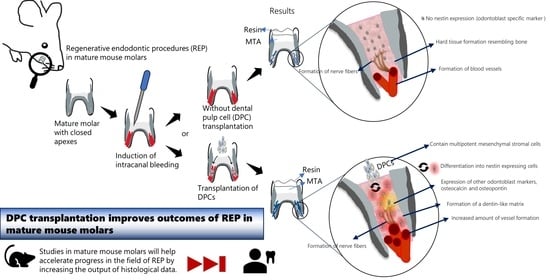
Dental Pulp Cell Transplantation Combined with Regenerative Endodontic Procedures Promotes Dentin Matrix Formation in Mature Mouse Molars
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Isolation and Culture of Transgenic Mouse DPCs
2.3. Immunocytochemistry of DPCs
2.4. Gene Expression of DPCs
2.5. REP and DPC Transplantation
2.6. Micro-CT (µCT) Analysis of REP Only- and REP + DPC-Treated Molars
2.7. Analysis of the Regenerated Pulp Tissue through H&E Staining and Immunohistochemistry
2.8. Statistical Analyses
3. Results
3.1. Isolation and Characteristics of Mouse DPCs
3.2. µCT Analysis of the Healing Response in the REP and REP + DPCs Groups
3.3. Histological Analysis of the Regenerated Tissue Using H&E Staining
3.4. Characterization of the Regenerated Tissue by IHC Staining
3.4.1. Detection of Odontoblast-Like Cells in the REP + DPC Group
3.4.2. Vasculogenesis in the Regenerated Dental Pulp-like Tissue
3.4.3. Neurogenesis in the Regenerated Dental Pulp-like Tissue
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Takashi, O. Pulp as a connective tissue. In Seltzer and Bender’s Dental Pulp, 2nd ed.; Hargreaves, M.K., Goodis, E.H., Tay, R.F., Eds.; Quintessence Publishing: Berlin, Germany, 2012; pp. 67–89. [Google Scholar]
- Yu, C.Y.; Abbott, P.V. Pulp microenvironment and mechanisms of pain arising from the dental pulp: From an endodontic perspective. Aust. Endod. J. 2018, 44, 82–98. [Google Scholar] [CrossRef]
- Guerrero, F.; Mendoza, A.; Ribas, D.; Aspiazu, K. Apexification: A systematic review. J. Conserv. Dent. 2018, 21, 462–465. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Nosrat, A.; Verma, P.; Udochukwu, O. Regenerative endodontic treatment or mineral trioxide aggregate apical plug in teeth with necrotic pulps and open apices: A systematic review and meta-analysis. J. Endod. 2017, 43, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Murray, P.E.; Garcia-Godoy, F.; Hargreaves, K.M. Regenerative endodontics: A review of current status and a call for action. J. Endod. 2007, 33, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Banchs, F.; Trope, M. Revascularization of immature permanent teeth with apical periodontitis: New treatment protocol? J. Endod. 2004, 30, 196–200. [Google Scholar] [CrossRef]
- Chrepa, V.; Henry, M.A.; Daniel, B.J.; Diogenes, A. Delivery of apical mesenchymal stem cells into root canals of mature teeth. J. Dent. Res. 2015, 94, 1653–1659. [Google Scholar] [CrossRef]
- Lovelace, T.W.; Henry, M.A.; Hargreaves, K.M.; Diogenes, A. Evaluation of the delivery of mesenchymal stem cells into the root canal space of necrotic immature teeth after clinical regenerative endodontic procedure. J. Endod. 2011, 37, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zheng, L.; Daraqel, B.; Liu, J.; Hu, Y. Treatment Outcome of Regenerative Endodontic Procedures for Necrotic Immature and Mature Permanent Teeth: A Systematic Review and Meta-Analysis Based on Randomised Controlled Trials. Oral Health Prev. Dent. 2023, 21, 141–152. [Google Scholar] [CrossRef]
- Digka, A.; Sakka, D.; Lyroudia, K. Histological assessment of human regenerative endodontic procedures (REP) of immature permanent teeth with necrotic pulp/apical periodontitis: A systematic review. Aust. Endod. J. 2020, 46, 140–153. [Google Scholar] [CrossRef]
- Wei, X.; Yang, M.; Yue, L.; Huang, D.; Zhou, X.; Wang, X.; Zhang, Q.; Qiu, L.; Huang, Z.; Wang, H.; et al. Expert Consensus on Regenerative Endodontic Procedures. Int. J. Oral Sci. 2022, 14, 55. [Google Scholar] [CrossRef]
- Arslan, H.; Şahin, Y.; Topçuoğlu, H.S.; Gündoğdu, B. Histologic evaluation of regenerated tissues in the pulp spaces of teeth with mature roots at the time of the regenerative endodontic procedures. J. Endod. 2019, 45, 1384–1389. [Google Scholar] [CrossRef]
- Garrido-Parada, S.; Castelo-Baz, P.; Feijoo-Pato, N.; Gaviño-Orduña, J.; Martín-Biedma, B. Endodontic Regenerative Procedures in Necrotic Adult Teeth. Appl. Sci. 2022, 12, 4212. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Nakata, K.; Nakashima, M. Age-Dependent Decline in Dental Pulp Regeneration after Pulpectomy in Dogs. Exp. Gerontol. 2014, 52, 39–45. [Google Scholar] [CrossRef]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage Potential of Adult Human Mesenchymal Stem Cells. Science 1999, 284, 5411. [Google Scholar] [CrossRef]
- Hilfiker, A.; Kasper, C.; Hass, R.; Haverich, A. Mesenchymal stem cells and progenitor cells in connective tissue engineering and regenerative medicine: Is there a future for transplantation? Langenbeck’s Arch. Surg. 2011, 396, 489–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Shao, J.Z.; Xiang, L.X.; Dong, X.J.; Zhang, G.R. Mesenchymal stem cells: A promising candidate in regenerative medicine. Int. J. Biochem. Cell Biol. 2008, 40, 815–820. [Google Scholar] [CrossRef] [PubMed]
- Bronckaers, A.; Hilkens, P.; Martens, W.; Gervois, P.; Ratajczak, J.; Struys, T.; Lambrichts, I. Mesenchymal stem/stromal cells as a pharmacological and therapeutic approach to accelerate angiogenesis. Pharmacol. Ther. 2014, 143, 181–196. [Google Scholar] [CrossRef]
- Miura, M.; Gronthos, S.; Zhao, M.; Lu, B.; Fisher, L.W.; Robey, P.G.; Shi, S. SHED: Stem Cells from Human Exfoliated Deciduous Teeth. Proc. Natl. Acad. Sci. USA 2003, 100, 5807–5812. [Google Scholar] [CrossRef]
- Morsczeck, C.; Götz, W.; Schierholz, J.; Zeilhofer, F.; Kühn, U.; Möhl, C.; Sippel, C.; Hoffmann, K.H. Isolation of Precursor Cells (PCs) from Human Dental Follicle of Wisdom Teeth. Matrix Biology 2005, 24, 155–165. [Google Scholar] [CrossRef]
- Huang, G.T.J.; Sonoyama, W.; Liu, Y.; Liu, H.; Wang, S.; Shi, S. The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J. Endod. 2008, 34, 645–651. [Google Scholar] [CrossRef]
- Seo, B.M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of Multipotent Postnatal Stem Cells from Human Periodontal Ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Luzuriaga, J.; Irurzun, J.; Irastorza, I.; Unda, F.; Ibarretxe, G.; Pineda, J.R. Vasculogenesis from Human Dental Pulp Stem Cells Grown in Matrigel with Fully Defined Serum-Free Culture Media. Biomedicines 2020, 8, 483. [Google Scholar] [CrossRef]
- Han, P.; Cui, Q.; Lu, W.; Yang, S.; Shi, M.; Li, Z.; Gao, P.; Xu, B.; Li, Z. Hepatocyte Growth Factor Plays a Dual Role in Tendon-Derived Stem Cell Proliferation, Migration, and Differentiation. J. Cell. Physiol. 2019, 234, 17382–17391. [Google Scholar] [CrossRef]
- Ding, G.; Niu, J.; Liu, Y. Dental Pulp Stem Cells Suppress the Proliferation of Lymphocytes via Transforming Growth Factor-Β1. Hum. Cell 2015, 28, 81–90. [Google Scholar] [CrossRef]
- Yamagata, M.; Yamamoto, A.; Kako, E.; Kaneko, N.; Matsubara, K.; Sakai, K.; Sawamoto, K.; Ueda, M. Human Dental Pulp-Derived Stem Cells Protect against Hypoxic-Ischemic Brain Injury in Neonatal Mice. Stroke 2013, 44, 551–554. [Google Scholar] [CrossRef]
- Carnevale, G.; Pisciotta, A.; Riccio, M.; Bertoni, L.; De Biasi, S.; Gibellini, L.; Zordani, A.; Cavallini, G.M.; La Sala, G.B.; Bruzzesi, G.; et al. Human Dental Pulp Stem Cells Expressing STRO-1, c-Kit and CD34 Markers in Peripheral Nerve Regeneration. J. Tissue Eng. Regen. Med. 2016, 12, e774–e785. [Google Scholar] [CrossRef]
- Delle Monache, S.; Pulcini, F.; Santilli, F.; Martellucci, S.; Santacroce, C.; Fabrizi, J.; Angelucci, A.; Sorice, M.; Mattei, V. Hypoxia Induces DPSC Differentiation versus a Neurogenic Phenotype by the Paracrine Mechanism. Biomedicines 2022, 10, 1056. [Google Scholar] [CrossRef] [PubMed]
- D’Aquino, R.; De Rosa, A.; Lanza, V.; Tirino, V.; Laino, L.; Graziano, A.; Desiderio, V.; Laino, G.; Papaccio, G. Human Mandible Bone Defect Repair by the Grafting of Dental Pulp Stem/Progenitor Cells and Collagen Sponge Biocomplexes. Eur. Cell Mater. 2009, 18, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kawase-Koga, Y.; Hojo, H.; Yano, F.; Sato, M.; Chung, U.I.; Ohba, S.; Chikazu, D. Bone Regeneration by Human Dental Pulp Stem Cells Using a Helioxanthin Derivative and Cell-Sheet Technology. Stem Cell Res. Ther. 2018, 9, 24. [Google Scholar] [CrossRef] [PubMed]
- Alsanea, R.; Ravindran, S.; Fayad, M.I.; Johnson, B.R.; Wenckus, C.S.; Hao, J.; George, A. Biomimetic approach to perforation repair using dental pulp stem cells and dentin matrix protein 1. J. Endod. 2011, 37, 1092–1097. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Potdar, P.D. Human dental pulp stem cells: Applications in future regenerative medicine. World J. Stem Cells 2015, 7, 839–851. [Google Scholar] [CrossRef]
- Iohara, K.; Utsunomiya, S.; Kohara, S.; Nakashima, M. Allogeneic transplantation of mobilized dental pulp stem cells with the mismatched dog leukocyte antigen type is safe and efficacious for total pulp regeneration. Stem Cell Res. Ther. 2018, 9, 116. [Google Scholar] [CrossRef]
- Xuan, K.; Li, B.; Guo, H.; Sun, W.; Kou, X.; He, X.; Zhang, Y.; Sun, J.; Liu, A.; Liao, L.; et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth. Sci. Transl. Med. 2018, 10, eaaf3227. [Google Scholar] [CrossRef] [PubMed]
- Aubeux, D.; Renard, E.; Pérez, F.; Tessier, S.; Geoffroy, V.; Gaudin, A. Review of animal models to study pulp inflammation. Front. Dent. Med. 2021, 2, 673552. [Google Scholar] [CrossRef]
- Dianat, O.; Mashhadi Abas, F.; Paymanpour, P.; Eghbal, M.J.; Haddadpour, S.; Bahrololumi, N. Endodontic Repair in immature dogs’ teeth with apical periodontitis: Blood clot vs plasma rich in growth factors scaffold. Dent. Traumatol. 2017, 33, 84–90. [Google Scholar] [CrossRef]
- Janebodin, K.; Horst, O.V.; Ieronimakis, N.; Balasundaram, G.; Reesukumal, K.; Pratumvinit, B.; Reyes, M. Isolation and characterization of neural crest-derived stem cells from dental pulp of neonatal mice. PLoS ONE 2011, 6, 11. [Google Scholar] [CrossRef] [PubMed]
- Kawashima, N.; Okiji, T. Odontoblasts: Specialized hard-tissue-forming cells in the dentin-pulp complex. Congenit. Anom. 2016, 56, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Janebodin, K.; Chavanachat, R.; Hays, A.; Reyes Gil, M. Silencing vegfr-2 hampers odontoblastic differentiation of dental pulp stem cells. Front. Cell Dev. Biol. 2021, 9, 665886. [Google Scholar] [CrossRef]
- Breyer, A.; Estharabadi, N.; Oki, M.; Ulloa, F.; Nelson-Holte, M.; Lien, L.; Jiang, Y. Multipotent adult progenitor cell isolation and culture procedures. Exp. Hematol. 2006, 34, 1596–1601. [Google Scholar] [CrossRef]
- Romero-Lanman, E.E.; Pavlovic, S.; Amlani, B.; Chin, Y.; Benezra, R. Id1 Maintains Embryonic Stem Cell Self-Renewal by up-Regulation of Nanog and Repression of Brachyury Expression. Stem Cells Dev. 2012, 21, 384–393. [Google Scholar] [CrossRef]
- Seki, Y.; Kurisaki, A.; Watanabe-Susaki, K.; Nakajima, Y.; Nakanishi, M.; Arai, Y.; Shiota, K.; Sugino, H.; Asashima, M. TIF1β Regulates the Pluripotency of Embryonic Stem Cells in a Phosphorylation-Dependent Manner. Proc. Natl. Acad. Sci. USA 2010, 107, 10926–10931. [Google Scholar] [CrossRef]
- Xu, W.; Jiang, S.; Chen, Q.; Ye, Y.; Chen, J.; Heng, B.C.; Jiang, Q.; Wu, B.; Ding, Z.; Zhang, C. Systemically transplanted bone marrow-derived cells contribute to dental pulp regeneration in a chimeric mouse model. J. Endod. 2016, 42, 263–268. [Google Scholar] [CrossRef]
- Tanaka, S.; Toriumi, T.; Ito, T.; Okuwa, Y.; Futenma, T.; Otake, K.; Akiyama, Y.; Kurita, K.; Nagao, T.; Honda, M. Histological analysis of dental pulp response in immature or mature teeth after extra-oral subcutaneous transplantation into mice dorsum. J. Oral Sci. 2021, 63, 184–190. [Google Scholar] [CrossRef]
- Siddiqui, Y.D.; Omori, K.; Ito, T.; Yamashiro, K.; Nakamura, S.; Okamoto, K.; Ono, M.; Yamamoto, T.; Dyke, T.E.V.; Takashiba, S. Resolvin D2 Induces Resolution of Periapical Inflammation and Promotes Healing of Periapical Lesions in Rat Periapical Periodotitis. Front. Immunol. 2019, 10, 307. [Google Scholar] [CrossRef]
- Lin, H.; Xu, L.; Liu, H.; Sun, Q.; Chen, Z.; Yuan, G.; Chen, Z. KLF4 promotes the odontoblastic differentiation of human dental pulp cells. J. Endod. 2011, 37, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Liu, H.; Sun, Q.; Yuan, G.; Zhang, L.; Chen, Z. KLF4 promoted odontoblastic differentiation of mouse dental papilla cells via regulation of DMP1. J. Cell Physiol. 2013, 228, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Butler, W.T.; Ritchie, H. The Nature and Functional Significance of Dentin Extracellular Matrix Proteins. Int. J. Dev. Biol. 1995, 39, 169–179. [Google Scholar]
- Miyazaki, T.; Kanatani, N.; Rokutanda, S.; Yoshida, C.; Toyosawa, S.; Nakamura, R.; Takada, S.; Komori, T. Inhibition of the Terminal Differentiation of Odontoblasts and Their Transdifferentiation into Osteoblasts in Runx2 Transgenic Mice. Arch. Histol. Cytol. 2008, 71, 131–146. [Google Scholar] [CrossRef] [PubMed]
- Linde, A.; Goldberg, M. Dentinogenesis. Crit. Rev. Oral Biol. Med. 1994, 4, 679–728. [Google Scholar] [CrossRef]
- Itoh, K. The distribution of nerves in human deciduous and permanent teeth. Arch. Histol. Jpn. 1976, 39, 379–399. [Google Scholar] [CrossRef]
- Fristad, I.; Bletsa, A.; Byers, M. Inflammatory nerve responses in the dental pulp. Endod. Top. 2007, 17, 12–41. [Google Scholar] [CrossRef]
- Byers, M.R.; Suzuki, H.; Maeda, T. Dental neuroplasticity, neuro-pulpal interactions, and nerve regeneration. Microsc. Res. Tech. 2003, 60, 503–515. [Google Scholar] [CrossRef]
- Sainio, M.T.; Rasila, T.; Molchanova, S.M.; Järvilehto, J.; Torregrosa-Muñumer, R.; Harjuhaahto, S.; Pennonen, J.; Huber, N.; Herukka, S.K.; Haapasalo, A.; et al. Neurofilament light regulates axon caliber, synaptic activity, and organelle trafficking in cultured human motor neurons. Front. Cell Dev. Biol. 2022, 9, 820105. [Google Scholar] [CrossRef]
- Dourou, V.; Lyroudia, K.; Karayannopoulou, G.; Papadimitriou, C.; Molyvdas, I. Comparative evaluation of neural tissue antigens—Neurofilament protein (NF), peripherin (PRP), S100B protein (S100B), neuron-specific enolase (NSE) and chromogranin-a (CGA)—In both normal and inflamed human mature dental pulp. Acta Histochem. 2006, 108, 343–350. [Google Scholar] [CrossRef]
- Iohara, K.; Murakami, M.; Takeuchi, N.; Osako, Y.; Ito, M.; Ishizaka, R.; Utunomiya, S.; Nakamura, H.; Matsushita, K.; Nakashima, M. A Novel Combinatorial Therapy With Pulp Stem Cells and Granulocyte Colony-Stimulating Factor for Total Pulp Regeneration. Stem Cells Transl. Med. 2013, 2, 521–533. [Google Scholar] [CrossRef]
- Fahmy, S.H.; Hassanien, E.E.S.; Nagy, M.M.; El Batouty, K.M.; Mekhemar, M.; Fawzy El Sayed, K.; Hassanein, E.H.; Wiltfang, J.; Dörfer, C. Investigation of the regenerative potential of necrotic mature teeth following different revascularisation protocols. Aust. Endod. J. 2017, 43, 73–82. [Google Scholar] [CrossRef]
- Komada, T.; Mitomo, K.; Ikarashi, T.; Shimono, M.; Jung, H.S.; Muramatsu, T. Periodontal ligament cells are involved in the formation of intracanal cementum-like tissue after regenerative endodontic procedures: A mouse in situ model. Front. Dent. Med. 2022, 3, 864406. [Google Scholar] [CrossRef]
- Halse, A.; Molven, O. Increased width of the apical periodontal membrane space in endodontically treated teeth may represent favourable healing. Int. Endod. J. 2004, 37, 552–560. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Garant, P.R. Structure and Organization of Odontoblasts. Anat. Rec. 1996, 245, 235–249. [Google Scholar] [CrossRef]
- Goldberg, M.; Kulkarni, A.B.; Young, M.; Boskey, A. Dentin: Structure, Composition and Mineralization. Front. Biosci.-Elite 2011, 3, 711–735. [Google Scholar] [CrossRef] [PubMed]
- Fujita, S.; Hideshima, K.; Ikeda, T. Nestin expression in odontoblasts and odontogenic ectomesenchymal tissue of odontogenic tumours. J. Clin. Pathol. 2006, 59, 240–245. [Google Scholar] [CrossRef] [PubMed]
- About, I.; Laurent-Maquin, D.; Lendahl, U.; Mitsiadis, T.A. Nestin expression in embryonic and adult human teeth under normal and pathological conditions. Am. J. Pathol. 2000, 157, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Kenmotsu, S.I.; Ohshima, H. The Expression of GM-CSF and Osteopontin in Immunocompetent Cells Precedes the Odontoblast Differentiation Following Allogenic Tooth Transplantation in Mice. J. Histochem. Cytochem. 2011, 59, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Saito, K.; Nakatomi, M.; Ida-Yonemochi, H.; Ohshima, H. Osteopontin Is Essential for Type i Collagen Secretion in Reparative Dentin. J. Dent. Res. 2016, 95, 1034–1041. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Sasaki, J.I.; Inubushi, T.; Abe, G.L.; Nör, J.E.; Yamashiro, T.; Imazato, S. Role of heparan sulfate in vasculogenesis of dental pulp stem cells. J. Dent. Res. 2023, 102, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Bergamo, M.T.; Zhang, Z.; Oliveira, T.M.; Nör, J.E. Vegfr1 Primes a Unique Cohort of Dental Pulp Stem Cells for Vasculogenic Differentiation. Eur. Cell Mater. 2021, 41, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wu, M.; Liu, S.; Liu, X.; Huan, Y.; Ye, Q.; Yang, X.; Guo, H.; Liu, A.; Huang, X.; et al. Apoptotic Vesicles Activate Autophagy in Recipient Cells to Induce Angiogenesis and Dental Pulp Regeneration. Mol. Ther. 2022, 30, 3193–3208. [Google Scholar] [CrossRef]
- Scelza, P.; Gonçalves, F.; Caldas, I.; Nunes, F.; Lourenço, E.S.; Tavares, S.; Magno, M.; Pintor, A.; Montemezzi, P.; Di Edoardo, E.; et al. Prognosis of regenerative endodontic procedures in mature teeth: A systematic review and meta-analysis of clinical and radiographic parameters. Materials 2021, 14, 4418. [Google Scholar] [CrossRef]
- Attik, G.N.; Villat, C.; Hallay, F.; Pradelle-Plasse, N.; Bonnet, H.; Moreau, K.; Colon, P.; Grosgogeat, B. In Vitro Biocompatibility of a Dentine Substitute Cement on Human MG63 Osteoblasts Cells: BiodentineTM versus MTA®. Int. Endod. J. 2014, 47, 1133–1141. [Google Scholar] [CrossRef]
- Nair, P.N.R.; Duncan, H.F.; Pitt Ford, T.R.; Luder, H.U. Histological, Ultrastructural and Quantitative Investigations on the Response of Healthy Human Pulps to Experimental Capping with Mineral Trioxide Aggregate: A Randomized Controlled Trial. Int. Endod. J. 2009, 42, 422–444. [Google Scholar] [CrossRef] [PubMed]







| Gene | Forward primer (5′ to 3′) | Reverse primer (5′ to 3′) |
|---|---|---|
| Gapdh | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG |
| Sox2 | CAAAAACCGTGATGCCGACT | CGCCCTCAGGTTTTCTCTGT |
| Nanog [38] | AAGCGGTGGCAGAAAAACC | GTGCTGAGCCCTTCTGAATCA |
| c-kit | GCTCGGGCTTCTGTACAACT | AAGGCTGACTAGGGAGGAGG |
| Klf4 | CCTGGCGAGTCTGACATGG | TCCTCACGCCAACGGTTAGT |
| Oct4 | GAGACTTTGCAGCCTGAGGG | CTTTCATGTCCTGGGACTCCTC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro Raudales, J.L.; Okuwa, Y.; Honda, M. Dental Pulp Cell Transplantation Combined with Regenerative Endodontic Procedures Promotes Dentin Matrix Formation in Mature Mouse Molars. Cells 2024, 13, 348. https://doi.org/10.3390/cells13040348
Montenegro Raudales JL, Okuwa Y, Honda M. Dental Pulp Cell Transplantation Combined with Regenerative Endodontic Procedures Promotes Dentin Matrix Formation in Mature Mouse Molars. Cells. 2024; 13(4):348. https://doi.org/10.3390/cells13040348
Chicago/Turabian StyleMontenegro Raudales, Jorge Luis, Yuta Okuwa, and Masaki Honda. 2024. "Dental Pulp Cell Transplantation Combined with Regenerative Endodontic Procedures Promotes Dentin Matrix Formation in Mature Mouse Molars" Cells 13, no. 4: 348. https://doi.org/10.3390/cells13040348
APA StyleMontenegro Raudales, J. L., Okuwa, Y., & Honda, M. (2024). Dental Pulp Cell Transplantation Combined with Regenerative Endodontic Procedures Promotes Dentin Matrix Formation in Mature Mouse Molars. Cells, 13(4), 348. https://doi.org/10.3390/cells13040348