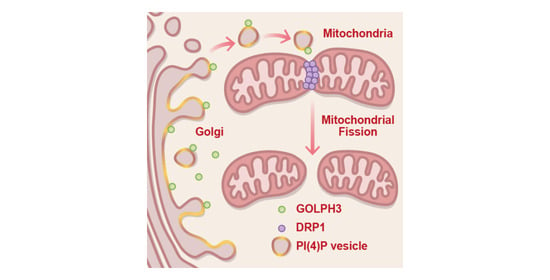
GOLPH3 Participates in Mitochondrial Fission and Is Necessary to Sustain Bioenergetic Function in MDA-MB-231 Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. DNA Constructs and Cell Reagents
2.2. Cell Culture and Transfection
2.3. Time-Lapse Microscopy and Image Processing
2.4. Generation of CRISPR-Cas9-Mediated GOLPH3 Knockout Cells
2.5. Antibodies
2.6. Immunofluorescence and Image Analysis
2.7. Transmission Electron Microscopy and Quantitative Analysis of Mitochondria
2.8. Preparation of Protein Extracts, SDS-PAGE, Immunoblotting, and Densitometric Quantification
2.9. ATP and ROS Quantification
2.10. Mitochondrial Membrane Potential
2.11. Oxygen Consumption
2.12. Statistical Analysis
3. Results
3.1. GFP-GOLPH3 Associates with the PI(4)P Reporter mCherry-PH-FAPP1 at Sites of Mitochondrial Fission
3.2. GFP-GOLPH3 Arrives at Mitochondrial Fission Sites after the Recruitment of YFP-DRP1
3.3. The Depletion of GOLPH3 in MDA-MB-231 Cells Disrupts the Structure of the Mitochondrial Network
3.4. The Depletion of GOLPH3 in MDA-MB-231 Cells Affects the Levels of Mitochondrial Fusion and Fission Proteins
3.5. The Depletion of GOLPH3 in MDA-MB-231 Cells Reduces the Mitochondrial Bioenergetic Capacity
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sarkar, S.; Ali, M.Z.; Choubey, S. Efficacy of Information Transmission in Cellular Communication. Phys. Rev. Res. 2023, 5, 013092. [Google Scholar] [CrossRef]
- David, Y.; Castro, I.G.; Schuldiner, M. The Fast and the Furious: Golgi Contact Sites. Contact 2021, 4, 1–15. [Google Scholar] [CrossRef]
- Hanada, K. Ceramide Transport from the Endoplasmic Reticulum to the Trans Golgi Region at Organelle Membrane Contact Sites. Adv. Exp. Med. Biol. 2017, 997, 69–81. [Google Scholar] [CrossRef]
- Clausmeyer, L.; Fröhlich, F. Mechanisms of Nonvesicular Ceramide Transport. Contact 2023, 6, 25152564231208250. [Google Scholar] [CrossRef]
- Scorrano, L.; De Matteis, M.A.; Emr, S.; Giordano, F.; Hajnóczky, G.; Kornmann, B.; Lackner, L.L.; Levine, T.P.; Pellegrini, L.; Reinisch, K.; et al. Coming Together to Define Membrane Contact Sites. Nat. Commun. 2019, 10, 1287. [Google Scholar] [CrossRef] [PubMed]
- Dolman, N.J.; Gerasimenko, J.V.; Gerasimenko, O.V.; Voronina, S.G.; Petersen, O.H.; Tepikin, A. V Stable Golgi-Mitochondria Complexes and Formation of Golgi Ca(2+) Gradients in Pancreatic Acinar Cells. J. Biol. Chem. 2005, 280, 15794–15799. [Google Scholar] [CrossRef] [PubMed]
- Nagashima, S.; Tábara, L.-C.; Tilokani, L.; Paupe, V.; Anand, H.; Pogson, J.H.; Zunino, R.; McBride, H.M.; Prudent, J. Golgi-Derived PI(4)P-Containing Vesicles Drive Late Steps of Mitochondrial Division. Science 2020, 367, 1366–1371. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wei, Y.; Zhang, M.; Zhang, W.; Huang, Y.; Zhang, Y.-H. PI4P-Containing Vesicles from Golgi Contribute to Mitochondrial Division by Coordinating with Polymerized Actin. Int. J. Mol. Sci. 2023, 24, 6593. [Google Scholar] [CrossRef]
- Wu, C.C.; Taylor, R.S.; Lane, D.R.; Ladinsky, M.S.; Weisz, J.A.; Howell, K.E. GMx33: A Novel Family of Trans-Golgi Proteins Identified by Proteomics. Traffic 2000, 1, 963–975. [Google Scholar] [CrossRef] [PubMed]
- Tenorio, M.J.; Ross, B.H.; Luchsinger, C.; Rivera-Dictter, A.; Arriagada, C.; Acuña, D.; Aguilar, M.; Cavieres, V.; Burgos, P.V.; Ehrenfeld, P.; et al. Distinct Biochemical Pools of Golgi Phosphoprotein 3 in the Human Breast Cancer Cell Lines MCF7 and MDA-MB-231. PLoS ONE 2016, 11, e0154719. [Google Scholar] [CrossRef]
- Wood, C.S.; Schmitz, K.R.; Bessman, N.J.; Setty, T.G.; Ferguson, K.M.; Burd, C.G. PtdIns4P Recognition by Vps74/GOLPH3 Links PtdIns 4-Kinase Signaling to Retrograde Golgi Trafficking. J. Cell Biol. 2009, 187, 967–975. [Google Scholar] [CrossRef]
- Dippold, H.C.; Ng, M.M.; Farber-Katz, S.E.; Lee, S.-K.; Kerr, M.L.; Peterman, M.C.; Sim, R.; Wiharto, P.A.; Galbraith, K.A.; Madhavarapu, S.; et al. GOLPH3 Bridges Phosphatidylinositol-4-Phosphate and Actomyosin to Stretch and Shape the Golgi to Promote Budding. Cell 2009, 139, 337–351. [Google Scholar] [CrossRef]
- Snyder, C.M.; Mardones, G.A.; Ladinsky, M.S.; Howell, K.E. GMx33 Associates with the Trans-Golgi Matrix in a Dynamic Manner and Sorts within Tubules Exiting the Golgi. Mol. Biol. Cell 2006, 17, 511–524. [Google Scholar] [CrossRef]
- Shewan, A.; Eastburn, D.J.; Mostov, K. Phosphoinositides in Cell Architecture. Cold Spring Harb. Perspect. Biol. 2011, 3, a004796. [Google Scholar] [CrossRef]
- Scott, K.L.; Kabbarah, O.; Liang, M.-C.; Ivanova, E.; Anagnostou, V.; Wu, J.; Dhakal, S.; Wu, M.; Chen, S.; Feinberg, T.; et al. GOLPH3 Modulates MTOR Signalling and Rapamycin Sensitivity in Cancer. Nature 2009, 459, 1085–1090. [Google Scholar] [CrossRef]
- Li, W.; Guo, F.; Gu, M.; Wang, G.; He, X.; Zhou, J.; Peng, Y.; Wang, Z.; Wang, X. Increased Expression of GOLPH3 Is Associated with the Proliferation of Prostate Cancer. J. Cancer 2015, 6, 420–429. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Pan, H.; Wei, W.; Yang, H.; Liu, J.; Yang, R. GOLPH3: A Novel Biomarker That Correlates with Poor Survival and Resistance to Chemotherapy in Breast Cancer. Oncotarget 2017, 8, 105155–105169. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Jiang, B.; Chen, L.; Di, J.; Cui, M.; Liu, M.; Ma, Y.; Yang, H.; Xing, J.; Zhang, C.; et al. GOLPH3 Predicts Survival of Colorectal Cancer Patients Treated with 5-Fluorouracil-Based Adjuvant Chemotherapy. J. Transl. Med. 2014, 12, 15. [Google Scholar] [CrossRef] [PubMed]
- Sechi, S.; Frappaolo, A.; Karimpour-Ghahnavieh, A.; Piergentili, R.; Giansanti, M.G. Oncogenic Roles of GOLPH3 in the Physiopathology of Cancer. Int. J. Mol. Sci. 2020, 21, 933. [Google Scholar] [CrossRef] [PubMed]
- Taft, M.H.; Behrmann, E.; Munske-Weidemann, L.-C.; Thiel, C.; Raunser, S.; Manstein, D.J. Functional Characterization of Human Myosin-18A and Its Interaction with F-Actin and GOLPH3. J. Biol. Chem. 2013, 288, 30029–30041. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.F.; Chachadi, V.B.; Petrosyan, A.; Cheng, P.-W. Golgi Phosphoprotein 3 Determines Cell Binding Properties under Dynamic Flow by Controlling Golgi Localization of Core 2 N-Acetylglucosaminyltransferase 1. J. Biol. Chem. 2012, 287, 39564–39577. [Google Scholar] [CrossRef] [PubMed]
- Isaji, T.; Im, S.; Gu, W.; Wang, Y.; Hang, Q.; Lu, J.; Fukuda, T.; Hashii, N.; Takakura, D.; Kawasaki, N.; et al. An Oncogenic Protein Golgi Phosphoprotein 3 Up-Regulates Cell Migration via Sialylation. J. Biol. Chem. 2014, 289, 20694–20705. [Google Scholar] [CrossRef] [PubMed]
- Pereira, N.A.; Pu, H.X.; Goh, H.; Song, Z. Golgi Phosphoprotein 3 Mediates the Golgi Localization and Function of Protein O-Linked Mannose β-1,2-N-Acetlyglucosaminyltransferase 1. J. Biol. Chem. 2014, 289, 14762–14770. [Google Scholar] [CrossRef]
- Farber-Katz, S.E.; Dippold, H.C.; Buschman, M.D.; Peterman, M.C.; Xing, M.; Noakes, C.J.; Tat, J.; Ng, M.M.; Rahajeng, J.; Cowan, D.M.; et al. DNA Damage Triggers Golgi Dispersal via DNA-PK and GOLPH3. Cell 2014, 156, 413–427. [Google Scholar] [CrossRef]
- Arriagada, C.; Luchsinger, C.; González, A.E.; Schwenke, T.; Arriagada, G.; Folch, H.; Ehrenfeld, P.; Burgos, P.V.; Mardones, G.A. The Knocking down of the Oncoprotein Golgi Phosphoprotein 3 in T98G Cells of Glioblastoma Multiforme Disrupts Cell Migration by Affecting Focal Adhesion Dynamics in a Focal Adhesion Kinase-Dependent Manner. PLoS ONE 2019, 14, e0212321. [Google Scholar] [CrossRef]
- Nakashima-Kamimura, N.; Asoh, S.; Ishibashi, Y.; Mukai, Y.; Shidara, Y.; Oda, H.; Munakata, K.; Goto, Y.-I.; Ohta, S. MIDAS/GPP34, a Nuclear Gene Product, Regulates Total Mitochondrial Mass in Response to Mitochondrial Dysfunction. J. Cell Sci. 2005, 118, 5357–5367. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.F.; Whitaker-Menezes, D.; Lin, Z.; Martinez-Outschoorn, U.E.; Tanowitz, H.B.; Al-Zoubi, M.S.; Howell, A.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Two-Compartment Tumor Metabolism: Autophagy in the Tumor Microenvironment and Oxidative Mitochondrial Metabolism (OXPHOS) in Cancer Cells. Cell Cycle 2012, 11, 2545–2559. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Parra, V.; Eisner, V.; Chiong, M.; Criollo, A.; Moraga, F.; Garcia, A.; Härtel, S.; Jaimovich, E.; Zorzano, A.; Hidalgo, C.; et al. Changes in Mitochondrial Dynamics during Ceramide-Induced Cardiomyocyte Early Apoptosis. Cardiovasc. Res. 2008, 77, 387–397. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Arrázola, M.S.; Inestrosa, N.C. Monitoring Mitochondrial Membranes Permeability in Live Neurons and Mitochondrial Swelling through Electron Microscopy Analysis. Methods Mol. Biol. 2015, 1254, 87–97. [Google Scholar] [CrossRef]
- Jara, C.; Aránguiz, A.; Cerpa, W.; Tapia-Rojas, C.; Quintanilla, R.A. Genetic Ablation of Tau Improves Mitochondrial Function and Cognitive Abilities in the Hippocampus. Redox Biol. 2018, 18, 279–294. [Google Scholar] [CrossRef]
- Torres, A.K.; Jara, C.; Olesen, M.A.; Tapia-Rojas, C. Pathologically Phosphorylated Tau at S396/404 (PHF-1) Is Accumulated inside of Hippocampal Synaptic Mitochondria of Aged Wild-Type Mice. Sci. Rep. 2021, 11, 4448. [Google Scholar] [CrossRef]
- Olesen, M.A.; Torres, A.K.; Jara, C.; Murphy, M.P.; Tapia-Rojas, C. Premature Synaptic Mitochondrial Dysfunction in the Hippocampus during Aging Contributes to Memory Loss. Redox Biol. 2020, 34, 101558. [Google Scholar] [CrossRef]
- Godi, A.; Di Campli, A.; Konstantakopoulos, A.; Di Tullio, G.; Alessi, D.R.; Kular, G.S.; Daniele, T.; Marra, P.; Lucocq, J.M.; De Matteis, M.A. FAPPs Control Golgi-to-Cell-Surface Membrane Traffic by Binding to ARF and PtdIns(4)P. Nat. Cell Biol. 2004, 6, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.; Gaume, B.; Bergmann-Leitner, E.S.; Leitner, W.W.; Robert, E.G.; Catez, F.; Smith, C.L.; Youle, R.J. The Role of Dynamin-Related Protein 1, a Mediator of Mitochondrial Fission, in Apoptosis. Dev. Cell 2001, 1, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.J.; Rimmer, K.A.; Mertens, H.D.T.; Waller, R.F.; Mulhern, T.D.; Lithgow, T.; Gooley, P.R. Structure, Topology and Function of the Translocase of the Outer Membrane of Mitochondria. Plant Physiol. Biochem. 2008, 46, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Donnarumma, E.; Kohlhaas, M.; Vimont, E.; Kornobis, E.; Chaze, T.; Gianetto, Q.G.; Matondo, M.; Moya-Nilges, M.; Maack, C.; Wai, T. Mitochondrial Fission Process 1 Controls Inner Membrane Integrity and Protects against Heart Failure. Nat. Commun. 2022, 13, 6634. [Google Scholar] [CrossRef] [PubMed]
- Benard, G.; Rossignol, R. Ultrastructure of the Mitochondrion and Its Bearing on Function and Bioenergetics. Antioxid. Redox Signal. 2008, 10, 1313–1342. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Hu, J. Mitochondrial Fusion: The Machineries in and out. Trends Cell Biol. 2021, 31, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Kashatus, J.A.; Nascimento, A.; Myers, L.J.; Sher, A.; Byrne, F.L.; Hoehn, K.L.; Counter, C.M.; Kashatus, D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell 2015, 57, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Chan, D.C. The Mitochondrial Fission Receptor Mff Selectively Recruits Oligomerized Drp1. Mol. Biol. Cell 2015, 26, 4466–4477. [Google Scholar] [CrossRef] [PubMed]
- Otera, H.; Wang, C.; Cleland, M.M.; Setoguchi, K.; Yokota, S.; Youle, R.J.; Mihara, K. Mff Is an Essential Factor for Mitochondrial Recruitment of Drp1 during Mitochondrial Fission in Mammalian Cells. J. Cell Biol. 2010, 191, 1141–1158. [Google Scholar] [CrossRef] [PubMed]
- Jeong, S.H.; Kim, H.K.; Song, I.-S.; Noh, S.J.; Marquez, J.; Ko, K.S.; Rhee, B.D.; Kim, N.; Mishchenko, N.P.; Fedoreyev, S.A.; et al. Echinochrome a Increases Mitochondrial Mass and Function by Modulating Mitochondrial Biogenesis Regulatory Genes. Mar. Drugs 2014, 12, 4602–4615. [Google Scholar] [CrossRef] [PubMed]
- Jhun, B.S.; O-Uchi, J.; Adaniya, S.M.; Mancini, T.J.; Cao, J.L.; King, M.E.; Landi, A.K.; Ma, H.; Shin, M.; Yang, D.; et al. Protein Kinase D Activation Induces Mitochondrial Fragmentation and Dysfunction in Cardiomyocytes. J. Physiol. 2018, 596, 827–855. [Google Scholar] [CrossRef] [PubMed]
- Lunetti, P.; Di Giacomo, M.; Vergara, D.; De Domenico, S.; Maffia, M.; Zara, V.; Capobianco, L.; Ferramosca, A. Metabolic Reprogramming in Breast Cancer Results in Distinct Mitochondrial Bioenergetics between Luminal and Basal Subtypes. FEBS J. 2019, 286, 688–709. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef]
- Vianello, C.; Dal Bello, F.; Shin, S.H.; Schiavon, S.; Bean, C.; Magalhães Rebelo, A.P.; Knedlík, T.; Esfahani, E.N.; Costiniti, V.; Lacruz, R.S.; et al. High-Throughput Microscopy Analysis of Mitochondrial Membrane Potential in 2D and 3D Models. Cells 2023, 12, 1089. [Google Scholar] [CrossRef]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial Electron Transport Chain: Oxidative Phosphorylation, Oxidant Production, and Methods of Measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef]
- St-Pierre, J.; Drori, S.; Uldry, M.; Silvaggi, J.M.; Rhee, J.; Jäger, S.; Handschin, C.; Zheng, K.; Lin, J.; Yang, W.; et al. Suppression of Reactive Oxygen Species and Neurodegeneration by the PGC-1 Transcriptional Coactivators. Cell 2006, 127, 397–408. [Google Scholar] [CrossRef] [PubMed]
- Zinovkin, R.A.; Lyamzaev, K.G.; Chernyak, B. V Current Perspectives of Mitochondria-Targeted Antioxidants in Cancer Prevention and Treatment. Front. Cell Dev. Biol. 2023, 11, 1048177. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Youle, R.J. The Role of Mitochondria in Apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.; Ryan, M.T. The Constriction and Scission Machineries Involved in Mitochondrial Fission. J. Cell Sci. 2017, 130, 2953–2960. [Google Scholar] [CrossRef]
- Buschman, M.D.; Xing, M.; Field, S.J. The GOLPH3 Pathway Regulates Golgi Shape and Function and Is Activated by DNA Damage. Front. Neurosci. 2015, 9, 362. [Google Scholar] [CrossRef] [PubMed]
- Sato, O.; Sakai, T.; Choo, Y.-Y.; Ikebe, R.; Watanabe, T.M.; Ikebe, M. Mitochondria-Associated Myosin 19 Processively Transports Mitochondria on Actin Tracks in Living Cells. J. Biol. Chem. 2022, 298, 101883. [Google Scholar] [CrossRef] [PubMed]
- Twig, G.; Shirihai, O.S. The Interplay between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef]
- van der Bliek, A.M.; Shen, Q.; Kawajiri, S. Mechanisms of Mitochondrial Fission and Fusion. Cold Spring Harb. Perspect. Biol. 2013, 5, a011072. [Google Scholar] [CrossRef]
- Cho, D.-H.; Nakamura, T.; Fang, J.; Cieplak, P.; Godzik, A.; Gu, Z.; Lipton, S.A. S-Nitrosylation of Drp1 Mediates Beta-Amyloid-Related Mitochondrial Fission and Neuronal Injury. Science 2009, 324, 102–105. [Google Scholar] [CrossRef]
- Che, T.-F.; Lin, C.-W.; Wu, Y.-Y.; Chen, Y.-J.; Han, C.-L.; Chang, Y.; Wu, C.-T.; Hsiao, T.-H.; Hong, T.-M.; Yang, P.-C. Mitochondrial Translocation of EGFR Regulates Mitochondria Dynamics and Promotes Metastasis in NSCLC. Oncotarget 2015, 6, 37349–37366. [Google Scholar] [CrossRef]
- Arriagada, C.; Cavieres, V.A.; Luchsinger, C.; González, A.E.; Muñoz, V.C.; Cancino, J.; Burgos, P.V.; Mardones, G.A. GOLPH3 Regulates EGFR in T98G Glioblastoma Cells by Modulating Its Glycosylation and Ubiquitylation. Int. J. Mol. Sci. 2020, 21, 8880. [Google Scholar] [CrossRef]
- Wang, K.; Qi, Y.; Wang, X.; Liu, Y.; Zhao, M.; Zhou, D.; Zhang, Y.; Wang, Y.; Yu, R.; Zhou, X. GOLPH3 Promotes Glioma Progression by Enhancing PHB2-Mediated Autophagy. Am. J. Cancer Res. 2021, 11, 2106–2123. [Google Scholar]
- Signorile, A.; Sgaramella, G.; Bellomo, F.; De Rasmo, D. Prohibitins: A Critical Role in Mitochondrial Functions and Implication in Diseases. Cells 2019, 8, 71. [Google Scholar] [CrossRef] [PubMed]
- Kasashima, K.; Ohta, E.; Kagawa, Y.; Endo, H. Mitochondrial Functions and Estrogen Receptor-Dependent Nuclear Translocation of Pleiotropic Human Prohibitin 2. J. Biol. Chem. 2006, 281, 36401–36410. [Google Scholar] [CrossRef] [PubMed]
- Merkwirth, C.; Dargazanli, S.; Tatsuta, T.; Geimer, S.; Löwer, B.; Wunderlich, F.T.; von Kleist-Retzow, J.-C.; Waisman, A.; Westermann, B.; Langer, T. Prohibitins Control Cell Proliferation and Apoptosis by Regulating OPA1-Dependent Cristae Morphogenesis in Mitochondria. Genes Dev. 2008, 22, 476–488. [Google Scholar] [CrossRef] [PubMed]
- Schleicher, M.; Shepherd, B.R.; Suarez, Y.; Fernandez-Hernando, C.; Yu, J.; Pan, Y.; Acevedo, L.M.; Shadel, G.S.; Sessa, W.C. Prohibitin-1 Maintains the Angiogenic Capacity of Endothelial Cells by Regulating Mitochondrial Function and Senescence. J. Cell Biol. 2008, 180, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, F.M.; Martínez-Koteski, N.; Cavieres, V.A.; Mardones, G.A.; Fidelio, G.D.; Vilcaes, A.A.; Daniotti, J.L. Golgi Phosphoprotein 3 Regulates the Physical Association of Glycolipid Glycosyltransferases. Int. J. Mol. Sci. 2022, 23, 10354. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, R.; Russo, D.; Kurokawa, K.; Sahu, P.; Lombardi, B.; Supino, D.; Zhukovsky, M.A.; Vocat, A.; Pothukuchi, P.; Kunnathully, V.; et al. Golgi Maturation-Dependent Glycoenzyme Recycling Controls Glycosphingolipid Biosynthesis and Cell Growth via GOLPH3. EMBO J. 2021, 40, e107238. [Google Scholar] [CrossRef] [PubMed]
- Frappaolo, A.; Karimpour-Ghahnavieh, A.; Sechi, S.; Giansanti, M.G. The Close Relationship between the Golgi Trafficking Machinery and Protein Glycosylation. Cells 2020, 9, 2652. [Google Scholar] [CrossRef]
- Zeng, Z.; Lin, H.; Zhao, X.; Liu, G.; Wang, X.; Xu, R.; Chen, K.; Li, J.; Song, L. Overexpression of GOLPH3 Promotes Proliferation and Tumorigenicity in Breast Cancer via Suppression of the FOXO1 Transcription Factor. Clin. Cancer Res. 2012, 18, 4059–4069. [Google Scholar] [CrossRef]
- Yuan, Z.; Zhao, L.; Zhang, Y.; Li, S.; Pan, B.; Hua, L.; Wang, Z.; Ye, C.; Lu, J.; Yu, R.; et al. Inhibition of Glioma Growth by a GOLPH3 SiRNA-Loaded Cationic Liposomes. J. Neurooncol. 2018, 140, 249–260. [Google Scholar] [CrossRef]
- Ye, C.; Pan, B.; Xu, H.; Zhao, Z.; Shen, J.; Lu, J.; Yu, R.; Liu, H. Co-Delivery of GOLPH3 SiRNA and Gefitinib by Cationic Lipid-PLGA Nanoparticles Improves EGFR-Targeted Therapy for Glioma. J. Mol. Med. 2019, 97, 1575–1588. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Zhang, Y.; Wang, Y.; Zhang, H.; Xie, S.; Xie, P.; Yu, R.; Zhou, X. Golgi Phosphoprotein 3 Sensitizes the Tumour Suppression Effect of Gefitinib on Gliomas. Cell Prolif. 2019, 52, e12636. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-P.; Wang, L.-P.; Hong, Z.-S.; Qiu, C.-Z.; Wang, M.-Z.; Chen, Z.-X.; Tang, L.-F.; Yu, W.-S.; Wang, C.-X. Silencing GOLPH3 Gene Expression Reverses Resistance to Cisplatin in HT29 Colon Cancer Cells via Multiple Signaling Pathways. Int. J. Oncol. 2018, 53, 1183–1192. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Z.; Qiu, C.-Z.; Yu, W.-S.; Guo, Y.-T.; Wang, C.-X.; Chen, Z.-X. GOLPH3 Expression Promotes the Resistance of HT29 Cells to 5-fluorouracil by Activating Multiple Signaling Pathways. Mol. Med. Rep. 2018, 17, 542–548. [Google Scholar] [CrossRef]
- Cui, G.-H.; Li, W.-X.; Lin, Z.-Y.; Zhang, W.-Q.; Hu, Z.-H.; Wang, L. Short Interfering RNA Directed against the GOLPH3 Gene Enhances the Effect of Chemotherapy against Oral Squamous Cell Carcinoma by Regulating Caspase3, Bcl2 and Cytochrome-c Expression. Int. J. Clin. Exp. Med. 2015, 8, 13007–13012. [Google Scholar] [PubMed]
- Chapdelaine, A.G.; Sun, G. Challenges and Opportunities in Developing Targeted Therapies for Triple Negative Breast Cancer. Biomolecules 2023, 13, 1207. [Google Scholar] [CrossRef]
- Ong, G.; Logue, S.E. Unfolding the Interactions between Endoplasmic Reticulum Stress and Oxidative Stress. Antioxidants 2023, 12, 981. [Google Scholar] [CrossRef]
- Feng, C.; Zhang, L.; Chang, X.; Qin, D.; Zhang, T. Regulation of Post-Translational Modification of PD-L1 and Advances in Tumor Immunotherapy. Front. Immunol. 2023, 14, 1230135. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Rodríguez-Enríquez, S.; Marín-Hernández, A.; Saavedra, E. Energy Metabolism in Tumor Cells. FEBS J. 2007, 274, 1393–1418. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, Y.; Song, W.; Jiang, X.; Deng, Z.; Xiong, W.; Shen, J. Metabolic Reprogramming Mediated PD-L1 Depression and Hypoxia Reversion to Reactivate Tumor Therapy. J. Control Release 2022, 352, 793–812. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, Z.; Hu, R.; Dong, M.; Zhou, X.; Ren, S.; Zhang, Y.; Chen, C.; Huang, R.; Zhu, M.; et al. Metabolic Intervention Liposome Boosted Lung Cancer Radio-Immunotherapy via Hypoxia Amelioration and PD-L1 Restraint. Adv. Sci. 2023, 10, e2207608. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polanco, C.M.; Cavieres, V.A.; Galarza, A.J.; Jara, C.; Torres, A.K.; Cancino, J.; Varas-Godoy, M.; Burgos, P.V.; Tapia-Rojas, C.; Mardones, G.A. GOLPH3 Participates in Mitochondrial Fission and Is Necessary to Sustain Bioenergetic Function in MDA-MB-231 Breast Cancer Cells. Cells 2024, 13, 316. https://doi.org/10.3390/cells13040316
Polanco CM, Cavieres VA, Galarza AJ, Jara C, Torres AK, Cancino J, Varas-Godoy M, Burgos PV, Tapia-Rojas C, Mardones GA. GOLPH3 Participates in Mitochondrial Fission and Is Necessary to Sustain Bioenergetic Function in MDA-MB-231 Breast Cancer Cells. Cells. 2024; 13(4):316. https://doi.org/10.3390/cells13040316
Chicago/Turabian StylePolanco, Catalina M., Viviana A. Cavieres, Abigail J. Galarza, Claudia Jara, Angie K. Torres, Jorge Cancino, Manuel Varas-Godoy, Patricia V. Burgos, Cheril Tapia-Rojas, and Gonzalo A. Mardones. 2024. "GOLPH3 Participates in Mitochondrial Fission and Is Necessary to Sustain Bioenergetic Function in MDA-MB-231 Breast Cancer Cells" Cells 13, no. 4: 316. https://doi.org/10.3390/cells13040316
APA StylePolanco, C. M., Cavieres, V. A., Galarza, A. J., Jara, C., Torres, A. K., Cancino, J., Varas-Godoy, M., Burgos, P. V., Tapia-Rojas, C., & Mardones, G. A. (2024). GOLPH3 Participates in Mitochondrial Fission and Is Necessary to Sustain Bioenergetic Function in MDA-MB-231 Breast Cancer Cells. Cells, 13(4), 316. https://doi.org/10.3390/cells13040316