Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model
Abstract
:1. Introduction
2. Advantages of the Protocol
3. Limitations
4. Detailed Protocol Including !CAUTION and #CRITICAL STEP
4.1. Materials
4.1.1. Reagents
- PneumaCultTM-Ex Plus Basal Medium (STEMCELL, Cologne, Germany, cat. no. 05041)
- PneumaCultTM-Ex Plus 50× Supplement (STEMCELL, Cologne, Germany, cat. no. 05042)
- PneumaCultTM- ALI Basal Medium (STEMCELL, Cologne, Germany, cat. no. 05002)
- PneumaCultTM- ALI 10× supplement (STEMCELL, Cologne, Germany, cat. no. 05003)
- PneumaCultTM- ALI 100× Maintenance supplement (STEMCELL, Cologne, Germany, cat. no. 05006)
- Hydrocortisone stock solution (STEMCELL, Cologne, Germany, cat. no. 07925)
- Animal Component-free cell dissociation kit (STEMCELL, Cologne, Germany, cat. no. 05426)
- D-PBS
- Hydrocortisone Solution (STEMCELL, Cologne, Germany, cat. no. 07925)
- Heparin Solution (STEMCELL, Cologne, Germany, cat. no. 07980)
- GrowDex® stock solution (UPM Biochemicals, Helsinki, Finland, cat. no. 100 103 005)
- Mowiol
- WGA 488 (Biotium, Fremont, CA, USA, cat.no. 29022-1)
- Hoechst 33342 (Cell signaling Technology, Frankfurt a. Main, Germany, cat. no. 4082)
- Mitotracker deep red (Life Technologies Austria, Vienna, Austria, cat.no. M22426)
- Recovery Cell culture freezing medium (Gibco™, Life Technologies Austria, Vienna, Austria, cat. no. 12648010)
- Human derived respiratory epithelial primary cells: NHBE: cat#: CC-2540-S, SAEC: cat#: CC-2547-S (Lonza, Cologne, Germany)
4.1.2. Equipment
- Tissue culture flasks, 75 cm2 (T75, Corning Costar, Amsterdam, The Netherlands)
- 6- and 24-well plates (Corning Costar, Amsterdam, The Netherlands)
- Transwell permeable supports, 6,5 mm Insert for 24 well plates (Corning Costar, Amsterdam, The Netherlands)
- Corning Falcon conical centrifuge tubes, 15 mL and 50 mL (Corning Costar, Amsterdam, The Netherlands
- Anatomical tweezers, scissors
- Assorted pipettes (2–20, 20–200 and 100–1000 µL) with sterile, disposable tips
- Centrifuge
- UV-sterilized tape
- Cell culture incubator, 37 °C and 5% CO2
- Water bath, 37 °C
- Neubauer chamber
- Vacuum pump, for medium aspiration
- Cryovials
- Cell culture hood
- Inverted microscope for cell culture approaches
- 1 mL serological pipettes
- 10 mL serological pipettes
- 25 mL serological pipettes
- Operetta CLS™ (Perkin Elmer Cellular Technologies Germany GmbH, Hamburg, Germany)
- Confocal microscope (LEICA SP5, Wetzlar, Germany)
- Specimen slides
- Cover slips
- Parafilm
4.2. Reagent Setup
4.3. Procedure
4.3.1. Seeding of Primary Epithelial Lung Cells to Cell Culture Flask; Expansion Phase
- (1)
- Prepare 100 mL of Complete PneumaCultTM-Ex Plus medium.
- (2)
- Pre-heat 25 mL of the medium to 37 °C (in this case, 25 mL can be transferred to a T75 cell culture flask and pre-heated in the incubator for a minimum of 30 min at 37 °C).
- (3)
- Thaw primary respiratory cells quickly in a water-bath at 37 °C for a maximum of 2 min.
- (4)
- After thawing, immediately transfer cells to pre-warmed growth medium (Complete PneumaCultTM-Ex Plus) in the cell culture flask—between 500,000 and 1 × 106 cells should be seeded to a T75 cell culture flask to guarantee an expansion rate up to 80% of the flask area after 3 days.
- (5)
- Rinse the cryovial with 500 µL of pre-warmed growth medium to make sure all cells are transferred to the T75 flask.
- (6)
- Incubate the flask (lying, not standing) at 37 °C and 5% CO2 until the cells expanded to a confluence of about 80%—this takes about 3 days. Using older passages > 4, the expansion phase also takes up to 5 days.
- (7)
- On day 2 of the expansion phase, 10 mL of the medium are exchanged. For this, pre-heat the medium at 37 °C in the water-bath. Aspirate 10 mL of the flask medium by using a serological pipette.
4.3.2. Harvesting of NHBE/SAE Cells from T75 Cell Culture Flasks
- (8)
- Pre-heat D-PBS (without Mg2+ and Ca2+) and Complete PneumaCult TM-Ex Plus Medium.
- (9)
- Aspirate the cell culture medium from the upstanding T75 cell culture flask.
- (10)
- Wash the cells with 5 mL pre-warmed D-PBS.
- (11)
- Aspirate the D-PBS and then add 6 mL of ACF Enzymatic dissociation solution.
- (12)
- Add 6 mL of ACF Enzymatic Inhibition solution and mix gently by moving the flask.
- (13)
- Incubate at 37 °C for 7–8 min until cells can be loosened from the bottom by gently tapping to the flask.
- (14)
- Collect the cells in a 15 mL Falcon using a 10 mL serological pipette.
- (15)
- Centrifuge the Falcon at 1400 RPM (350× g) for 5 min.
- (16)
- Aspirate the supernatant and re-suspend in pre-warmed Complete PneumaCult TM-Ex Plus Medium.
- (17)
- Count the cells using a Neubauer Chamber.
- (18)
- In the next step, calculate the cell numbers needed for the number of Transwells that are prepared.
- (19)
- By using the first three passages of NHBE and SAE cells, which can be frozen without losing any of the cellular features (see Freezing of non-seeded cells), between 10–13 × 106 cells are harvested from the T75 cell culture flask after the first expansion phase. Then, 1 × 106 cells are added to a new T75 cell culture flask and further expanded (repeat steps from 4) on), while the rest is frozen (see 20) to 24)) or seeded on Transwell filters (see Section 4.3.4 Upside-down seeding of NHBE/SAE cells (Figure 6)).
4.3.3. Freezing of Non-Seeded Cells
- (20)
- Cells that are not immediately used for further culture can be frozen up to a passage of 4, without losing any features (ciliogenesis, proliferative capacities).
- (21)
- Cryovials are prepared with correct labeling.
- (22)
- The calculated cell number seeded on Transwell membranes or further expanded in a T75 flask is separated from cells to be frozen, which are then spun at 1400 RPM (350× g).
- (23)
- The supernatant is aspirated, and the pellet is directly re-suspended using 500 µL Recovery cell culture freezing medium per million cells.
- (24)
- Cells are transferred to cryovials before being transmitted into a freezing container for cell preservation to ensure reproducible controlled-rate freezing.
4.3.4. Upside-Down Seeding of NHBE/SAE Cells
- (25)
- Per Transwell insert, 1 × 105 cells are seeded. The number of used Transwells has to be determined first to calculate cell numbers mixed with the GrowDex® solution.
- (26)
- Per upside-down seeded Transwell, 100 µL of 0.5% GrowDex® solution containing cells is used. To get this concentration, 333 µL of GrowDex® stock solution (1.5%) is mixed with 567 µL diluent (Complete PneumaCult TM-Ex Plus Basal Medium) and 100 µL cell suspension.
- (27)
- After the last centrifugation step (see 18), when the calculated cell numbers for seeding are pelleted, the cell pellet is directly solved in pre-heated (37 °C) Complete PneumaCult TM-Ex Plus Basal Medium.
- (28)
- The cell-containing GrowDex® solution is incubated at 37 °C until Transwell inserts are readily prepared for seeding.
- (29)
- To seed the cells upside-down, the Transwells are fixed in 6-well plates upside-down.
- (30)
- When all Transwells are fixed in 6-well plates in the upside-down position, 100 µL of 0.5% GrowDex® solution/cell mixture is added to the membranes.
- (31)
- When cells are freshly seeded to Transwells, they, again, are undergoing an expansion phase during the first 3–4 days. For this, cells have to be cultured under submerged conditions. During the first hours of overnight incubation of upside-down seeded cells, it is not a problem to cover cells with medium from the apical side because there is medium added to the GrowDex® solution as diluent.
- (32)
- In the next step, wrap parafilm around the plate so that the lid does not touch the GrowDex/cell drop on top of the membranes
- (33)
- After an overnight incubation at 37 °C and 5% CO2, flip the Transwells back to the normal position and insert them to 24-well plates.
- (34)
- Add 500 µL pre-warmed Complete PneumaCult TM-Ex Plus Basal Medium to the basal chamber of the wells, which will then be the apical layer of the cells to be put in the air phase. Medium is also added to the inside of the Transwells to guarantee submerged conditions (liquid–liquid phase conditions).
- (35)
- Change the medium every second day.
- (36)
- Shift cells to ALI when confluency of 80% is reached, which is normally after about 3 days.

4.3.5. Shift to ALI (Air Liquid Interphase)
- (37)
- For the shift to the ALI state, prepare PneumaCultTM-ALI Basal Medium and PneumaCultTM- ALI Maintenance medium.
- (38)
- For every upside-down seeded Transwell, 250 µL of medium is needed.
- (39)
- Pre-heat the Maintenance medium at 37 °C.
- (40)
- Aspirate the medium at both sides of the membrane and shift the Transwells into new wells.
- (41)
- !CAUTION The ALI-Maintenance medium is, from now on, only added to the basolateral side of the cells. So in the case of the upside-down seeded cells, the medium has to be added inside the Transwell and not into the 24-well plate directly under standard culture conditions.
- (42)
- Change the medium every second day after 21 days in ALI culture, and on collagen, epithelial cells are completely differentiated independent on the seeding type (normal or upside-down) and are ready for further experiments. In contrast, cells grown in GrowDex® can be used from day 14 and on in ALI.
- (43)
- !CAUTION If culturing cells over a long period of time, the mucous has to be carefully aspirated once a week using a 100 µL tip.
4.3.6. Preparing Cells for Live Cell Imaging
- (44)
- For preparing the master mix, all compounds were diluted in D-PBS. Small amounts of Master mixes (50–100 µL/well) are added to the bottom of a glass-bottom dish at concentrations found in Table 1.
- (45)
- Optional: To guarantee improved staining of the cells, and in case the mucous is not of interest for imaging, carefully aspirate the mucus layer on the apical side of the cells.
- (46)
- Transfer the Transwell directly to the Master mix in the glass-bottom dish.
- (47)
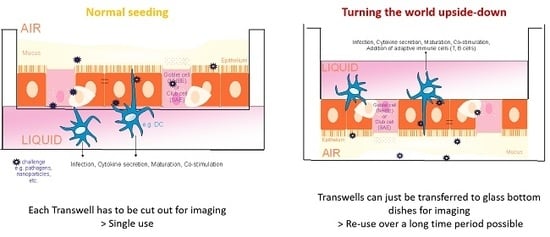
- Imaging can be immediately started, but fluorescence intensities increase with time. The advantage of seeding cells upside-down, concerning live cell imaging approaches, is that the complete Transwell can be transferred to a sterile 6- or 12-well glass-bottom plate without cutting the membrane. Upside-down seeded Transwells can—after the imaging period—be taken back into the culture without any harm to the cells.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Gamm, U.A.; Huang, B.K.; Mis, E.K.; Khokha, M.K.; Choma, M.A. Visualization and quantification of injury to the ciliated epithelium using quantitative flow imaging and speckle variance optical coherence tomography. Sci. Rep. 2017, 7, 15115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chateau, S.; D’Ortona, U.; Poncet, S.; Favier, J. Transport and mixing induced by beating cilia in human airways. Front. Physiol. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Puchelle, E.; Zahm, J.-M.; Tournier, J.-M.; Coraux, C. Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2006, 3, 726–733. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.; Robbins, R.; Romberger, D.; Sisson, J.; Spurzem, J.; Teschler, H.; Rennard, S. Immunological functions of the pulmonary epithelium. Eur. Respir. J. 1995, 8, 127–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- A Whitsett, J.; Alenghat, T. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat. Immunol. 2015, 16, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Huang, S.X.; De Carvalho, A.L.R.T.; Ho, S.-H.; Islam, M.N.; Volpi, S.; Notarangelo, L.D.; Ciancanelli, M.; Casanova, J.-L.; Bhattacharya, J.; et al. A three-dimensional model of human lung development and disease from pluripotent stem cells. Nature 2017, 19, 542–549. [Google Scholar] [CrossRef] [PubMed]
- Chandorkar, P.; Posch, W.; Zaderer, V.; Blatzer, M.; Steger, M.; Ammann, C.G.; Binder, U.; Hermann, M.; Hörtnagl, P.; Lass-Flörl, C.; et al. Fast-track development of an in vitro 3D lung/immune cell model to study Aspergillus infections. Sci. Rep. 2017, 7, 11644. [Google Scholar] [CrossRef] [PubMed]
- Rayner, R.E.; Makena, P.; Prasad, G.L.; Cormet-Boyaka, E. Optimization of normal human bronchial epithelial (NHBE) cell 3D cultures for in vitro lung model studies. Sci. Rep. 2019, 9, 500. [Google Scholar] [CrossRef] [PubMed]
- Bergeron, A.; Rossi, A. Imaging of Cellular Fluorescence on Corning’s Transwell® Permeable Supports. Available online: https://www.corning.com/catalog/cls/documents/application-notes/CLS-AN-521-A4.pdf (accessed on 18 October 2019).
- Pijuan, J.; Barceló, C.; Moreno, D.F.; Maiques, O.; Sisó, P.; Marti, R.M.; Macià, A.; Panosa, A. In vitro Cell Migration, Invasion, and Adhesion Assays: From Cell Imaging to Data Analysis. Front. Cell Dev. Boil. 2019, 7, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Antibody | Final Concentration |
|---|---|
| Hoechst 33342 | 2 µg/mL |
| WGA-488 | 5 µg/mL |
| Mitotracker in far red | 100–500 nM |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaderer, V.; Hermann, M.; Lass-Flörl, C.; Posch, W.; Wilflingseder, D. Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model. Cells 2019, 8, 1292. https://doi.org/10.3390/cells8101292
Zaderer V, Hermann M, Lass-Flörl C, Posch W, Wilflingseder D. Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model. Cells. 2019; 8(10):1292. https://doi.org/10.3390/cells8101292
Chicago/Turabian StyleZaderer, Viktoria, Martin Hermann, Cornelia Lass-Flörl, Wilfried Posch, and Doris Wilflingseder. 2019. "Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model" Cells 8, no. 10: 1292. https://doi.org/10.3390/cells8101292
APA StyleZaderer, V., Hermann, M., Lass-Flörl, C., Posch, W., & Wilflingseder, D. (2019). Turning the World Upside-Down in Cellulose for Improved Culturing and Imaging of Respiratory Challenges within a Human 3D Model. Cells, 8(10), 1292. https://doi.org/10.3390/cells8101292