1. Introduction
Solid tumors are often considered as abnormal organs not only composed of the tumor cells but also the surrounding tumor microenvironment which mainly contains fibroblasts, immune cells, blood vessels, lymphatic vessels, and the extracellular matrix [
1,
2]. The tumor microenvironment plays a very important role in tumor development and resistance to drug treatment. The functional and physical interaction of the tumor microenvironment with cancer cells plus the variations of the vascular networks within the tumor contribute to inter- and intratumoral heterogeneity and ultimately influence clinical outcomes [
3,
4]. Therefore, for personalized medicine in precision oncology, it is crucial that a preclinical model captures the complexity and heterogeneity of tumor biology ex vivo to individually predict in vivo therapy of tumors. Precision-cut tumor slices (PCTS) of 200–300 µm thickness maintain both the three-dimensional architecture and tumor heterogeneity, in addition to preserving the native microenvironment concerning different cell types and the extracellular matrix [
2]. There have been a growing number of publications using tumor slices as a model to study the tumor microenvironment and address the response of drug treatment [
5,
6,
7,
8,
9,
10,
11].
Davies et al. [
12] described a standardized workflow for systematic comparison of different tumor slice cultivation methods and showed that the cultivation of tumor slices requires organotypic support materials and atmospheric oxygen to maintain the viability and structure of the tumor slices. The Millipore filter (MF) support culture system under atmosphere was found as one of the promising systems, but it is still associated with significant temporal and loco-regional changes in protein expression such as estrogen receptor (ER) and hypoxia-inducible factor α (HIF1α) in slices of the MCF-7 cell line-derived xenograft (CDX). The development of loco-regional heterogeneity during slice culture makes the data interpretation more complicated in the studies especially with pharmacological perturbation [
12,
13]. Due to the lack of functional vasculature in the tumor slices, the diffusion of oxygen, nutrients, and drugs are influenced by several variables. Still, it is not yet established in common tumor slice cultures to combine possible perfusion systems to mimic the vasculature on both sides of the tumor slice and further optimize oxygen and nutrients supply and drug distribution [
12,
14].
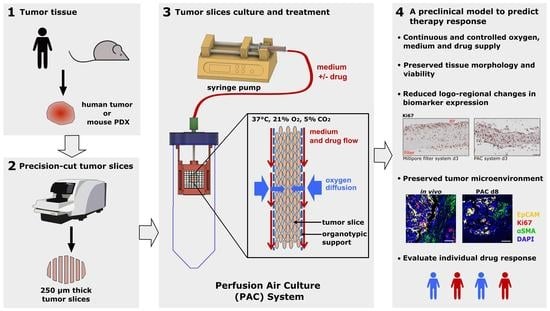
Recently, we have developed a perfusion air culture (PAC) system which integrates a perfusion system to partially mimic the vasculature in tumor slices with a continuous and controlled oxygen, medium, and drug supply. In the PAC system, precision-cut tumor slices (PCTS) were kept in between two organotypic supports fixed in a special chamber and placed inside of a 50 mL tube with air exchange capacity housed in a standard CO
2-incubator. Cotton meshes were used as organotypic supports to cover both sides of the tumor slice in the system. Due to their material structure, the cotton fibers can function as capillaries to supply medium containing nutrients and drugs to the tumor slice. Meanwhile, the relatively big open pores of the cotton mesh allow the oxygen to be easily diffused into the tumor slice from both sides during cultivation. It has been stated that the key parameters governing tissue viability are organotypic filter supports and oxygen levels [
12]. The PAC system fulfills these criteria and can supply the oxygen, nutrients, and drugs from the same direction to the tumor slice imitating the in vivo situation. For the in vivo tumor, viable tumor cells were not observed at distances greater than 160 μm from blood vessels [
15]. In addition, it was demonstrated that the oxygen can only be diffused to a distance of about 100 µm from a blood vessel [
16,
17]. In the PAC system, the two cotton meshes supported on both sides of the tumor slice work to mimic the vasculature in a tumor and guarantee the equal distribution and diffusion of oxygen, nutrients, and drugs from both sides of the tumor slices into the deeper cell layers.
Tumor tissue is complex and dynamic, and there are interactions between cancer cells and immune cells in the tumor [
1]. In recent years, there has been an increasing interest in using tumor slice cultures as a model to study the tumor immune microenvironment and the immune response of the tumor to the drug treatment [
10,
18]. Immune cell survival after slice culture in pancreatic cancer has been reported [
10]. The immune cells in the slices responded predictably to an immuno-modulator and anti-programmed death- ligand 1 (PD-L1) checkpoint inhibitor blockade [
18]. To test whether the PAC system is also suitable to assess functional immune response in tumor slices, we analyzed the immune cells of tumor slices before and after cultivation. The patient-specific immune cells and their composition were preserved throughout the culture period in the PAC system. Increased expression of PD-L1 was observed in the tumor slices after cisplatin treatment.
In this study, we introduce a novel PAC system to culture the tumor slices. It overcomes the problems of the static MF culture system, better represents the complexities of tumor biology, and facilitates the homogenous and controlled supply of oxygen, nutrients, and drugs. It allows the long-term culture of tumor tissue and the analysis of therapy response, including immune therapy, and is thus suitable for individual testing of drug efficacy to predict patient response and enhance drug selection for clinical trials.
2. Materials and Methods
Perfusion air culture system construction: The designed chambers (
Figure 1) were printed by a 3D printer (Printrbot Simple Metal, Printbot, Lincoln, CA, USA) with polylactic acid (PLA) using the stock thermoplastic extruder. The software used for printing the STL file was Cura Ultimaker. The printed chambers were immersed in 70% ethanol for 15 min, followed by drying out under the cell culture hood for sterilization. The chamber consists of two main components (a and b,
Supplementary Figure S1a), which can be assembled very easily under sterile conditions using a click system. Both components together form the holder for the organotypic supports (component c in
Supplementary Figure S1a) for the tumor slices. The filter papers (Whatman™ Cellulose Blotting Papers, Grade GB003, #10426892 Cytiva, Marlborough, MA, USA), which are shown as component d in
Supplementary Figure S1a, with the property of diffusion were fixed in the chambers (components a and b) and served as a reservoir for the nutrient fluid. The tumor slices were kept in between two organotypic supports and fixed in the chamber (
Supplementary Figure S1b). The organotypic supports used in this study were cotton mesh (ES-Kompressen, #2050040 HARTMANN, Heidenheim, Germany, for xenograft tumor slice cultivation and aluderm
® Kompressen #KR03029, SÖHNGEN, Taunusstein, Germany, for human ovarian tumor slice cultivation). The decellularized porcine intestine scaffold which was kindly provided by Prof. Heike Walles (Otto-von-Guericke-University, Magdeburg, Germany) was used for the long-term cultivation of human ovarian tumor slices in the PAC system. The chamber was settled vertically inside of a 50 mL tube with air exchange capacity (TubeSpin
® Bioreactor 50 with Septum, TPP, Trasadingen, Switzerland). A needle went through the lid of the tube and was inserted in the top of the chamber. The needle was connected to a silicone tube with an inside diameter (ID) of 0.5 mm and an outside diameter (OD) of 2.5 mm. The 1 m-long gas-permeable silicone tube was further connected to a syringe pump for delivery of the medium (
Figure 1). The commercially available syringe pump allows precise, low-speed perfusion that can be matched to blood perfusion rates in capillaries (e.g., 50–100 µL/hour;
Figure 1). Cultivation was performed in a regular cell culture incubator at 37 °C and 5% CO
2 under atmospheric oxygen (21% oxygen) conditions.
Cell-line-derived xenograft (CDX) tumor samples: The local committees approved all of the animal facilities and handling protocols on the ethics of animal experiments, as required in each country, and adhered to the European Convention for Protection of Vertebrate Animals used for Experimental Purposes (Directive 2010/63/EU). Experiments with mice performed at AstraZeneca were compliant with the UK Animals (Scientific Procedures) Act, which is consistent with EU Directive 2010/63/EU and had undergone internal ethical review. At Charles River Germany GmbH, experiments carried out with mice were scrutinized by the Committee on the Ethics of Animal Experiments of the regional council (Regierungspräsidium Freiburg, Abt. Landwirtschaft, Ländlicher Raum, Veterinär- und Lebensmittelwesen).
The breast CDX MCF-7 was derived by subcutaneous injection of 5 × 106 MCF-7 cells (ATCC-HTB-22) per 0.1 mL in 50:50 basal media Matrigel (#356234, BD Biosciences, San Jose, CA, USA) into the left flank of male SCID mice (SCID/CB17). The animals were implanted with 0.5 mg/21 day 17β oestradiol pellets (Innovative Research of America) one day before cell implant. The lung CDX NCI-H1437 was derived by subcutaneous injection of 5 × 106 NCI-H1437 cells (ATCC-CRL-5872) into the flank of 4–6-week-old NMRI nu/nu mice. All of the cell lines were routinely (every 3 months) checked for mycoplasma contamination and the master stock of the cells was authenticated using STR analysis before injected being into the mice.
Tumors from mouse xenografts were harvested when the volume reached between 0.4–1 cm3. The animals were euthanized by cervical dislocation and tumors were excised. A small sample of the tumor was either snap-frozen using liquid nitrogen or fixed in 10% neutral buffered formalin immediately after resection. Tumors were placed into ice-cold MACS Tissue Storage Solution (#130-100-008, Miltenyi Biotec, Bergisch Gladbach, Germany) before tissue slicing.
Primary human ovarian tumors: Sterile fresh tissue was obtained during debulking surgery in case of ovarian cancer first detected by a frozen section and confirmed by final histological examination at the Robert Bosch Hospital, Stuttgart. Immediately after surgical resection, the tumor tissue was maintained in ice-cold MACS Tissue Storage Solution (#130-100-008, Miltenyi Biotec) until use. The procedure had been approved by the local ethics committee (397/2016BO1) and informed consent from all participating subjects was obtained.
Tumor slice preparation and cultivation: Preparation of tumor slices was performed as described previously [
12]. The tumors were mounted onto the magnetic specimen holder of a Leica VT1200S vibrating blade microtome using cyanoacrylate adhesive. The tumor slices were prepared at a thickness of 250 μm (MCF-7 CDX and H1437 CDX) and 280 μm (primary OV) using the precision-cut vibratome. The slices were visually inspected whilst being cut to ensure the tissue was not compressed or torn, resulting in an inconsistent slice thickness. Generally, 15–25 slices can be obtained from one tumor depending on the size and condition of the tumor tissue. MCF-7 CDX slices were cultivated in DMEM (#31053-028, Gibco, Grand Island, NY, USA); H1437 CDX and primary OV slices were cultivated in RPMI 1640 (# F1215, Biochrom AG, Berlin, Germany). The medium was supplemented with glutamine (2 mM; Gibco), penicillin (100 U/mL) and streptomycin (100 μg/mL; Gibco), and 10% fetal bovine serum (FBS; #10082, Gibco).
Cultivation was performed at 37 °C and 5% CO
2 in a humidified atmosphere under atmospheric oxygen (21% oxygen) conditions. The tumor slices were maintained on a Millipore filter (Millicell Cell Culture inserts, #PICM ORG 50, pore size 0.4 μm, Merck Millipore, PTFE, Merck KGaA, Darmstadt, Germany) with an air-liquid interface in a six-well plate with 1.5 mL medium under the filter in each well and one drop of medium on the top of each slice. The tumor slices were alternatively cultured on the self-made perfusion air culture (PAC) system illustrated in
Figure 1. The slices were harvested at different time points. The snap-frozen samples were either collected for RNA isolation (three to four slices per condition) or fixed in 10% neutral buffered formalin for immunohistochemistry (IHC) (one slice per condition). Overall, at least 12–15 slices were analyzed per tumor. The tissue fixed immediately after surgical resection was defined as the in vivo sample. The tissue after the slicing process and before tumor slice cultivation was defined as the d0 sample. The fixed slices were embedded in paraffin in vertical orientation as published in Davies et al., 2015 [
12].
Drug treatment: Tumor slices from both xenografts and primary human ovarian tumors were treated with cisplatin (Teva®, Ulm, Germany) in both MF and PAC systems. With a final concentration of 13 µM, cisplatin was applied in 1.5 mL medium in the MF system with a drop of medium containing cisplatin on the top of the tumor slices. In the PAC system, the cisplatin in the medium was prepared in a 10 mL syringe and continually applied to the tumor slices through a silicone tube. In order to minimize the influence of tumor heterogeneity, the control and treated slices were always adjacent slices in the tumor.
Immunohistochemical staining: The fixed tumor slices were cut into 4 μm serial sections by Rotary Microtome (Leica RM2255, Wetzlar, Germany). The paraffin sections were stained with hematoxylin (#1.09253.0500, Merck KGaA, Darmstadt, Germany) and eosin (R03040, Merck KGaA) (H&E) for histopathological examination. IHC was carried out by standard protocols as previously described. Briefly, the sections were deparaffinized in Microclear and rehydrated in graded ethanol followed by use of a Dako Envision Kit (#K5007, Agilent Technologies, Glostrup, Denmark) according to the manufacturer’s manual. Epitope retrieval was achieved in a steam heater for 30 min with either citric acid buffer pH 6 or Tris/EDTA buffer pH9 (Agilent Technologies, Dako). The primary antibodies were as follows: Ki67 (Clone MIB-1, Dako), hypoxia-inducible actor 1 α (HIF1α, #610959, BD Biosciences), cleaved-caspase 3 (CC3, #9661, Cell Signaling Technology, Danvers, MA, USA), an estrogen receptor (ER, Clone 6F11, #PA1051, LeicaBond), phospho-histone H2A.X (γH2AX, #2577, Cell Signaling Technology), CD8 (SP16, Cell Marque, Rocklin, CA, USA), CD4 (SP35, Cell Marque), CD68 (Kp-1, Cell Marque), FOXP3 (236A/E7, eBioscience), PD-1 (NAT105, Cell Marque), and PD-L1 (E1L3N, Cell Signaling Technology). The antibodies were visualized using 3,3′-diaminobenzidine (DAB) chromogen and counterstained with hematoxylin. Images were taken by an Olympus slide scanner VS120.
Multiplex immunohistochemical staining: The tissue sections were prepared, deparaffinized, rehydrated, subjected to heat-induced epitope retrieval, and incubated with primary and secondary antibodies as described for immunohistochemical staining. The antibodies were visualized using fluorescent tyramide. The process of epitope retrieval and staining was repeated sequentially for different primary antibody and fluorescent tyramide combinations. The following tyramide dyes were used: CF®488 (#92171, Biotium, Fremont, CA, USA), CF®555 (#96021, Biotium), and CF®640R (#92175, Biotium). After all of the staining steps, the sections were mounted using a DAPI-containing mounting medium (EverBrite™, Biotium). Images were acquired using a Leica TCS SP8 confocal microscope.
Quantification of immunohistochemical staining: The quantification of immunohistochemical staining images was performed in whole tissue sections using the computer-aided image analysis software Tissue Studio from Definiens and QuPath. These programs allow quantification of the positively stained cell numbers in user-defined regions of interest (ROIs). Within these ROIs, algorithms were used that detect nuclei, membranous, and cytoplasmic staining. The mean percentages of positively stained tumor cells from IHC quantification were calculated across tumor types from at least three independent experiments. The Wilcoxon matched-pairs test was used for statistical analysis in GraphPad Prism (GraphPad Software, San Diego, CA, USA). Loco-regional changes in biomarker expression across different areas of the tumor slices were quantified by splitting the tumor slice longitudinally into three layers using ROIs (MF: Filter side, Middle, Air side; PAC: Air side-1, Middle, Air side-2). The percentage difference (|Difference|/Average × 100%) in the positively stained cells between the two outer layers was calculated (MF: Filter side and Air side; PAC: Air side-1 and Air side-2). The average was defined as the average of positively stained cells of the three layers in the tumor slice. Tumor slices with a percentage difference of >20% were defined as having a gradient for this biomarker. The detailed workflow for determining the biomarker expression gradient is shown in
Supplementary Figure S2.
High-throughput TaqMan-based qPCR Fluidigm: Snap-frozen tumor slices were used for total RNA extraction. Lysing matrix D tubes were used to prepare tissue lysates in the FastPrep sample preparation system (MP Biomedicals, Santa Ana, CA, USA). Subsequently, RNA extraction was carried out using an RNeasy Mini Kit (Qiagen, Hilden, Germany) and on-column DNase (Qiagen) digestion was performed to eliminate genomic DNA contamination. M-MLV reverse transcriptase (Promega, Madison, WI, USA) was used to generate the cDNA. Expression analysis was performed on the BioMark HD System (Fluidigm, South San Francisco, CA, USA) according to the manufacturer´s instructions. The TaqMan assays were purchased from Applied Biosystems as previously described [
12].
Gene expression analysis: Gene expression qPCR data were normalized to a housekeeping gene and converted to log2 values. Differentially expressed genes were found using the limma package in R statistics environment [
19]. Tumor number was included in the linear model to take heterogeneity between the tumors into account. Genes with an FDR-corrected p-value less than 0.05 and a log-fold change of at least 1 were considered as significant.
Euclidean distance (square root of the sum of square differences) was used to measure the difference of the slice models from the in vivo situation. The function removeBatchEffect from limma R package was used to remove the effect of the tumor number before calculating Euclidean distances and completing principal component analysis (PCA) [
20]. PCA was completed using the prcomp function in base R. Genes containing any missing values were removed before calculating principal components. The scatterplot showed the first two components (those with the largest and second-largest variance).
4. Discussion
Precision-cut tumor slice culture has the ability to closely recapitulate the architecture and heterogeneity of the original tumors. Therefore, it can be used as a platform to study the tumor microenvironment and evaluate the preclinical efficacy of drug treatment. As a model, the tumor slice culture also has limitations that cannot be overcome with the currently available culture methods. A major limitation is the lack of functional vasculature in the tumor slice [
14]. Due to the loss of the intact circulatory network in the tumor slice, the availability of oxygen, nutrients, or drugs in tumor slices is strictly limited to diffusion [
12,
23]. In in vivo tumors, oxygen is provided by hemoglobin in red blood cells. Hemoglobin can deliver large amounts of oxygen to cells at relatively low oxygen tension. This unique property simply cannot be replicated in vitro [
24]. In the cell culture medium, an efficient oxygen carrier such as hemoglobin does not exist. It was shown that the oxygen concentration in the medium is about 2% of the oxygen content in the ambient atmosphere [
25]. The total amount of oxygen that can be supplied from the medium to the tumor slice is very limited. Studies have tried to increase the amount of dissolved oxygen in culture medium by using up to 95% oxygen in the culture. However, the increase in the oxygen concentration is still limited and may induce hyperoxia problems in the tissue [
24,
26]. Because of hemoglobin, blood as a liquid has the ability to offer comparable amounts of oxygen as the atmosphere, which has approximately 10 times higher oxygen content (delta of artery and venous blood oxygen content) than the medium (saturated with atmospheric oxygen) even under lower oxygen partial pressure with 100 mmHg [
27,
28]. Therefore, in vivo, the oxygen can be quickly transported and rapidly released to the tissue even in a low oxygen partial pressure environment. The special ability of hemoglobin ensures sufficient oxygen supply to the tissue without the problem of hyperoxia. The tumor slices do not have a functional blood supply; the oxygen must be diffused from ambient gases into the medium and then into the tumor slices. The PAC system has two sides of air-liquid interfaces, which can minimize the diffusion distance from the ambient gases to the tumor slice; therefore, it can provide a sufficient oxygen supply to the tumor slices during cultivation under a low oxygen tension. For the experiments, a normal tumor slice with 200–300 µm thickness and 5–10 mm diameter contains more than 5 million cells, which is about the same cell number of a confluent T75 cell culture flask. Normally, up to 20 mL medium is used for the 2D monolayer cell culture with the T75 flask. The commonly used Millipore filter (MF) system for tumor slice culture can only apply about 1.5 mL medium under static conditions, which is much less than the 2D monolayer cell culture system. This also explains the gradients from the air to the filter interface of the tumor slice cultured in the MF system. The tumor slice cultivated in the MF system has two different sides, the air-interface side and filter-liquid side. In the in vivo tumor, the oxygen, nutrients, or drugs can diffuse from the blood vessels to the cells [
1]. In the MF system, they are mainly provided from two different directions to the tumor slices. The oxygen can only diffuse mainly from the air-liquid interface side of the tumor slice, and the nutrients and drugs are mostly supplied from the filter-liquid side and have to first pass through the filter to reach the tumor slices. Considering that the MF system is a static culture, the exchange of nutrients has low efficiency. It cannot faithfully recapitulate the in vivo situation. The development of loco-regional heterogeneity in the MF culture system has been reported for both xenografts and patient tumors [
12]. This can also be observed in our data. Although gradients of oxygen tension are a common feature of solid tumors [
29], prudence is required when interpreting the data from the MF system culture with the gradients, especially after drug treatment, because the oxygen, nutrients, or drugs may be supplied from two separate sides of the tumor slice.
The PAC system overcomes this problem. The oxygen, nutrients, and drugs are supplied from both sides of the tumor slices with the same direction, which is closer to the in vivo situation. The cotton meshes on both sides of the tumor slice work not only as a support structure but also as vasculature in tumor slices. With this special structure, the thickness of the tumor slice can be increased and is not limited to 200–300 µm for cultivation. The hypoxic condition can also be created in the middle of the tumor slices when changing the oxygen supply to hypoxia in the PAC system. As one of the key parameters governing tissue viability, organotypic supports must fulfill two functions. One is to mimic the vasculature and supply sufficient oxygen and nutrients to the tumor tissue continuously; the other is to act as the extracellular matrix (ECM) to protect the thin and friable slice from the stress of fluidic flow and keep the stiffness of the tumor slices. It has been well known that tumors are much stiffer than normal tissues because of the changes in their ECM. The relative stiffness can have profound effects on cellular function [
1]. We have compared many different materials as organotypic supports for the PAC system. Nylon meshes with different pore sizes (89 µm, 41 µm, and 29 µm), polycarbonate membranes with a 12 µm pore size, and hydrogels did not show good oxygen and nutrients supply because of their small pore sizes and material structures (data not shown). Cotton meshes with 500 µm pore size showed the best oxygen, nutrient, and drug supply. Because of their big pore size, some cells on the surface of the slices were washed away during the culture in the PAC system. Therefore, we mainly used cotton meshes as organotypic supports for 3-day, short-term experiments. The decellularized scaffold from the porcine intestine can better protect the slices from the shear stress of fluidic flow. We used it together with cotton meshes for long-term cultivation of the tumor slices. Thus, the primary OV tumor slices can be cultured for up to 8 days within the PAC system. Although the cotton mesh showed better properties than the other organotypic supports tested, it still has drawbacks. It may wash away cells from tumor slices during culture and it is difficult to section the cotton fibers together with the tissue in the formalin-fixed paraffin-embedded (FFPE) samples for IHC staining. The scaffold from the porcine intestine does not have this problem, but it still requires the addition of a cotton mesh to maintain a better distribution of the medium to the slices. In addition, it was presumed that atmospheric concentrations of oxygen can produce hyperoxic conditions at the air-liquid interface side to allow a supply of just sufficient oxygen to deeper cell layers [
12]. In the PAC system, if the oxygen can be supplied from both sides of the tumor slice, the oxygen concentration in the atmosphere can be reduced to avoid hyperoxic damage to the tumor slices. Overall, the PAC system still needs to be further optimized especially with regards to supports and a suitable oxygen concentration surrounding the tumor slices during culture.
Cultivation of tumor slices induces similar changes in key stress pathways in the MF and PAC systems. This result can be confirmed with the Euclidean distance of stress gene biomarker expression. The in vivo primary OV samples showed a high heterogeneity of the cluster pattern in the PCA plots, making it hard to draw a conclusion from the primary OV tissue data. Therefore, IHC staining is a better method to evaluate the primary OV tumor slices. Because of the highly inter- and intratumoral heterogeneity of the patient tumors, the tissue structure and the spatial distribution of biomarker expression in tumors are more important.
Unlike the CDX tumor slices, the primary OV tumor slices showed gradient patterns in the MF system culture in only 9 out of 15 cases. There could be two reasons for this. One is the heterogeneity of the primary OV of patients; the other is that the proliferation rate of the tumor cells in primary OV is much slower than the tumor cells in CDX. This can also explain why the cisplatin treatment induced Ki67 expression in H1437 CDX but not in primary OV tumor slices. Different primary OV tumors showed different responses to cisplatin treatment. This also reflects the heterogeneity of the primary OV and indicates the necessity of personalized therapy. Cisplatin treatment was accompanied by a minor increase in γH2AX in both MF and PAC systems while strongly enhanced CC3 was observed only in the PAC system. This might reflect higher aerobic metabolism in the PAC system [
22]. This indicates that the functional response to drug treatment is more sensitive in the PAC system. Further studies will need to be undertaken which correlate the responses of primary OV tumor slices to cisplatin treatment with clinical outcomes to predict patient response, especially the large difference in CC3 induction between primary OV tumor slices.
Several reports have shown that tumor slices can be used to study the immune microenvironment and test the immune responses of drug treatment [
10,
18]. Here, we provide evidence that the tumor slices cultured in the PAC system are suitable for studying the tumor immune cell environment and the immune response of drug treatment. Interestingly, PD-L1 expression was increased individually after cisplatin treatment compared to the untreated control group in primary OV tumors (
Figure 10d,e). The PAC system showed comparable and even more sensitive data in comparison to the MF system (
Supplementary Figure S5). Other studies have shown that non-small cell lung cancer patients who received cisplatin-based neoadjuvant chemotherapy followed by surgery have significantly increased PD-L1 expression in both tumor cells and immune cells from the microenvironment [
30]. Our data also demonstrated that not only the EpCAM+ cells showed increased PD-L1 expression. It is also reported that cisplatin induces PD-L1 over-expression in hepatoma H22 cells [
31]. Cisplatin also upregulates PD-L1 expression in vitro and in vivo in ovarian cancer mouse models [
32]. Our results on tumor slices support the evidence from previous observations and indicate that the PAC system is a reliable system for studying drug treatment and predicting in vivo drug response.