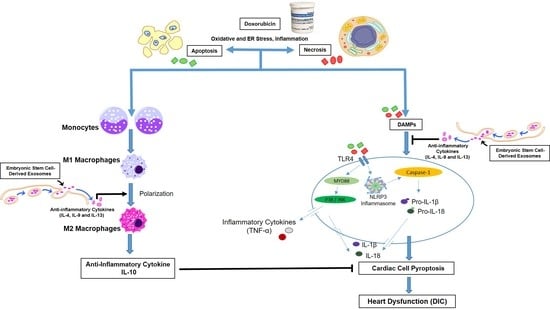
Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Exosomes Preparation
2.2. Animal Preparation
2.3. Immunohistochemistry (IHC) Staining
2.4. SDS-PAGE and Western Blot Analysis
2.5. cDNA Synthesis and RT-PCR Reaction
2.6. Determination of Cytoplasmic Vacuolization, Myofibrillar Loss, and Cardiac Hypertrophy
2.7. Determination of Intestinal and Vascular Fibrosis
2.8. Echocardiography
2.9. Statistical Analysis
3. Results
3.1. Effect of ESCs or ES-Exos on Mice Weight Following Dox Administration
3.2. Expression of Inflammasome Formation Proteins (TLR4 and NLRP3) after ESCs or ES-Exos Treatment
3.3. Expression of Pyroptotic Markers Caspase-1, IL-1β, and IL-18, after ESCs or ES-Exos Treatment
3.4. ES-Exos Reduces Pro-Inflammatory M1 Macrophages and Enhances Anti-Inflammatory M2 Macrophages
3.5. Effects of ESCs or ES-Exos Treatment on Mitogen-Activated Protein Kinase (MAPK) Cell Signaling
3.6. Effects of ESCs or ES-Exos on Cytoplasmic Vacuolization, Myofibril Loss, and Cardiac Hypertrophy
3.7. Effects of ESCs or ES-Exos on Fibrosis and Pro-Fibrotic Protein MMP-9
3.8. ES-Exos Improves Heart Function in DIC Murine Model
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BW | body weight |
| DAPI | 4′,6-diamino-2-phenylindole |
| DIC | doxorubicin-induced cardiotoxicity |
| DMEM | dulbecco’s modified eagle’s medium |
| Dox | doxorubicin |
| EDV | end diastolic volume |
| EF | ejection fraction |
| ER | endoplasmic reticulum |
| ESCs | embryonic stem cells |
| ESV | end systolic volume |
| Exos | exosomes |
| FBS | fetal bovine serum |
| FS | fractional shortening |
| H&E | hematoxylin and eosin |
| HW | heart weight |
| IHC | immunohistochemistry |
| IL | interleukin |
| iNOS | induced nitric oxide synthase |
| IP | intraperitoneal |
| LVIDd | left ventricular internal dimension-diastole |
| LVIDs | left ventricular internal dimension-systole |
| MI | myocardial infarction |
| miRNA | microRNA |
| MMP | matrix metalloproteinase |
| PBS | phosphate buffered saline |
| PS | penicillin/streptomycin |
| PVDF | polyvinylidene difluoride |
| RIPA | radio-immunoprecipitation assay |
| RT-PCR | real time-polymerase chain reaction |
| Src-α-actin | sarcomeric alpha actin |
| SDS | sodium dodecyl sulfate |
| SEM | standard error of mean |
References
- Tavakoli Dargani, Z.; Singla, D.K. Embryonic stem cell-derived exosomes inhibit doxorubicin-induced TLR4-NLRP3-mediated cell death-pyroptosis. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H460–H471. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.F.; Oshiro, C.; Marsh, S.; Hernandez-Boussard, T.; McLeod, H.; Klein, T.E.; Altman, R.B. Doxorubicin pathways: Pharmacodynamics and adverse effects. Pharmacogenetics Genom. 2011, 21, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Octavia, Y.; Tocchetti, C.G.; Gabrielson, K.L.; Janssens, S.; Crijns, H.J.; Moens, A.L. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J. Mol. Cell. Cardiol. 2012, 52, 1213–1225. [Google Scholar] [CrossRef] [Green Version]
- Jain, D.; Ahmad, T.; Cairo, M.; Aronow, W. Cardiotoxicity of cancer chemotherapy: Identification, prevention and treatment. Ann. Transl. Med. 2017, 5, 348. [Google Scholar] [CrossRef]
- Zhang, Y.-W.; Shi, J.; Li, Y.-J.; Wei, L. Cardiomyocyte death in doxorubicin-induced cardiotoxicity. Arch. Immunol. et Ther. Exp. 2009, 57, 435–445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merino, H.; Singla, D.K. Notch-1 Mediated Cardiac Protection following Embryonic and Induced Pluripotent Stem Cell Transplantation in Doxorubicin-Induced Heart Failure. PLoS ONE 2014, 9, e101024. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, K.; Zhang, J.; Honbo, N.; Karliner, J.S. Doxorubicin cardiomyopathy. Cardiology 2010, 115, 155–162. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K. Akt—mTOR Pathway Inhibits Apoptosis and Fibrosis in Doxorubicin-Induced Cardiotoxicity following Embryonic Stem Cell Transplantation. Cell Transplant. 2015, 24, 1031–1042. [Google Scholar] [CrossRef] [PubMed]
- Wu, S. Adriamycin-induced Cardiomyocyte and Endothelial Cell Apoptosis: In Vitro and In Vivo Studies. J. Mol. Cell. Cardiol. 2002, 34, 1595–1607. [Google Scholar] [CrossRef] [PubMed]
- Kalyanaraman, B.; Kotamraju, S.; Konorev, E.A.; Joseph, J. Doxorubicin-induced Apoptosis in Endothelial Cells and Cardiomyocytes Is Ameliorated by Nitrone Spin Traps and Ebselen. J. Boil. Chem. 2000, 275, 33585–33592. [Google Scholar] [Green Version]
- Johnson, T.A.; Singla, D.K. PTEN inhibitor VO-OHpic attenuates inflammatory M1 macrophages and cardiac remodeling in doxorubicin-induced cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2018, 315, H1236–H1249. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Raheem, I.T.; Taye, A.; Abouzied, M.M.; Abdel-Raheem, I.T. Cardioprotective Effects of Nicorandil, a Mitochondrial Potassium Channel Opener against Doxorubicin-Induced Cardiotoxicity in Rats. Basic Clin. Pharmacol. Toxicol. 2013, 113, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Farías, J.G.; Molina, V.M.; Carrasco, R.A.; Zepeda, A.B.; Figueroa, E.; Letelier, P.; Castillo, R.L. Antioxidant Therapeutic Strategies for Cardiovascular Conditions Associated with Oxidative Stress. Nutrients 2017, 9, 966. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.; Sahebkar, A. Protective effects of curcumin against doxorubicin-induced toxicity and resistance: A review. Crit. Rev. Oncol. Hematol. 2018, 122, 30–51. [Google Scholar] [CrossRef]
- Priya, L.B.; Baskaran, R.; Huang, C.Y.; Padma, V.V. Neferine ameliorates cardiomyoblast apoptosis induced by doxorubicin: Possible role in modulating NADPH oxidase/ROS-mediated NFkappaB redox signaling cascade. Sci. Rep. 2017, 7, 12283. [Google Scholar] [CrossRef] [PubMed]
- QuanJun, Y.; Genjin, Y.; Lili, W.; Yonglong, H.; Yan, H.; Jie, L.; Jinlu, H.; Jin, L.; Run, G.; Cheng, G. Protective Effects of Dexrazoxane against Doxorubicin-Induced Cardiotoxicity: A Metabolomic Study. PLoS ONE 2017, 12, e0169567. [Google Scholar] [CrossRef]
- Tian, X.-Q.; Ni, X.-W.; Xu, H.-L.; Zheng, L.; Zhuge, D.-L.; Chen, B.; Lu, C.-T.; Yuan, J.-J.; Zhao, Y.-Z. Prevention of doxorubicin-induced cardiomyopathy using targeted MaFGF mediated by nanoparticles combined with ultrasound-targeted MB destruction. Int. J. Nanomed. 2017, 12, 7103–7119. [Google Scholar] [CrossRef]
- Viswanatha Swamy, A.H.; Wangikar, U.; Koti, B.C.; Thippeswamy, A.H.; Ronad, P.M.; Manjula, D.V. Cardioprotective effect of ascorbic acid on doxorubicin-induced myocardial toxicity in rats. Indian J. Pharmacol. 2011, 43, 507–511. [Google Scholar] [CrossRef]
- Singla, D.K.; Ahmed, A.; Singla, R.; Yan, B. Embryonic stem cells improve cardiac function in Doxorubicin-induced cardiomyopathy mediated through multiple mechanisms. Cell Transplant. 2012, 21, 1919–1930. [Google Scholar] [CrossRef]
- Nussbaum, J.; Minami, E.; Laflamme, M.A.; Virag, J.A.I.; Ware, C.B.; Masino, A.; Muskheli, V.; Pabon, L.; Reinecke, H.; Murry, C.E. Transplantation of undifferentiated murine embryonic stem cells in the heart: Teratoma formation and immune response. FASEB J. 2007, 21, 1345–1357. [Google Scholar] [CrossRef]
- Swijnenburg, R.-J.; Tanaka, M.; Vogel, O.H.; Baker, J.; Kofidis, T.; Gunawan, F.; Lebl, D.R.; Caffarelli, A.D.; De Bruin, J.L.; Fedoseyeva, E.V.; et al. Embryonic stem cell immunogenicity increases upon differentiation after transplantation into ischemic myocardium. Circulation 2005, 112, I166–I172. [Google Scholar] [PubMed]
- Abdelwahid, E.; Kalvelyte, A.; Stulpinas, A.; De Carvalho, K.A.T.; Guarita-Souza, L.C.; Földes, G. Stem cell death and survival in heart regeneration and repair. Apoptosis 2016, 21, 252–268. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K. Stem cells and exosomes in cardiac repair. Curr. Opin. Pharmacol. 2016, 27, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.G.; Wu, J.C. Exosomes as potential alternatives to stem cell therapy in mediating cardiac regeneration. Circ. Res. 2015, 117, 7–9. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.G.-E.; Cheng, K.; Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep. 2014, 2, 606–619. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Y.; Liu, H.; Tang, W.H. Exosomes: Biogenesis, biologic function and clinical potential. Cell Biosci. 2019, 9, 19. [Google Scholar] [CrossRef] [PubMed]
- Jella, K.K.; Nasti, T.H.; Li, Z.; Malla, S.R.; Buchwald, Z.S.; Khan, M.K. Exosomes, Their Biogenesis and Role in Inter-Cellular Communication, Tumor Microenvironment and Cancer Immunotherapy. Vaccines 2018, 6, 69. [Google Scholar] [CrossRef]
- Khan, M.; Nickoloff, E.; Abramova, T.; Johnson, J.; Verma, S.K.; Krishnamurthy, P.; Mackie, A.R.; Vaughan, E.; Garikipati, V.N.S.; Benedict, C.; et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015, 117, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Tavakoli Dargani, Z.; Singla, R.; Johnson, T.; Kukreja, R.; Singla, D.K. Exosomes derived from embryonic stem cells inhibit doxorubicin and inflammation-induced pyroptosis in muscle cells. Can. J. Physiol. Pharmacol. 2018, 96, 304–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Singla, R.D.; Abdelli, L.S.; Singal, P.K.; Singla, D.K. Regulation of PTEN/Akt pathway enhances cardiomyogenesis and attenuates adverse left ventricular remodeling following thymosin beta4 Overexpressing embryonic stem cell transplantation in the infarcted heart. PLoS ONE 2013, 8, e75580. [Google Scholar]
- Miao, E.A.; Rajan, J.V.; Aderem, A. Caspase-1-induced pyroptotic cell death. Immunol. Rev. 2011, 243, 206–214. [Google Scholar] [CrossRef] [PubMed]
- Singla, D.K.; Singla, R.D.; Abdelli, L.S.; Glass, C. Fibroblast Growth Factor-9 Enhances M2 Macrophage Differentiation and Attenuates Adverse Cardiac Remodeling in the Infarcted Diabetic Heart. PLOS ONE 2015, 10, e0120739. [Google Scholar] [CrossRef] [PubMed]
- Parisi, L.; Gini, E.; Baci, D.; Tremolati, M.; Fanuli, M.; Bassani, B.; Farronato, G.; Bruno, A.; Mortara, L. Macrophage Polarization in Chronic Inflammatory Diseases: Killers or Builders? J. Immunol. Res. 2018, 2018, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Urbina, P.; Singla, D.K. BMP-7 attenuates adverse cardiac remodeling mediated through M2 macrophages in prediabetic cardiomyopathy. Am. J. Physiol. Circ. Physiol. 2014, 307, H762–H772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.; Yuan, Y.C.; Li, J.Y.; Gionfriddo, M.R.; Huang, R.C. Tumor necrosis factor-alpha and its role as a mediator in myocardial infarction: A brief review. Chronic. Dis. Transl. Med. 2015, 1, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.; Shankar, N. Roles of TLR/MyD88/MAPK/NF-kappaB Signaling Pathways in the Regulation of Phagocytosis and Proinflammatory Cytokine Expression in Response to E. faecalis Infection. PLoS ONE 2015, 10, e0136947. [Google Scholar] [CrossRef] [PubMed]
- Marchant, D.; Singhera, G.K.; Utokaparch, S.; Hackett, T.L.; Boyd, J.H.; Luo, Z.; Si, X.; Dorscheid, D.R.; McManus, B.M.; Hegele, R.G. Toll-Like Receptor 4-Mediated Activation of p38 Mitogen-Activated Protein Kinase Is a Determinant of Respiratory Virus Entry and Tropism. J. Virol. 2010, 84, 11359–11373. [Google Scholar] [CrossRef] [Green Version]
- Du, Q.; Zhu, B.; Zhai, Q.; Yu, B. Sirt3 attenuates doxorubicin-induced cardiac hypertrophy and mitochondrial dysfunction via suppression of Bnip3. Am. J. Transl. Res. 2017, 9, 3360–3373. [Google Scholar]
- Glass, C.; Singla, D.K. MicroRNA-1 transfected embryonic stem cells enhance cardiac myocyte differentiation and inhibit apoptosis by modulating the PTEN/Akt pathway in the infarcted heart. Am. J. Physiol. Circ. Physiol. 2011, 301, H2038–H2049. [Google Scholar] [CrossRef] [Green Version]
- Faiella, W.; Atoui, R. Therapeutic use of stem cells for cardiovascular disease. Clin. Transl. Med. 2016, 5, 34. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Li, X.; Liu, M.; Zeng, Y.; Chen, S.; Zhang, P. Advances in stem cell therapy for cardiovascular disease (Review). Int. J. Mol. Med. 2016, 38, 23–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, B.; Singla, D.K. Transplanted Induced Pluripotent Stem Cells Mitigate Oxidative Stress and Improve Cardiac Function through the Akt Cell Survival Pathway in Diabetic Cardiomyopathy. Mol. Pharm. 2013, 10, 3425–3432. [Google Scholar] [CrossRef] [PubMed]
- Orlic, D.; Kajstura, J.; Chimenti, S.; Jakoniuk, I.; Anderson, S.M.; Li, B.; Pickel, J.; McKay, R.; Nadal-Ginard, B.; Bodine, D.M.; et al. Bone marrow cells regenerate infarcted myocardium. Nature 2001, 410, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Abushouk, A.I.; Salem, A.M.A.; Saad, A.; Afifi, A.M.; Afify, A.Y.; Afify, H.; Salem, H.S.; Ghanem, E.; Abdel-Daim, M.M. Mesenchymal Stem Cell Therapy for Doxorubicin-Induced Cardiomyopathy: Potential Mechanisms, Governing Factors, and Implications of the Heart Stem Cell Debate. Front. Pharmacol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Cai, X.; Liu, J.; Bai, B.; Li, X. Sphingosine 1-phosphate promotes mesenchymal stem cell-mediated cardioprotection against myocardial infarction via ERK1/2-MMP-9 and Akt signaling axis. Life Sci. 2018, 215, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Garbade, J.; Dhein, S.; Lipinski, C.; Aupperle, H.; Arsalan, M.; Borger, M.A.; Barten, M.J.; Lehmann, S.; Walther, T.; Mohr, F.-W. Bone Marrow-Derived Stem Cells Attenuate Impaired Contractility and Enhance Capillary Density in a Rabbit Model of Doxorubicin-Induced Failing Hearts. J. Card. Surg. 2009, 24, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Haider, H.; Ashraf, M. Bone marrow cell transplantation in clinical perspective. J. Mol. Cell. Cardiol. 2005, 38, 225–235. [Google Scholar] [CrossRef] [PubMed]
- Du Pre, B.C.; Doevendans, P.A.; van Laake, L.W. Stem cells for cardiac repair: An introduction. J. Geriatr. Cardiol. 2013, 10, 186–197. [Google Scholar] [PubMed]
- Blum, B.; Benvenisty, N. The Tumorigenicity of Human Embryonic Stem Cells. Adv. Cancer Res. 2008, 100, 133–158. [Google Scholar] [PubMed]
- Hentze, H.; Soong, P.L.; Wang, S.T.; Phillips, B.W.; Putti, T.C.; Dunn, N.R. Teratoma formation by human embryonic stem cells: Evaluation of essential parameters for future safety studies. Stem Cell Res. 2009, 2, 198–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arslan, F.; Lai, R.C.; Smeets, M.B.; Akeroyd, L.; Choo, A.; Aguor, E.N. Mesenchymal stem cell-derived exosomes increase ATP levels, decrease oxidative stress and activate PI3K/Akt pathway to enhance myocardial viability and prevent adverse remodeling after myocardial ischemia/reperfusion injury. Stem Cell Res. 2013, 10, 301–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, J.; Pan, Y.; Li, X.H.; Yang, X.Y.; Feng, Y.L.; Tan, H.H.; Jiang, L.; Feng, J.; Yu, X.Y. Cardiac progenitor cell-derived exosomes prevent cardiomyocytes apoptosis through exosomal miR-21 by targeting PDCD4. Cell Death Dis. 2016, 7, e2277. [Google Scholar] [CrossRef] [PubMed]
- Barile, L.; Milano, G.; Vassalli, G. Beneficial effects of exosomes secreted by cardiac-derived progenitor cells and other cell types in myocardial ischemia. Stem Cell Investig. 2017, 4, 93. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Du, W.; Liu, J.; Ma, W.; Zhang, L.; Du, Z.; Cai, B. Stem Cell-Derived Exosome in Cardiovascular Diseases: Macro Roles of Micro Particles. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.L.; Cookson, B.T. Apoptosis, Pyroptosis, and Necrosis: Mechanistic Description of Dead and Dying Eukaryotic Cells. Infect. Immun. 2005, 73, 1907–1916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Obregon, C.; Dreher, D.; Kok, M.; Cochand, L.; Kiama, G.S.; Nicod, L.P. Human alveolar macrophages infected by virulent bacteria expressing SipB are a major source of active interleukin-18. Infect. Immun. 2003, 71, 4382–4388. [Google Scholar] [CrossRef] [PubMed]
- Sansonetti, P.J.; Phalipon, A.; Arondel, J.; Thirumalai, K.; Banerjee, S.; Akira, S.; Takeda, K.; Zychlinsky, A. Caspase-1 activation of IL-1beta and IL-18 are essential for Shigella flexneri-induced inflammation. Immunity 2000, 12, 581–590. [Google Scholar] [CrossRef]
- Qiu, Z.; Lei, S.; Zhao, B.; Wu, Y.; Su, W.; Liu, M.; Meng, Q.; Zhou, B.; Leng, Y.; Xia, Z.-Y. NLRP3 Inflammasome Activation-Mediated Pyroptosis Aggravates Myocardial Ischemia/Reperfusion Injury in Diabetic Rats. Oxidative Med. Cell. Longev. 2017, 2017, 1–17. [Google Scholar] [CrossRef]
- Latz, E.; Xiao, T.S.; Stutz, A. Activation and regulation of the inflammasomes. Nat. Rev. Immunol. 2013, 13, 397–411. [Google Scholar] [CrossRef]
- Fleetwood, A.J.; Lee, M.K.; Singleton, W.; Achuthan, A.; Lee, M.-C.; O’Brien-Simpson, N.M.; Cook, A.D.; Murphy, A.J.; Dashper, S.G.; Reynolds, E.C.; et al. Metabolic Remodeling, Inflammasome Activation, and Pyroptosis in Macrophages Stimulated by Porphyromonas gingivalis and Its Outer Membrane Vesicles. Front. Microbiol. 2017, 7, 351. [Google Scholar] [CrossRef]
- Dong, W.; Zhu, Q.; Yang, B.; Qin, Q.; Wang, Y.; Xia, X.; Zhu, X.; Liu, Z.; Song, E.; Song, Y. Polychlorinated Biphenyl Quinone Induces Caspase 1-Mediated Pyroptosis through Induction of Pro-inflammatory HMGB1-TLR4-NLRP3-GSDMD Signal Axis. Chem. Res. Toxicol. 2019, 32, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Man, S.M.; Karki, R.; Kanneganti, T.-D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, S.; Zhou, X.; Ge, Z.; Song, Y.; Wang, H.; Liu, X.; Zhang, D. Exosomes from adipose-derived mesenchymal stem cells ameliorate cardiac damage after myocardial infarction by activating S1P/SK1/S1PR1 signaling and promoting macrophage M2 polarization. Int. J. Biochem. Cell Boil. 2019, 114, 105564. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Qiao, B.; Gao, N.; Lin, N.; He, W. Oral squamous cell carcinoma-derived exosomes promote M2 subtype macrophage polarization mediated by exosome-enclosed miR-29a-3p. Am. J. Physiol. Physiol. 2019, 316, C731–C740. [Google Scholar] [CrossRef] [PubMed]
- Bardi, G.T.; Smith, M.A.; Hood, J.L. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine 2018, 105, 63–72. [Google Scholar] [CrossRef]
- El-Agamy, D.S.; El-Harbi, K.M.; Khoshhal, S.; Ahmed, N.; Elkablawy, M.A.; Shaaban, A.A.; Abo-Haded, H.M. Pristimerin protects against doxorubicin-induced cardiotoxicity and fibrosis through modulation of Nrf2 and MAPK/NF-kB signaling pathways. Cancer Manag. Res. 2019, 11, 47–61. [Google Scholar] [CrossRef] [PubMed]
- Kaminska, B.; Gozdz, A.; Zawadzka, M.; Ellert-Miklaszewska, A.; Lipko, M. MAPK signal transduction underlying brain inflammation and gliosis as therapeutic target. Anat. Rec. 2009, 292, 1902–1913. [Google Scholar] [CrossRef]
- Zhang, B.; Ramesh, G.; Uematsu, S.; Akira, S.; Reeves, W.B. TLR4 signaling mediates inflammation and tissue injury in nephrotoxicity. J. Am. Soc. Nephrol. 2008, 19, 923–932. [Google Scholar] [CrossRef]
- Ramalho, B.D.S.; De Almeida, F.M.; Sales, C.M.; De Lima, S.; Martinez, A.M.B. Injection of bone marrow mesenchymal stem cells by intravenous or intraperitoneal routes is a viable alternative to spinal cord injury treatment in mice. Neural Regen. Res. 2018, 13, 1046–1053. [Google Scholar]
- Wang, M.; Liang, C.; Hu, H.; Zhou, L.; Xu, B.; Wang, X.; Han, Y.; Nie, Y.; Jia, S.; Liang, J.; et al. Intraperitoneal injection (IP), Intravenous injection (IV) or anal injection (AI)? Best way for mesenchymal stem cells transplantation for colitis. Sci. Rep. 2016, 6, 30696. [Google Scholar] [CrossRef]
- Marzban, M.; Mousavizadeh, K.; Bakhshayesh, M.; Vousooghi, N.; Vakilzadeh, G.; Torkaman-Boutorabi, A. Effect of Multiple Intraperitoneal Injections of Human Bone Marrow Mesenchymal Stem Cells on Cuprizone Model of Multiple Sclerosis. Iran. Biomed. J. 2018, 22, 312–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singla, D.K.; Abdelli, L.S. Embryonic Stem Cells and Released Factors Stimulate c-kit(+)/FLK-1(+) Progenitor Cells and Promote Neovascularization in Doxorubicin-Induced Cardiomyopathy. Cell Transplant. 2015, 24, 1043–1052. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.K.; Chetty, C.; Balasubramaniam, V.; Centanni, R.; Haraldsdottir, K.; Hematti, P.; Eldridge, M.W. Intraperitoneal injection of MSC-derived exosomes prevent experimental bronchopulmonary dysplasia. Biochem. Biophys. Res. Commun. 2018, 503, 2653–2658. [Google Scholar] [CrossRef] [PubMed]













| Target | Forward Primer | Reverse Primer |
|---|---|---|
| GAPDH | 5′-ACCCAGAAGACTGTGGATGG-3′ | 5′-CACATTGGGGGTAGGAACAC-3′ |
| Caspase-1 | 5′-GAAACGCCATGGCTGACAAG-3′ | 5′-CGTGCCTTGTCCATAGCAGT-3′ |
| IL-1β | 5′-AACCTGCTGGTGTGTGACTTC-3′ | 5′-CAGCACGAGGCTTTTTTGT-3′ |
| IL-18 | 5′-ACTTTGGCCGACTTCACTGT-3′ | 5′-GTCTGGTCTGGGGTTCACTG-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singla, D.K.; Johnson, T.A.; Tavakoli Dargani, Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells 2019, 8, 1224. https://doi.org/10.3390/cells8101224
Singla DK, Johnson TA, Tavakoli Dargani Z. Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells. 2019; 8(10):1224. https://doi.org/10.3390/cells8101224
Chicago/Turabian StyleSingla, Dinender K., Taylor A. Johnson, and Zahra Tavakoli Dargani. 2019. "Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy" Cells 8, no. 10: 1224. https://doi.org/10.3390/cells8101224
APA StyleSingla, D. K., Johnson, T. A., & Tavakoli Dargani, Z. (2019). Exosome Treatment Enhances Anti-Inflammatory M2 Macrophages and Reduces Inflammation-Induced Pyroptosis in Doxorubicin-Induced Cardiomyopathy. Cells, 8(10), 1224. https://doi.org/10.3390/cells8101224