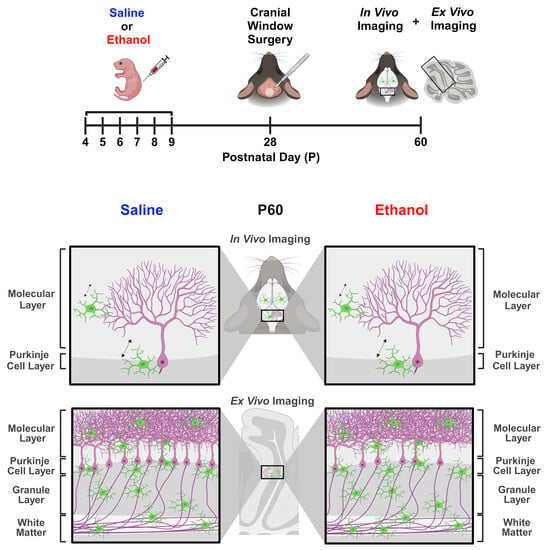
Figure 1.
Dosing paradigm, mouse weights, and P10 mRNA expression. (
A) Timeline of third-trimester equivalent dosing. Pups were subcutaneously given 5.0 g/kg/day of either ethanol solution or saline twice a day, two hours apart. Mice were aged to adolescence (~P28) and cranial windows were implanted over lobule IV/V of the cerebellar vermis. Some animals underwent in-vivo imaging at this age as reported in [
24]. They were further aged to young adulthood (~P60) and underwent in-vivo two-photon imaging, followed by fixation. Brains were harvested, sectioned, and imaged on a confocal microscope. (
B) Mice were weighed every day before dosing (saline 9M; 7F, ethanol 8M; 6F), as well as before surgery and perfusion. When sexes were pooled, there was a main effect of age (F (1.791, 50.16) = 1757,
p < 0.0001) and main effect of treatment (F (1, 28) = 8.182,
p = 0.0079) on body weight over time, and a trend towards an interaction between age and treatment (F (7, 196) = 1.980,
p = 0.0596). Saline-dosed animals gained weight every day (
p < 0.0001) and ethanol-dosed animals gained weight every day (
p < 0.05), except for P4–P5 (
p > 0.99) when sexes were pooled. From P7–P9, saline-dosed pups weighed significantly more than ethanol-dosed pups (
p < 0.05), but there were no significant differences in weight between saline- and ethanol-dosed animals at any other ages (
p > 0.05) when sexes were pooled. Saline-dosed males weighed significantly more than ethanol-dosed males at P28 (
p = 0.0226), but not any other ages (
p > 0.05). There were no significant differences between saline- and ethanol-dosed female (
p > 0.05) weights at any age. (
C–
E) The effect of P4–P9 developmental ethanol exposure on the P10 (24 h after the final dose of ethanol) cerebellum in a separate cohort of mice (saline 10M; 9F, ethanol 9M; 9F) was examined for IL-1β (
C), TNF-α (
D), and CCL2 (
E) mRNA expression. (
C) IL-1β expression was significantly increased in ethanol-dosed pups (F (1, 33) = 19.44,
p = 0.0001), an effect that was significant for both males (
p = 0.0043) and females (
p = 0.0129). There was no main effect for sex (F (1, 33) = 0.4066,
p = 0.5281), and no treatment–sex interaction (F (1, 33) = 0.05975,
p = 0.8084). (
D) There was a trend for increased TNF-α expression in ethanol pups (F (1, 33) = 3.409,
p = 0.0738), but no main effect for sex (F (1, 33) = 1.161,
p = 0.2892), and no treatment–sex interaction (F (1, 33) = 0.2171,
p = 0.6443). (
E) CCL2 expression was significantly increased in ethanol-dosed pups (F (1, 33) = 8.859,
p = 0.0054) and there was a significant treatment–sex interaction (F (1, 33) = 4.317,
p = 0.0456), as the effect was significant in males (
p = 0.0019), but not females (
p ≥ 0.9999). There was no main effect for sex (F (1, 33) = 0.1921,
p = 0.6641). (
B–
E) Males (M) are shown with closed shapes and females (F) with open shapes. Data are presented as the mean ± SEM. (
B) Each datapoint represents a treatment group and sex. Two-way ANOVA, repeated measures with Bonferroni post-hoc comparisons. (
C–
E) Each datapoint represents an individual animal. Two-way ANOVA with Bonferroni post-hoc comparisons.
Figure 1.
Dosing paradigm, mouse weights, and P10 mRNA expression. (
A) Timeline of third-trimester equivalent dosing. Pups were subcutaneously given 5.0 g/kg/day of either ethanol solution or saline twice a day, two hours apart. Mice were aged to adolescence (~P28) and cranial windows were implanted over lobule IV/V of the cerebellar vermis. Some animals underwent in-vivo imaging at this age as reported in [
24]. They were further aged to young adulthood (~P60) and underwent in-vivo two-photon imaging, followed by fixation. Brains were harvested, sectioned, and imaged on a confocal microscope. (
B) Mice were weighed every day before dosing (saline 9M; 7F, ethanol 8M; 6F), as well as before surgery and perfusion. When sexes were pooled, there was a main effect of age (F (1.791, 50.16) = 1757,
p < 0.0001) and main effect of treatment (F (1, 28) = 8.182,
p = 0.0079) on body weight over time, and a trend towards an interaction between age and treatment (F (7, 196) = 1.980,
p = 0.0596). Saline-dosed animals gained weight every day (
p < 0.0001) and ethanol-dosed animals gained weight every day (
p < 0.05), except for P4–P5 (
p > 0.99) when sexes were pooled. From P7–P9, saline-dosed pups weighed significantly more than ethanol-dosed pups (
p < 0.05), but there were no significant differences in weight between saline- and ethanol-dosed animals at any other ages (
p > 0.05) when sexes were pooled. Saline-dosed males weighed significantly more than ethanol-dosed males at P28 (
p = 0.0226), but not any other ages (
p > 0.05). There were no significant differences between saline- and ethanol-dosed female (
p > 0.05) weights at any age. (
C–
E) The effect of P4–P9 developmental ethanol exposure on the P10 (24 h after the final dose of ethanol) cerebellum in a separate cohort of mice (saline 10M; 9F, ethanol 9M; 9F) was examined for IL-1β (
C), TNF-α (
D), and CCL2 (
E) mRNA expression. (
C) IL-1β expression was significantly increased in ethanol-dosed pups (F (1, 33) = 19.44,
p = 0.0001), an effect that was significant for both males (
p = 0.0043) and females (
p = 0.0129). There was no main effect for sex (F (1, 33) = 0.4066,
p = 0.5281), and no treatment–sex interaction (F (1, 33) = 0.05975,
p = 0.8084). (
D) There was a trend for increased TNF-α expression in ethanol pups (F (1, 33) = 3.409,
p = 0.0738), but no main effect for sex (F (1, 33) = 1.161,
p = 0.2892), and no treatment–sex interaction (F (1, 33) = 0.2171,
p = 0.6443). (
E) CCL2 expression was significantly increased in ethanol-dosed pups (F (1, 33) = 8.859,
p = 0.0054) and there was a significant treatment–sex interaction (F (1, 33) = 4.317,
p = 0.0456), as the effect was significant in males (
p = 0.0019), but not females (
p ≥ 0.9999). There was no main effect for sex (F (1, 33) = 0.1921,
p = 0.6641). (
B–
E) Males (M) are shown with closed shapes and females (F) with open shapes. Data are presented as the mean ± SEM. (
B) Each datapoint represents a treatment group and sex. Two-way ANOVA, repeated measures with Bonferroni post-hoc comparisons. (
C–
E) Each datapoint represents an individual animal. Two-way ANOVA with Bonferroni post-hoc comparisons.
![Cells 13 00386 g001]()
Figure 2.
Cerebellar microglia density and distribution in lobule IV/V. (A) 10× confocal stitched image of lobule IV/V of the vermis. White lines indicate different layers (Molecular Layer (ML)+ Purkinje Cell Layer (PCL) combined, Granule Layer (GL), White Matter (WM)). (B) For the entire lobule IV/V (all layers combined), microglia density (number of microglia/μm2) was unchanged between saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice. There was no main effect for treatment (F (1, 16) = 1.836, p = 0.1943) or sex (F (1, 16) = 0.02769, p = 0.8699), and no interaction between treatment and sex (F (1, 16) = 0.6661, p = 0.4264). (C) For the entire lobule IV/V (all layers combined), the microglia spacing index ((nearest neighbor)2xmicroglia density) was unchanged between saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice. There was no main effect for treatment (F (1, 16) = 0.7948, p = 0.3859) or sex (F (1, 16) = 0.01861, p = 0.8932), and no interaction between treatment and sex (F (1, 16) = 0.6416, p = 0.4349). (D) When broken down by layer (ML + PCL, GL, WM), microglia density remained largely unaltered. In the ML and PCL combined, there was no main effect for treatment (F (1, 16) = 1.441, p = 0.2474) or sex (F (1, 16) = 0.01629, p = 0.9000), and no interaction between treatment and sex (F (1, 16) = 0.3860, p = 0.5432). In the GL, there was no main effect for treatment (F (1, 16) = 0.9490, p = 0.3445) or sex (F (1, 16) = 0.6814, p = 0.4213), and no interaction between treatment and sex (F (1, 16) = 0.1797, p = 0.6773). However, in the WM there was a trend towards increased microglia density in ethanol-dosed animals (F (1, 16) = 3.707, p = 0.0722), with a trend in females (p = 0.0674), but not males (p > 0.99). There was no main effect for sex (F (1, 16) = 0.03950, p = 0.8450) and no interaction between treatment and sex (F (1, 16) = 1.850, p = 0.1927). (B–D) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 100 μm.
Figure 2.
Cerebellar microglia density and distribution in lobule IV/V. (A) 10× confocal stitched image of lobule IV/V of the vermis. White lines indicate different layers (Molecular Layer (ML)+ Purkinje Cell Layer (PCL) combined, Granule Layer (GL), White Matter (WM)). (B) For the entire lobule IV/V (all layers combined), microglia density (number of microglia/μm2) was unchanged between saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice. There was no main effect for treatment (F (1, 16) = 1.836, p = 0.1943) or sex (F (1, 16) = 0.02769, p = 0.8699), and no interaction between treatment and sex (F (1, 16) = 0.6661, p = 0.4264). (C) For the entire lobule IV/V (all layers combined), the microglia spacing index ((nearest neighbor)2xmicroglia density) was unchanged between saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice. There was no main effect for treatment (F (1, 16) = 0.7948, p = 0.3859) or sex (F (1, 16) = 0.01861, p = 0.8932), and no interaction between treatment and sex (F (1, 16) = 0.6416, p = 0.4349). (D) When broken down by layer (ML + PCL, GL, WM), microglia density remained largely unaltered. In the ML and PCL combined, there was no main effect for treatment (F (1, 16) = 1.441, p = 0.2474) or sex (F (1, 16) = 0.01629, p = 0.9000), and no interaction between treatment and sex (F (1, 16) = 0.3860, p = 0.5432). In the GL, there was no main effect for treatment (F (1, 16) = 0.9490, p = 0.3445) or sex (F (1, 16) = 0.6814, p = 0.4213), and no interaction between treatment and sex (F (1, 16) = 0.1797, p = 0.6773). However, in the WM there was a trend towards increased microglia density in ethanol-dosed animals (F (1, 16) = 3.707, p = 0.0722), with a trend in females (p = 0.0674), but not males (p > 0.99). There was no main effect for sex (F (1, 16) = 0.03950, p = 0.8450) and no interaction between treatment and sex (F (1, 16) = 1.850, p = 0.1927). (B–D) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 100 μm.
![Cells 13 00386 g002]()
Figure 3.
Cerebellar microglia morphology in lobule IV/V in fixed tissue. (
A) 40× confocal image within lobule IV/V of the vermis. White lines indicate different layers (ML, PCL, GL, WM). Concentric rings were drawn with increasing radii around each microglia (example in GL) to examine process ramification. (
B) Example Sholl curve graph. X axis corresponds to radii from the soma center (concentric circles in (
A)) and y axis corresponds to process intersections across the circles. The Sholl curve is labeled with factors that give information about its behavior: before the change-point (α1); after the change-point (α2); branch maximum (eτ); change-point (γ) (from [
24]). (
C–
E) Sholl curves for saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice for all layers combined when sexes were combined (
C) or separated into males (
D) and females (
E) show no differences in ramification. (
F) Individual Sholl curves were fit using a hierarchical Bayesian approach to capture variation at each level of the experimental hierarchy. Credible intervals of 95% for effects on each parameter from (
B) were calculated across treatments and sexes when all layers were combined. (
C–
E) Each datapoint represents a treatment group and sex. Data are presented as the mean ± SEM. Scale bar = 50 μm.
Figure 3.
Cerebellar microglia morphology in lobule IV/V in fixed tissue. (
A) 40× confocal image within lobule IV/V of the vermis. White lines indicate different layers (ML, PCL, GL, WM). Concentric rings were drawn with increasing radii around each microglia (example in GL) to examine process ramification. (
B) Example Sholl curve graph. X axis corresponds to radii from the soma center (concentric circles in (
A)) and y axis corresponds to process intersections across the circles. The Sholl curve is labeled with factors that give information about its behavior: before the change-point (α1); after the change-point (α2); branch maximum (eτ); change-point (γ) (from [
24]). (
C–
E) Sholl curves for saline-dosed (5M, 5F) and ethanol-dosed (5M, 5F) mice for all layers combined when sexes were combined (
C) or separated into males (
D) and females (
E) show no differences in ramification. (
F) Individual Sholl curves were fit using a hierarchical Bayesian approach to capture variation at each level of the experimental hierarchy. Credible intervals of 95% for effects on each parameter from (
B) were calculated across treatments and sexes when all layers were combined. (
C–
E) Each datapoint represents a treatment group and sex. Data are presented as the mean ± SEM. Scale bar = 50 μm.
![Cells 13 00386 g003]()
Figure 4.
Cerebellar microglia dynamics in vivo. (A) Example in-vivo two-photon image of cerebellar microglia. Each time point was binarized (example shows time point 1 (T1) and time point 2 (T2). Each time point was compared to its adjacent time point to find the motility index by taking the sum of the retracted (blue) and extended (pink) pixels divided by the stable (white) pixels. To determine the surveillance ratio, a maximum projection of all 12 time points was created and the total number of microglia pixels in the projection was normalized to the number of microglia pixels in T1 (blue). (B,C) Microglia motility was unchanged by treatment or sex in both the ML (B) and PCL (C). There was no interaction between treatment and sex in either the ML ((B); F (1, 16) = 2.080, p = 0.1685) or PCL (C; F (1, 16) = 1.109, p = 0.3079). There was no main effect for treatment in either the ML ((B); F (1, 16) = 2.343, p = 0.1454) or PCL ((C); F (1, 16) = 0.5477, p = 0.4700). There was no main effect for sex in either the ML ((B); F (1, 16) = 0.6480, p = 0.4326) or PCL ((C); F (1, 16) = 0.003263, p = 0.9552). (D,E) Similarly, microglia surveillance was also unaltered in both the ML (D) and PCL (E). There was no interaction between treatment and sex in either the ML ((D); F (1, 16) = 1.625, p = 0.2207) or PCL ((E); F (1, 16) = 0.3599, p = 0.5570). There was no main effect for treatment in either the ML ((D); F (1, 16) = 2.322, p = 0.1471) or PCL ((E); F (1, 16) = 0.1977, p = 0.6625). There was no main effect for sex in either the ML ((D); F (1, 16) = 1.282, p = 0.2742) or PCL ((E); F (1, 16) = 0.6310, p = 0.4386). (F–K) The motility (F–H) and surveillance (I–K) of microglia in the ML and PCL of the same animal were compared. PCL microglia were significantly more motile than ML microglia when sexes were pooled ((F); saline: p = 0.0006, EtOH: p = 0.0028). In males (G), there was more motility in PCL microglia than ML microglia, an effect which was significant in saline-dosed males (p = 0.0269) or exhibited a trend towards significance in ethanol-dosed males (p = 0.0582). In females (H), PCL microglia were significantly more motile than ML microglia in both saline-dosed (p = 0.0200), and ethanol-dosed (p = 0.0482) animals. PCL microglia surveyed significantly more area than ML microglia when sexes were pooled ((I); saline: p = 0.0057, EtOH: p = 0.0352). In males (J), saline-dosed animals had a higher surveillance ratio in the PCL than the ML (p = 0.0291), but there were no differences in ethanol-dosed males (p = 0.3767). In females (K), there were trends towards higher surveillance in the PCL than ML (saline: p = 0.0570, EtOH: p = 0.0546). (B–E) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. (F–K) Each pair of datapoints connected by a line represents an individual animal. Paired t-tests. Scale bar = 25 μm.
Figure 4.
Cerebellar microglia dynamics in vivo. (A) Example in-vivo two-photon image of cerebellar microglia. Each time point was binarized (example shows time point 1 (T1) and time point 2 (T2). Each time point was compared to its adjacent time point to find the motility index by taking the sum of the retracted (blue) and extended (pink) pixels divided by the stable (white) pixels. To determine the surveillance ratio, a maximum projection of all 12 time points was created and the total number of microglia pixels in the projection was normalized to the number of microglia pixels in T1 (blue). (B,C) Microglia motility was unchanged by treatment or sex in both the ML (B) and PCL (C). There was no interaction between treatment and sex in either the ML ((B); F (1, 16) = 2.080, p = 0.1685) or PCL (C; F (1, 16) = 1.109, p = 0.3079). There was no main effect for treatment in either the ML ((B); F (1, 16) = 2.343, p = 0.1454) or PCL ((C); F (1, 16) = 0.5477, p = 0.4700). There was no main effect for sex in either the ML ((B); F (1, 16) = 0.6480, p = 0.4326) or PCL ((C); F (1, 16) = 0.003263, p = 0.9552). (D,E) Similarly, microglia surveillance was also unaltered in both the ML (D) and PCL (E). There was no interaction between treatment and sex in either the ML ((D); F (1, 16) = 1.625, p = 0.2207) or PCL ((E); F (1, 16) = 0.3599, p = 0.5570). There was no main effect for treatment in either the ML ((D); F (1, 16) = 2.322, p = 0.1471) or PCL ((E); F (1, 16) = 0.1977, p = 0.6625). There was no main effect for sex in either the ML ((D); F (1, 16) = 1.282, p = 0.2742) or PCL ((E); F (1, 16) = 0.6310, p = 0.4386). (F–K) The motility (F–H) and surveillance (I–K) of microglia in the ML and PCL of the same animal were compared. PCL microglia were significantly more motile than ML microglia when sexes were pooled ((F); saline: p = 0.0006, EtOH: p = 0.0028). In males (G), there was more motility in PCL microglia than ML microglia, an effect which was significant in saline-dosed males (p = 0.0269) or exhibited a trend towards significance in ethanol-dosed males (p = 0.0582). In females (H), PCL microglia were significantly more motile than ML microglia in both saline-dosed (p = 0.0200), and ethanol-dosed (p = 0.0482) animals. PCL microglia surveyed significantly more area than ML microglia when sexes were pooled ((I); saline: p = 0.0057, EtOH: p = 0.0352). In males (J), saline-dosed animals had a higher surveillance ratio in the PCL than the ML (p = 0.0291), but there were no differences in ethanol-dosed males (p = 0.3767). In females (K), there were trends towards higher surveillance in the PCL than ML (saline: p = 0.0570, EtOH: p = 0.0546). (B–E) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. (F–K) Each pair of datapoints connected by a line represents an individual animal. Paired t-tests. Scale bar = 25 μm.
![Cells 13 00386 g004a]()
![Cells 13 00386 g004b]()
Figure 5.
Cerebellar microglia injury response in vivo. (A) Example in-vivo two-photon images of cerebellar microglia and the injury core (bright area of autofluorescent debris) over time after a brief laser ablation was inflicted. Outlined in white in each image are the ablation core and the “microglia front,” which decreases over time as microglia converge on the injury. (B–D) Quantification of microglial convergence on the injury core over 60 min of imaging. No significant differences between ethanol- and saline-dosed animals for convergence were observed when sexes were combined (B), or separated into males (C) and females (D). When sexes were pooled (B), there was a main effect of time (F (2.326, 41.87) = 209.2, p < 0.0001) on microglia normalized proximity, but no main effect of treatment (F (1, 18) = 0.3516, p = 0.5606) and no interaction between time and treatment (F (11, 198) = 0.3193, p = 0.9811). In males (C), there was a main effect of time (F (11, 88) = 85.68, p < 0.0001), but no main effect of treatment (F (1, 8) = 0.001843, p = 0.9668) and no interaction between time and treatment (F (11, 88) = 1.104, p = 0.3680). In females (D), there was a main effect of time (F (2.877, 23.02) = 117.3, p < 0.0001), but no main effect of treatment (F (1, 8) = 1.188, p = 0.3075) and no interaction between time and treatment (F (11, 88) = 1.031, p = 0.4263). (B–D) Each datapoint represents a treatment group and sex. Data are presented as the mean ± SEM. Two-way ANOVA, repeated measures with Bonferroni post-hoc comparisons. Scale bar = 25 μm.
Figure 5.
Cerebellar microglia injury response in vivo. (A) Example in-vivo two-photon images of cerebellar microglia and the injury core (bright area of autofluorescent debris) over time after a brief laser ablation was inflicted. Outlined in white in each image are the ablation core and the “microglia front,” which decreases over time as microglia converge on the injury. (B–D) Quantification of microglial convergence on the injury core over 60 min of imaging. No significant differences between ethanol- and saline-dosed animals for convergence were observed when sexes were combined (B), or separated into males (C) and females (D). When sexes were pooled (B), there was a main effect of time (F (2.326, 41.87) = 209.2, p < 0.0001) on microglia normalized proximity, but no main effect of treatment (F (1, 18) = 0.3516, p = 0.5606) and no interaction between time and treatment (F (11, 198) = 0.3193, p = 0.9811). In males (C), there was a main effect of time (F (11, 88) = 85.68, p < 0.0001), but no main effect of treatment (F (1, 8) = 0.001843, p = 0.9668) and no interaction between time and treatment (F (11, 88) = 1.104, p = 0.3680). In females (D), there was a main effect of time (F (2.877, 23.02) = 117.3, p < 0.0001), but no main effect of treatment (F (1, 8) = 1.188, p = 0.3075) and no interaction between time and treatment (F (11, 88) = 1.031, p = 0.4263). (B–D) Each datapoint represents a treatment group and sex. Data are presented as the mean ± SEM. Two-way ANOVA, repeated measures with Bonferroni post-hoc comparisons. Scale bar = 25 μm.
![Cells 13 00386 g005]()
Figure 6.
Cerebellar Purkinje cell linear frequency in lobule IV/V. (A) 10× confocal stitched image of lobule IV/V of the vermis. White line indicates the length of the PCL. (B,C) Purkinje cell linear frequency (number of Purkinje cells divided by the length of the PCL) was significantly lower in ethanol-dosed animals when sexes were combined ((B), p = 0.0376) and when separated by sex ((C), F (1, 16) = 4.884, p = 0.0420), although this effect seemed to be caused by females where the post-hoc test showed a trend towards significance (p = 0.0610), whereas it did not in males (p = 0.9255). There was no main effect of sex (F (1, 16) = 0.1277, p = 0.7255) and no interaction between treatment and sex (F (1, 16) = 1.313, p = 0.2687). (B,C) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. (B) Unpaired t-test. (C) Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 100 μm.
Figure 6.
Cerebellar Purkinje cell linear frequency in lobule IV/V. (A) 10× confocal stitched image of lobule IV/V of the vermis. White line indicates the length of the PCL. (B,C) Purkinje cell linear frequency (number of Purkinje cells divided by the length of the PCL) was significantly lower in ethanol-dosed animals when sexes were combined ((B), p = 0.0376) and when separated by sex ((C), F (1, 16) = 4.884, p = 0.0420), although this effect seemed to be caused by females where the post-hoc test showed a trend towards significance (p = 0.0610), whereas it did not in males (p = 0.9255). There was no main effect of sex (F (1, 16) = 0.1277, p = 0.7255) and no interaction between treatment and sex (F (1, 16) = 1.313, p = 0.2687). (B,C) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. (B) Unpaired t-test. (C) Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 100 μm.
Figure 7.
Cerebellar microglia–Purkinje cell interactions in lobule IV/V in fixed tissue. (A) 40× confocal images within lobule IV/V of the vermis showing microglia (left), Purkinje cells (middle), and composite (right). White lines indicate different layers that were analyzed (ML (B–E), PCL (F–I), WM (J–M)). (B,C,F,G,J,K) The number of microglia (B,F,J) or Purkinje cell (C,G,K) pixels was divided by the volume of either the ML (B,C), PCL (F,G), or WM (J,K) to obtain the volume fraction. The microglial volume fraction was unaltered in all layers. There was no main effect for treatment in the ML ((B); F (1, 16) = 0.1135, p = 0.7406), PCL ((F); F (1, 16) = 0.3775, p = 0.5476), or WM ((J); F (1, 16) = 0.8330, p = 0.3750). There was no main effect for sex in the ML ((B); F (1, 16) = 0.7162, p = 0.4099), PCL ((F); F (1, 16) = 0.07930, p = 0.7819), or WM ((J); F (1, 16) = 0.005239, p = 0.9432). There was no interaction between treatment and sex in the ML ((B); F (1, 16) = 0.5903, p = 0.4535), PCL ((F); F (1, 16) = 0.09619, p = 0.7605), or WM ((J); F (1, 16) = 0.8679, p = 0.3654). For Purkinje cell volume fraction, there was no main effect for treatment in the ML ((C); F (1, 16) = 1.897, p = 0.1874) or WM ((K); F (1, 16) = 0.001421, p = 0.9704). However, there was significantly less Purkinje cell volume fraction in the PCL in ethanol-dosed mice compared to saline-treated mice ((G); F (1, 16) = 10.63, p = 0.0049), an effect which was significant in females (p = 0.0180), but not males (p = 0.2410). There was no main effect for sex in the ML ((C); F (1, 16) = 0.1437, p = 0.7096), PCL ((G); F (1, 16) = 0.07170, p = 0.7923), or WM ((K); F (1, 16) = 0.2196, p = 0.6457). There was no interaction between treatment and sex in the ML ((C); F (1, 16) = 0.2023, p = 0.6589) or PCL ((G); F (1, 16) = 0.8862, p = 0.3605). However, there was a trend towards a treatment–sex interaction effect for the Purkinje cell volume fraction in the WM ((K); F (1, 16) = 3.628, p = 0.0750). (D,H,L) The number of pixels representing the overlap between the microglia and Purkinje cell pixels was unaltered by ethanol exposure in every layer. For microglia–Purkinje cell interactions, there was no main effect for treatment in the ML ((D); F (1, 16) = 0.3242, p = 0.5770), PCL ((H); F (1, 16) = 0.0009879, p = 0.9753), or WM ((L); F (1, 16) = 1.703, p = 0.2103). There was no main effect for sex in the ML ((D); F (1, 16) = 2.718, p = 0.1187), PCL ((H); F (1, 16) = 0.1628, p = 0.6920), or WM ((L); F (1, 16) = 0.6698, p = 0.4251). There was no interaction effect between treatment and sex in the ML ((D); F (1, 16) = 0.5222, p = 0.4804), PCL ((H); F (1, 16) = 0.002974, p = 0.9572) or WM ((L); F (1, 16) = 1.282, p = 0.2742). (E,I,M) The number of pixels representing the overlap between the microglia and Purkinje cell pixels was also normalized to the number of the microglia pixels to determine microglia–Purkinje cell interaction. For microglia–Purkinje cell interactions normalized to microglia pixels, there was no main effect for treatment in the ML ((E); F (1, 16) = 0.05929, p = 0.8107), PCL ((I); F (1, 16) = 0.08791, p = 0.7707), or WM ((M); F (1, 16) = 0.8391, p = 0.3732). There was no main effect for sex in the ML ((E); F (1, 16) = 2.472, p = 0.1354) or WM ((M); F (1, 16) = 0.09420, p = 0.7629). However, in the PCL (I), there was a trend towards a decrease in microglia–Purkinje cell interactions in females compared to males (F (1, 16) = 3.572, p = 0.0770) and there was a significant treatment–sex interaction (F (1, 16) = 6.499, p = 0.0214), with female ethanol-dosed mice having fewer microglia–Purkinje cell interactions than male ethanol-dosed mice (p = 0.0127). There was no interaction effect between treatment and sex in the ML ((E); F (1, 16) = 0.01089, p= 0.9182) or WM ((M); F (1, 16) = 1.229, p = 0.2841). (B–M) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 50 μm.
Figure 7.
Cerebellar microglia–Purkinje cell interactions in lobule IV/V in fixed tissue. (A) 40× confocal images within lobule IV/V of the vermis showing microglia (left), Purkinje cells (middle), and composite (right). White lines indicate different layers that were analyzed (ML (B–E), PCL (F–I), WM (J–M)). (B,C,F,G,J,K) The number of microglia (B,F,J) or Purkinje cell (C,G,K) pixels was divided by the volume of either the ML (B,C), PCL (F,G), or WM (J,K) to obtain the volume fraction. The microglial volume fraction was unaltered in all layers. There was no main effect for treatment in the ML ((B); F (1, 16) = 0.1135, p = 0.7406), PCL ((F); F (1, 16) = 0.3775, p = 0.5476), or WM ((J); F (1, 16) = 0.8330, p = 0.3750). There was no main effect for sex in the ML ((B); F (1, 16) = 0.7162, p = 0.4099), PCL ((F); F (1, 16) = 0.07930, p = 0.7819), or WM ((J); F (1, 16) = 0.005239, p = 0.9432). There was no interaction between treatment and sex in the ML ((B); F (1, 16) = 0.5903, p = 0.4535), PCL ((F); F (1, 16) = 0.09619, p = 0.7605), or WM ((J); F (1, 16) = 0.8679, p = 0.3654). For Purkinje cell volume fraction, there was no main effect for treatment in the ML ((C); F (1, 16) = 1.897, p = 0.1874) or WM ((K); F (1, 16) = 0.001421, p = 0.9704). However, there was significantly less Purkinje cell volume fraction in the PCL in ethanol-dosed mice compared to saline-treated mice ((G); F (1, 16) = 10.63, p = 0.0049), an effect which was significant in females (p = 0.0180), but not males (p = 0.2410). There was no main effect for sex in the ML ((C); F (1, 16) = 0.1437, p = 0.7096), PCL ((G); F (1, 16) = 0.07170, p = 0.7923), or WM ((K); F (1, 16) = 0.2196, p = 0.6457). There was no interaction between treatment and sex in the ML ((C); F (1, 16) = 0.2023, p = 0.6589) or PCL ((G); F (1, 16) = 0.8862, p = 0.3605). However, there was a trend towards a treatment–sex interaction effect for the Purkinje cell volume fraction in the WM ((K); F (1, 16) = 3.628, p = 0.0750). (D,H,L) The number of pixels representing the overlap between the microglia and Purkinje cell pixels was unaltered by ethanol exposure in every layer. For microglia–Purkinje cell interactions, there was no main effect for treatment in the ML ((D); F (1, 16) = 0.3242, p = 0.5770), PCL ((H); F (1, 16) = 0.0009879, p = 0.9753), or WM ((L); F (1, 16) = 1.703, p = 0.2103). There was no main effect for sex in the ML ((D); F (1, 16) = 2.718, p = 0.1187), PCL ((H); F (1, 16) = 0.1628, p = 0.6920), or WM ((L); F (1, 16) = 0.6698, p = 0.4251). There was no interaction effect between treatment and sex in the ML ((D); F (1, 16) = 0.5222, p = 0.4804), PCL ((H); F (1, 16) = 0.002974, p = 0.9572) or WM ((L); F (1, 16) = 1.282, p = 0.2742). (E,I,M) The number of pixels representing the overlap between the microglia and Purkinje cell pixels was also normalized to the number of the microglia pixels to determine microglia–Purkinje cell interaction. For microglia–Purkinje cell interactions normalized to microglia pixels, there was no main effect for treatment in the ML ((E); F (1, 16) = 0.05929, p = 0.8107), PCL ((I); F (1, 16) = 0.08791, p = 0.7707), or WM ((M); F (1, 16) = 0.8391, p = 0.3732). There was no main effect for sex in the ML ((E); F (1, 16) = 2.472, p = 0.1354) or WM ((M); F (1, 16) = 0.09420, p = 0.7629). However, in the PCL (I), there was a trend towards a decrease in microglia–Purkinje cell interactions in females compared to males (F (1, 16) = 3.572, p = 0.0770) and there was a significant treatment–sex interaction (F (1, 16) = 6.499, p = 0.0214), with female ethanol-dosed mice having fewer microglia–Purkinje cell interactions than male ethanol-dosed mice (p = 0.0127). There was no interaction effect between treatment and sex in the ML ((E); F (1, 16) = 0.01089, p= 0.9182) or WM ((M); F (1, 16) = 1.229, p = 0.2841). (B–M) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 50 μm.
![Cells 13 00386 g007]()
Figure 8.
Cerebellar microglia–Purkinje cell interactions in vivo. (A) In-vivo two-photon images in the ML (top) and PCL (bottom) showing microglia (left), Purkinje cells (middle), and interactions (right). White overlay indicates microglia–Purkinje cell overlap. (B,C) The overlap between the whole microglia and whole Purkinje cell pixels was normalized to the number of the microglia pixels to determine microglia–Purkinje cell interaction. For in-vivo whole-microglia–whole-Purkinje cell interactions, there was no main effect for treatment in the ML ((B); F (1, 16) = 1.465, p = 0.2438) or PCL ((C); F (1, 16) = 0.4931, p = 0.4926). There was no main effect for sex in the ML ((B); F (1, 16) = 0.1041, p = 0.7511). In the PCL (C), there were significantly more interactions in female mice than in male mice (F (1, 16) = 4.832, p = 0.0430), with post-hoc testing showing a trend in saline-dosed mice (p = 0.0649). In the ML (B), there was a trend towards a significant treatment–sex interaction (F (1, 16) = 3.397, p = 0.0839), with ethanol-dosed female mice trending towards having more microglia–Purkinje cell interactions than saline-dosed females (p = 0.0928). There was no interaction effect between treatment and sex in the PCL ((C); F (1, 16) = 1.242, p = 0.2816). (D) To determine the dynamic interaction index, the binarized whole-microglia–whole-Purkinje cell overlap (example from ML in (A)) in each of the 12 time points was compared to adjacent time points by taking the sum of the retracted (blue) and extended (pink) pixels and dividing by the stable (white) pixels. For the coverage index, the binarized interaction images at all time points were summed (white) and divided by the total pixels (white + black). (E,G) For the dynamic interaction index, there was no main effect for treatment in either the ML ((E); F (1, 16) = 0.4659, p = 0.5047) or PCL ((G); F (1, 16) = 2.216, p = 0.1560). There was no main effect for sex in either the ML ((E); F (1, 16) = 0.05820, p = 0.8124) or PCL ((G); F (1, 16) = 2.947, p = 0.1053). In the ML (E), there was a trend towards significance for a treatment–sex interaction effect for the dynamic interaction index (F (1, 16) = 3.533, p = 0.0785). There was no interaction between treatment and sex in the PCL ((G); F (1, 16) = 0.5999, p = 0.4499). (F,H) For the coverage index, there was no main effect for treatment in either the ML ((F); F (1, 16) = 0.9049, p = 0.3556) or PCL ((H); F (1, 16) = 0.4976, p = 0.4907). There was no main effect for sex in the ML ((F); F (1, 16) = 2.240, p = 0.1540). In the PCL (H), female mice had a significantly higher coverage index than male mice (F (1, 16) = 4.556, p = 0.0486), with post-hoc testing showing a trend in saline-dosed animals (p = 0.0720). There was no interaction between treatment and sex in the ML ((F); F (1, 16) = 1.033, p = 0.3246) or PCL ((H); F (1, 16) = 1.216, p = 0.2864). (B,C,E–H) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 25 μm.
Figure 8.
Cerebellar microglia–Purkinje cell interactions in vivo. (A) In-vivo two-photon images in the ML (top) and PCL (bottom) showing microglia (left), Purkinje cells (middle), and interactions (right). White overlay indicates microglia–Purkinje cell overlap. (B,C) The overlap between the whole microglia and whole Purkinje cell pixels was normalized to the number of the microglia pixels to determine microglia–Purkinje cell interaction. For in-vivo whole-microglia–whole-Purkinje cell interactions, there was no main effect for treatment in the ML ((B); F (1, 16) = 1.465, p = 0.2438) or PCL ((C); F (1, 16) = 0.4931, p = 0.4926). There was no main effect for sex in the ML ((B); F (1, 16) = 0.1041, p = 0.7511). In the PCL (C), there were significantly more interactions in female mice than in male mice (F (1, 16) = 4.832, p = 0.0430), with post-hoc testing showing a trend in saline-dosed mice (p = 0.0649). In the ML (B), there was a trend towards a significant treatment–sex interaction (F (1, 16) = 3.397, p = 0.0839), with ethanol-dosed female mice trending towards having more microglia–Purkinje cell interactions than saline-dosed females (p = 0.0928). There was no interaction effect between treatment and sex in the PCL ((C); F (1, 16) = 1.242, p = 0.2816). (D) To determine the dynamic interaction index, the binarized whole-microglia–whole-Purkinje cell overlap (example from ML in (A)) in each of the 12 time points was compared to adjacent time points by taking the sum of the retracted (blue) and extended (pink) pixels and dividing by the stable (white) pixels. For the coverage index, the binarized interaction images at all time points were summed (white) and divided by the total pixels (white + black). (E,G) For the dynamic interaction index, there was no main effect for treatment in either the ML ((E); F (1, 16) = 0.4659, p = 0.5047) or PCL ((G); F (1, 16) = 2.216, p = 0.1560). There was no main effect for sex in either the ML ((E); F (1, 16) = 0.05820, p = 0.8124) or PCL ((G); F (1, 16) = 2.947, p = 0.1053). In the ML (E), there was a trend towards significance for a treatment–sex interaction effect for the dynamic interaction index (F (1, 16) = 3.533, p = 0.0785). There was no interaction between treatment and sex in the PCL ((G); F (1, 16) = 0.5999, p = 0.4499). (F,H) For the coverage index, there was no main effect for treatment in either the ML ((F); F (1, 16) = 0.9049, p = 0.3556) or PCL ((H); F (1, 16) = 0.4976, p = 0.4907). There was no main effect for sex in the ML ((F); F (1, 16) = 2.240, p = 0.1540). In the PCL (H), female mice had a significantly higher coverage index than male mice (F (1, 16) = 4.556, p = 0.0486), with post-hoc testing showing a trend in saline-dosed animals (p = 0.0720). There was no interaction between treatment and sex in the ML ((F); F (1, 16) = 1.033, p = 0.3246) or PCL ((H); F (1, 16) = 1.216, p = 0.2864). (B,C,E–H) Each datapoint represents an individual animal. Data are presented as the mean ± SEM. Two-way ANOVA with Bonferroni post-hoc comparisons. Scale bar = 25 μm.
![Cells 13 00386 g008]()