Yao, Y.; Yang, H.; Lu, F. Exosomes treating osteoarthritis: Hope with challenge. Helyon 2023, 9, e13152. [Google Scholar] [CrossRef]
- Mianehsaz, E.; Mirzaei, H.R.; Mahjoubin-Tehran, M.; Rezaee, A.; Sahebnasagh, R.; Pourhanifeh, M.H.; Mirzaei, H.; Hamblin, M.R. Mesenchymal stem cell-derived exosomes: A new therapeutic approach to osteoarthritis? Stem Cell Res. Ther. 2019, 10, 340. [Google Scholar] [CrossRef]
- Yin, H.; Li, M.; Tian, G.; Ma, Y.; Ning, C.; Yan, Z.; Wu, J.; Ge, Q.; Sui, X.; Liu, S.; et al. The role of extracellular vesicles in osteoarthritis treatment via microenvironment regulation. Biomater. Res. 2022, 26, 52. [Google Scholar] [CrossRef]
- Seyhan, A.A. Lost in translation: The valley of death across preclinical and clinical divide-identification of problems and overcoming obstacles. Transl. Med. Commun. 2019, 4, 18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Francesco, F.; Zingaretti, N.; Parodi, P.C.; Riccio, M. The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine. Cells 2023, 12, 2567. https://doi.org/10.3390/cells12212567
De Francesco F, Zingaretti N, Parodi PC, Riccio M. The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine. Cells. 2023; 12(21):2567. https://doi.org/10.3390/cells12212567
Chicago/Turabian StyleDe Francesco, Francesco, Nicola Zingaretti, Pier Camillo Parodi, and Michele Riccio. 2023. "The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine" Cells 12, no. 21: 2567. https://doi.org/10.3390/cells12212567
APA StyleDe Francesco, F., Zingaretti, N., Parodi, P. C., & Riccio, M. (2023). The Evolution of Current Concept of the Reconstructive Ladder in Plastic Surgery: The Emerging Role of Translational Medicine. Cells, 12(21), 2567. https://doi.org/10.3390/cells12212567