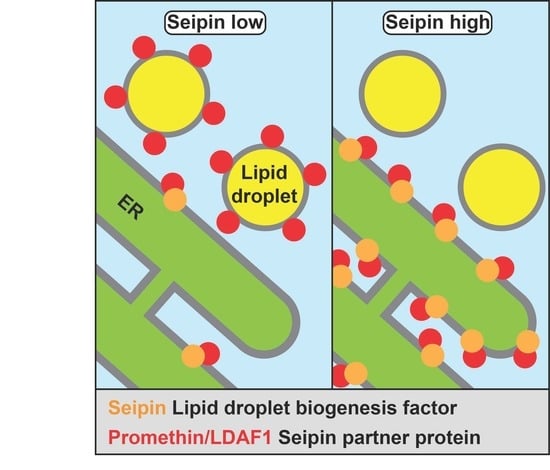
Promethin Is a Conserved Seipin Partner Protein
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mammalian Cell Culture and Plasmids
2.2. Yeast Growth, Strains, and Plasmids
2.3. RNA Extraction and Quantification
2.4. Immunofluorescence
2.5. Immunoprecipitation
2.6. Microscopy of Cultured Mammalian Cells and Yeast
3. Results
3.1. Promethin is an LD-associated Protein That Is Induced during Adipogenesis
3.2. Promethin Is a Novel Seipin Partner Protein
3.3. Promethin Subcellular Localization Is Modulated by Seipin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Walther, T.C.; Chung, J.; Farese, R.V. Lipid Droplet Biogenesis. Annu. Rev. Cell Dev. Biol. 2017, 33, 491–510. [Google Scholar] [CrossRef]
- Chen, X.; Goodman, J.M. The Collaborative Work of Droplet Assembly. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 1205–1211. [Google Scholar] [CrossRef]
- Magré, J.; Delépine, M.; Khallouf, E.; Gedde-Dahl, T.; Van Maldergem, L.; Sobel, E.; Papp, J.; Meier, M.; Mégarbané, A.; Bachy, A.; et al. Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat. Genet. 2001, 28, 365–370. [Google Scholar] [CrossRef]
- Patel, H.; Hart, P.E.; Warner, T.T.; Houlston, R.S.; Patton, M.A.; Jeffery, S.; Crosby, A.H. The Silver Syndrome Variant of Hereditary Spastic Paraplegia Maps to Chromosome 11q12-q14, with Evidence for Genetic Heterogeneity within This Subtype. Am. J. Hum. Genet. 2001, 69, 209–215. [Google Scholar] [CrossRef]
- Berardinelli, W. An undiagnosed endocrinometabolic syndrome: Report of 2 cases. J. Clin. Endocrinol. Metab. 1954, 14, 193–204. [Google Scholar] [CrossRef]
- Seip, M. Lipodystrophy and gigantism with associated endocrine manifestation: A new diencephalic syndrome. Acta Paediatr. Scand. 1959, 48, 455–474. [Google Scholar] [CrossRef]
- Windpassinger, C.; Wagner, K.; Petek, E.; Fischer, R.; Auer-Grumbach, M. Refinement of the “Silver syndrome locus” on chromosome 11q12-q14 in four families and exclusion of eight candidate genes. Hum. Genet. 2003, 114, 99–109. [Google Scholar] [CrossRef]
- Lundin, C.; Nordström, R.; Wagner, K.; Windpassinger, C.; Andersson, H.; von Heijne, G.; Nilsson, I. Membrane topology of the human seipin protein. FEBS Lett. 2006, 580, 2281–2284. [Google Scholar] [CrossRef]
- Ito, D.; Fujisawa, T.; Iida, H.; Suzuki, N. Characterization of seipin/BSCL2, a protein associated with spastic paraplegia 17. Neurobiol. Dis. 2008, 31, 266–277. [Google Scholar] [CrossRef]
- Ito, D.; Suzuki, N. Molecular pathogenesis of seipin/BSCL2-related motor neuron diseases. Ann. Neurol. 2007, 61, 237–250. [Google Scholar] [CrossRef]
- Wee, K.; Yang, W.; Sugii, S.; Han, W. Towards a mechanistic understanding of lipodystrophy and seipin functions. Biosci. Rep. 2014, 34, 583–591. [Google Scholar] [CrossRef]
- Mcilroy, G.D.; Suchacki, K.; Roelofs, A.J.; Yang, W.; Fu, Y.; Bai, B.; Wallace, R.J.; De Bari, C.; Cawthorn, W.P.; Han, W.; et al. Adipose specific disruption of seipin causes early-onset generalised lipodystrophy and altered fuel utilisation without severe metabolic disease. Mol. Metab. 2018, 10, 55–65. [Google Scholar] [CrossRef]
- Prieur, X.; Dollet, L.; Takahashi, M.; Nemani, M.; Pillot, B.; Le May, C.; Mounier, C.; Takigawa-Imamura, H.; Zelenika, D.; Matsuda, F.; et al. Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 2013, 56, 1813–1825. [Google Scholar] [CrossRef]
- Chen, W.; Chang, B.; Saha, P.; Hartig, S.M.; Li, L.; Reddy, V.T.; Yang, Y.; Yechoor, V.; Mancini, M.A.; Chan, L. Berardinelli-Seip Congenital Lipodystrophy 2/Seipin Is a Cell-Autonomous Regulator of Lipolysis Essential for Adipocyte Differentiation. Mol. Cell. Biol. 2012, 32, 1099–1111. [Google Scholar] [CrossRef]
- Cui, X.; Wang, Y.; Tang, Y.; Liu, Y.; Zhao, L.; Deng, J.; Xu, G.; Peng, X.; Ju, S.; Liu, G.; et al. Seipin ablation in mice results in severe generalized lipodystrophy. Hum. Mol. Genet. 2011, 20, 3022–3030. [Google Scholar] [CrossRef]
- Binns, D.; Lee, S.; Hilton, C.L.; Jiang, Q.X.; Goodman, J.M. Seipin is a discrete homooligomer. Biochemistry 2010, 49, 10747–10755. [Google Scholar] [CrossRef]
- Sim, M.F.; Talukder, M.M.; Dennis, R.J.; O’Rahilly, S.; Edwardson, J.M.; Rochford, J.J. Analysis of naturally occurring mutations in the human lipodystrophy protein seipin reveals multiple potential pathogenic mechanisms. Diabetologia 2013, 56, 2498–2506. [Google Scholar] [CrossRef]
- Yan, R.; Qian, H.; Lukmantara, I.; Gao, M.; Du, X.; Yan, N.; Yang, H. Human SEIPIN Binds Anionic Phospholipids. Dev. Cell 2018, 47, 248–256. [Google Scholar] [CrossRef]
- Sui, X.; Arlt, H.; Brook, K.P.; Lai, Z.W.; DiMaio, F.; Marks, D.S.; Liao, M.; Farese, R.V.; Walther, T.C. Cryo-electron microscopy structure of the lipid droplet-formation protein seipin. J. Cell Biol. 2018, 217, 4080–4091. [Google Scholar] [CrossRef]
- Szymanski, K.M.; Binns, D.; Bartz, R.; Grishin, N.V.; Li, W.-P.; Agarwal, A.K.; Garg, A.; Anderson, R.G.W.; Goodman, J.M. The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc. Natl. Acad. Sci. USA 2007, 104, 20890–20895. [Google Scholar] [CrossRef]
- Salo, V.T.; Belevich, I.; Li, S.; Karhinen, L.; Vihinen, H.; Vigouroux, C.; Magré, J.; Thiele, C.; Hölttä-Vuori, M.; Jokitalo, E.; et al. Seipin regulates ER–lipid droplet contacts and cargo delivery. EMBO J. 2016, 35, 2699–2716. [Google Scholar] [CrossRef] [PubMed]
- Grippa, A.; Buxó, L.; Mora, G.; Funaya, C.; Idrissi, F.Z.; Mancuso, F.; Gomez, R.; Muntanyà, J.; Sabidó, E.; Carvalho, P. The seipin complex Fld1/Ldb16 stabilizes ER-lipid droplet contact sites. J. Cell Biol. 2015, 211, 829–844. [Google Scholar] [CrossRef] [PubMed]
- Schuldiner, M.; Bohnert, M. A different kind of love–lipid droplet contact sites. Biochim. Biophys. Acta-Mol. Cell Biol. Lipids 2017, 1862, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Thein, S.; Wang, X.; Bi, X.; Ericksen, R.E.; Xu, F.; Han, W. BSCL2/seipin regulates adipogenesis through actin cytoskeleton remodelling. Hum. Mol. Genet. 2014, 23, 502–513. [Google Scholar] [CrossRef] [PubMed]
- Talukder, M.M.; Sim, M.F.; O’Rahilly, S.; Edwardson, J.M.; Rochford, J.J. Seipin oligomers can interact directly with AGPAT2 and lipin 1, physically scaffolding critical regulators of adipogenesis. Mol. Metab. 2015, 4, 199–209. [Google Scholar] [CrossRef]
- Bi, J.; Wang, W.; Liu, Z.; Hueng, X.; Jiang, Q.; Liu, G.; Wang, Y.; Huang, X. Seipin promotes adipose tissue fat storage through the ER Ca2+-ATPase SERCA. Cell Metab. 2014, 19, 861–871. [Google Scholar] [CrossRef]
- Sim, M.F.; Dennis, R.J.; Aubry, E.M.; Ramanathan, N.; Sembongi, H.; Saudek, V.; Ito, D.; O’Rahilly, S.; Siniossoglou, S.; Rochford, J.J. The human lipodystrophy protein seipin is an ER membrane adaptor for the adipogenic PA phosphatase lipin 1. Mol. Metab. 2012, 2, 38–46. [Google Scholar] [CrossRef]
- Pagac, M.; Cooper, D.E.; Qi, Y.; Lukmantara, I.E.; Mak, H.Y.; Wu, Z.; Tian, Y.; Liu, Z.; Lei, M.; Du, X.; et al. SEIPIN Regulates Lipid Droplet Expansion and Adipocyte Development by Modulating the Activity of Glycerol-3-phosphate Acyltransferase. Cell Rep. 2016, 17, 1546–1559. [Google Scholar] [CrossRef] [PubMed]
- Lounis, M.A.; Lalonde, S.; Rial, S.A.; Bergeron, K.F.; Ralston, J.C.; Mutch, D.M.; Mounier, C. Hepatic BSCL2 (Seipin) Deficiency Disrupts Lipid Droplet Homeostasis and Increases Lipid Metabolism via SCD1 Activity. Lipids 2017, 52, 129–150. [Google Scholar] [CrossRef] [PubMed]
- Renvoisé, B.; Malone, B.; Falgairolle, M.; Munasinghe, J.; Stadler, J.; Sibilla, C.; Park, S.H.; Blackstone, C. Reep1 null mice reveal a converging role for hereditary spastic paraplegia proteins in lipid droplet regulation. Hum. Mol. Genet. 2016, 25, 5111–5125. [Google Scholar]
- Eisenberg-Bord, M.; Mari, M.; Weill, U.; Rosenfeld-Gur, E.; Moldavski, O.; Castro, I.G.; Soni, K.G.; Harpaz, N.; Levine, T.P.; Futerman, A.H.; et al. Identification of seipin-linked factors that act as determinants of a lipid droplet subpopulation. J. Cell Biol. 2018, 217, 269–282. [Google Scholar] [CrossRef]
- Teixeira, V.; Johnsen, L.; Martínez-Montañés, F.; Grippa, A.; Buxó, L.; Idrissi, F.Z.; Ejsing, C.S.; Carvalho, P. Regulation of lipid droplets by metabolically controlled Ldo isoforms. J. Cell Biol. 2018, 217, 127–138. [Google Scholar] [CrossRef]
- Payne, V.A.; Grimsey, N.; Tuthill, A.; Virtue, S.; Gray, S.L.; Nora, E.D.; Semple, R.K.; O’Rahilly, S.; Rochford, J.J. The human lipodystrophy gene BSCL2/Seipin may be essential for normal adipocyte differentiation. Diabetes 2008, 57, 2055–2060. [Google Scholar] [CrossRef]
- Longtine, S.M.; Pringle, R.J. Additional Modules for Versatile and Economical PCR-based Gene Deletion and Modification in Saccharomyces cerevisiae. Yeast 1998, 14, 953–961. [Google Scholar] [CrossRef]
- Brachmann, C.B.; Davies, A.; Cost, G.J.; Caputo, E.; Li, J.; Hieter, P.; Boeke, J.D. Designer deletion strains derived fromSaccharomyces cerevisiae S288C: A useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 1998, 14, 115–132. [Google Scholar] [CrossRef]
- Janke, C.; Magiera, M.M.; Rathfelder, N.; Taxis, C.; Reber, S.; Maekawa, H.; Moreno-Borchart, A.; Doenges, G.; Schwob, E.; Schiebel, E.; et al. A versatile toolbox for PCR-based tagging of yeast genes: New fluorescent proteins, more markers and promoter substitution cassettes. Yeast 2004, 21, 947–962. [Google Scholar] [CrossRef]
- Gietz, R.D.; Woods, R.A. Yeast Transformation by the LiAc/SS Carrier DNA/PEG Method. In Yeast Protocols; Humana Press: Totowa, NJ, USA, 2006; pp. 107–120. [Google Scholar]
- Yu, S.; Viswakarma, N.; Batra, S.K.; Sambasiva Rao, M.; Reddy, J.K. Identification of promethin and PGLP as two novel up-regulated genes in PPARγ1-induced adipogenic mouse liver. Biochimie 2004, 86, 743–761. [Google Scholar] [CrossRef]
- Wang, C.-W.; Miao, Y.-H.; Chang, Y.-S. Control of lipid droplet size in budding yeast requires the collaboration between Fld1 and Ldb16. J. Cell Sci. 2014, 127, 1214–1228. [Google Scholar] [CrossRef]
- Wang, H.; Becuwe, M.; Housden, B.E.; Chitraju, C.; Porras, A.J.; Graham, M.M.; Liu, X.N.; Thiam, A.R.; Savage, D.B.; Agarwal, A.K.; et al. Seipin is required for converting nascent to mature lipid droplets. Elife 2016, 5, e16582. [Google Scholar] [CrossRef]
- Fei, W.; Shui, G.; Gaeta, B.; Du, X.; Kuerschner, L.; Li, P.; Brown, A.J.; Wenk, M.R.; Parton, R.G.; Yang, H. Fld1p, a functional homologue of human seipin, regulates the size of lipid droplets in yeast. J. Cell Biol. 2008, 180, 473–482. [Google Scholar] [CrossRef]
- Kory, N.; Farese, R.V.; Walther, T.C. Targeting Fat: Mechanisms of Protein Localization to Lipid Droplets. Trends Cell Biol. 2016, 26, 535–546. [Google Scholar] [CrossRef]
- Bohnert, M. Wrapping up the fats—A structure of the lipid droplet biogenesis protein seipin. J. Cell Biol. 2018, 217, 4053–4054. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castro, I.G.; Eisenberg-Bord, M.; Persiani, E.; Rochford, J.J.; Schuldiner, M.; Bohnert, M. Promethin Is a Conserved Seipin Partner Protein. Cells 2019, 8, 268. https://doi.org/10.3390/cells8030268
Castro IG, Eisenberg-Bord M, Persiani E, Rochford JJ, Schuldiner M, Bohnert M. Promethin Is a Conserved Seipin Partner Protein. Cells. 2019; 8(3):268. https://doi.org/10.3390/cells8030268
Chicago/Turabian StyleCastro, Inês G., Michal Eisenberg-Bord, Elisa Persiani, Justin J. Rochford, Maya Schuldiner, and Maria Bohnert. 2019. "Promethin Is a Conserved Seipin Partner Protein" Cells 8, no. 3: 268. https://doi.org/10.3390/cells8030268
APA StyleCastro, I. G., Eisenberg-Bord, M., Persiani, E., Rochford, J. J., Schuldiner, M., & Bohnert, M. (2019). Promethin Is a Conserved Seipin Partner Protein. Cells, 8(3), 268. https://doi.org/10.3390/cells8030268